Abstract
Methylating agents are widely distributed environmental carcinogens. Moreover, they are being used in cancer chemotherapy. The primary target of methylating agents is DNA, and therefore, DNA repair is the first-line barrier in defense against their toxic and carcinogenic effects. Methylating agents induce in the DNA O6-methylguanine (O6MeG) and methylations of the ring nitrogens of purines. The lesions are repaired by O6-methylguanine-DNA methyltransferase (Mgmt) and by enzymes of the base excision repair (BER) pathway, respectively. Whereas O6MeG is well established as a pre-carcinogenic lesion, little is known about the carcinogenic potency of base N-alkylation products such as N3-methyladenine and N3-methylguanine. To determine their role in cancer formation and the role of BER in cancer protection, we checked the response of mice with a targeted gene disruption of Mgmt or N-alkylpurine-DNA glycosylase (Aag) or both Mgmt and Aag, to azoxymethane (AOM)-induced colon carcinogenesis, using non-invasive mini-colonoscopy. We demonstrate that both Mgmt- and Aag-null mice show a higher colon cancer frequency than the wild-type. With a single low dose of AOM (3 mg/kg) Aag-null mice showed an even stronger tumor response than Mgmt-null mice. The data provide evidence that both BER initiated by Aag and O6MeG reversal by Mgmt are required for protection against alkylation-induced colon carcinogenesis. Further, the data indicate that non-repaired N-methylpurines are not only pre-toxic but also pre-carcinogenic DNA lesions.
Introduction
Methylating agents that are widely distributed in the environment cause a significant contribution to tumor formation in human beings. Human exposure to alkylating carcinogens can result from cigarette smoke, fuel combustion, the presence of heterocyclic amines in the diet and from endogenous nitrosation of amides and amines mediated by enteric bacteria and the reaction of secondary amines with nitrite (1–4). Moreover, methylating agents are also used in tumor chemotherapy, e.g. for the treatment of malignant gliomas (5) and metastatic malignant melanomas (6).
Methylating agents, notably the so-called SN1-type agents, produce a wide spectrum of DNA adducts, including O6-methylguanine (O6MeG), O4-methylthymine, N7-methylguanine, N3-methyladenine and N3-methylguanine (7). O6MeG and O4-methylthymine are instructive lesions causing base mispairing and thus lead to point mutations (8). Since O4-methylthymine is induced by SN1 agents in very low amounts (<0.3% of total DNA methylation products compared with 8% of O6MeG) (7), its contribution to mutagenesis and carcinogenesis is usually neglected. Thus, the current paradigm ascribes O6MeG as the major mutagenic and carcinogenic DNA adduct induced by methylating agents. This is supported by the finding that O6-methylguanine-DNA methyltransferase (Mgmt), which repairs O6MeG in a single-step suicide reaction (9), is highly efficient in suppressing point mutations and genotoxicity in vitro and in vivo (for review see ref. 10). Mgmt was also shown to prevent from cancer formation induced by O6MeG-producing agents. Thus, human Mgmt expressed in mice reduced N-methyl-N-nitrosourea (MNU)-induced thymomas (11) and liver tumors upon dimethylnitrosamine exposure (12). It also protected against lung carcinogenesis (13) and azoxymethane (AOM)-induced aberrant crypt foci and mutations in K-ras (14). Mice expressing human Mgmt in skin were protected from skin tumor formation induced by MNU and the chloroethylating anticancer drug ACNU (nimustine), using the two-stage tumor initiation–promotion protocol in which 12-O-tetradecanoylphorbol 13-acetate was applied as tumor promoter (15,16). Mgmt transgenic overexpression also protected against MNU-induced conversion of benign into malignant tumors (17). In contrast, Mgmt-lacking mice are more sensitive than isogenic wild-type (WT) mice to the genotoxic effects of methylating agents (18–20). They are also highly vulnerable to cancer induction by alkylating agents, which was shown for the formation of thymic lymphomas (21) and colonic aberrant crypt foci (22).
O6MeG is not only a pre-mutagenic and pre-carcinogenic but also a pre-cytotoxic DNA lesion. Toxicity triggered by O6MeG is dependent on the processing of O6MeG/thymine mispairs by MutSα-dependent mismatch repair, in which thymine is excised and then reinserted opposite the O6MeG lesion during synthesis of the repair patch. This leads to a repetitive futile process that likely allows the formation of long stretches of gapped DNA that interferes with DNA replication causing DNA double-strand breaks that in turn trigger apoptosis (for review see ref. 10). In fact, Mgmt-deficient cells in vitro (21,23) and Mgmt-null mice (22,24) are highly sensitive to the toxic effect of SN1 methylating agents compared with the isogenic Mgmt-expressing cells and individuals. Further support for this model was provided by mismatch repair-deficient cells and mice, which are highly refractory to the killing effect of SN1 methylating agents (25). As expected, Mgmt/mismatch repair-double-knockout mice are resistant to the toxic effect of SN1 methylating agents, but at the same time show a high tumor incidence upon methylating agent treatment (22,26).
While these studies clearly demonstrated that O6MeG is a key node in cancer formation and Mgmt most important in its defense, the role of N-methylation products in carcinogenesis has not yet been elucidated in detail. N-methylation products such as N7-methylguanine, N3-methyladenine and N3-methylguanine are the major adducts formed in the DNA by both SN1 and SN2 alkylating agents, amounting to 70, 9 and 2%, respectively, of total methylation products induced in the DNA by MNU in vitro (7). These adducts are repaired by base excision repair (BER) (for review see ref. 27) that represents the major pathway for their removal from DNA (28). No human repair-deficient disorders have been described so far suffering from a complete deficiency in BER, which may be taken to indicate that BER is essential for human development and survival.
The N-methylpurines noted above are recognized and removed from DNA by N-methylpurine-DNA glycosylase (MPG, alias N-alkylpurine-DNA glycosylase, Aag). Aag is a type I DNA glycosylase that, upon release of the methylpurine from the DNA, leaves an abasic site in the DNA that is subsequently repaired by the other components of BER (for review see ref. 29). Aag-null mice are viable and, similar to Mgmt-null mice (18), do not show a spontaneous pathological phenotype (30). Mouse fibroblasts derived from Aag-null mice are sensitive to methylating agents (31) indicating that in this cell type, unrepaired N-methylpurines contribute to the cytotoxicity of methylating agents. Interestingly, Aag-deficient mice treated with methyl methanesulfonate that produces predominantly base N-methylations do not suffer from retinal degeneration, whereas WT mice do (32). This indicates that in some cell types in the body, even in the absence of replication, BER intermediates may cause cytotoxic effects, whereas non-repaired N-methylated bases can be tolerated to some extent.
Although it is clear that N-methylpurines are toxic and genotoxic (33), the contribution to carcinogenicity of N-methylated bases has been a matter of controversy for many years. Thus, SN2-type agents producing predominantly N-methylations such as methyl methanesulfonate exhibit only weak carcinogenic potency (34) and were not tumor initiating in two-stage skin carcinogenesis, but rather triggered tumor promotion (35). On the other hand, the finding that Aag-deficient mice are more resistant than WT mice to retinal degeneration following methyl methanesulfonate (32) indicates that organ specificity in the genotoxic and putative carcinogenic response to methylating agents has to be taken into account. Here, we ascertained the response of Aag-null mice to colon cancer formation, and compared it with Mgmt-null mice and Aag/Mgmt-double knockouts (DKOs), lacking both DNA repair proteins. We made use of mini-colonoscopy where neoplastic changes in the colon can be detected from very early stages without killing the animals (36). We demonstrate that Aag-deficient mice are more susceptible than Mgmt-deficient mice to colon cancer formation induced by a low non-toxic dose of the SN1 methylating agent AOM followed by promotion with dextran sulfate sodium (DSS). Our data demonstrate that not only repair of O6MeG by Mgmt but also the repair of N-methylation lesions by Aag is highly important for the defense against colon cancer.
Materials and methods
Mice and induction of colorectal carcinogenesis
Mgmt- and Aag-null mice on a C57BL/6 background were described previously (18,30). Twelve- to fourteen-week-old sex-matched Mgmt, Aag, Mgmt/Aag-double-null (DKO) and C57BL/6 WT control mice were used in the study. The genotype was checked routinely by PCR. Animal protocols were approved by the Animal Care and Use Committee of the University of Mainz. DSS-induced colitis or colitis-associated colorectal cancer was performed as described previously (37) and outlined in Figure 1A. In brief, mice received a single intraperitoneal injection of the mutagenic agent AOM (Sigma–Aldrich, Deisendorf, Germany) in phosphate-buffered saline (PBS) (3 or 10 mg/kg body weight; freshly prepared before administration) on day 0. Starting on day 2, colitis was induced by two cycles of 1 % DSS. For analysis of toxic dose and acute inflammation (38), 2% DSS (MP Biomedicals, Illkirch, France) was administered in drinking water followed by normal drinking water.
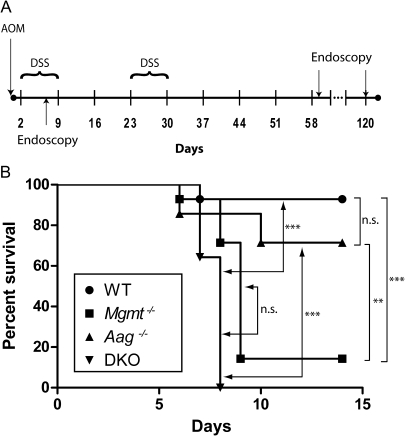
Fig. 1.
Experimental protocol and mortality of WT, Mgmt−/−, Aag−/− and Mgmt−/−/Aag−/− mice in the AOM/DSS model. (A) Schematic outline of the experimental setup for the induction of AOM/DSS-induced colon carcinogenesis. (B) Survival analysis of mice that received AOM (10 mg/kg) and 2 % DSS (n = 14 per group). Statistical analysis of survival was performed using log rank test. ***P < 0.001, **P < 0.01; n.s. not significant.
Mouse endoscopy
For the continuous monitoring of colonic inflammation and tumorigenesis, a high-resolution video miniendoscope (Karl Storz, Tuttlingen, Germany) was used. Endoscopic scoring of colitis activity was based on the murine endoscopic index of colitis severity scoring system that includes classification of mucosal translucency, vascularity, granularity, fibrin deposition and stool consistency (36). In some experiments, the Exera II CV-180 narrow band imaging system from Olympus was used to analyse colonic changes in the microvasculature and changes of the crypt pattern (38). Scoring of tumor development was based on tumor size and the number of tumors, as described previously (36). Briefly, tumor sizes were graded as grade 1 (very small but detectable tumor), grade 2 (tumor covering up to one-eighth of the colonic circumference), grade 3 (tumor covering up to a quarter of the colonic circumference), grade 4 (tumor covering up to half of the colonic circumference) and grade 5 (tumor covering more than half of the colonic circumference).
Histopathology
Colons were removed, flushed with PBS, fixed in 10% neutral buffered formalin overnight, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin for histopathological evaluation of inflammation and neoplasia. The degree of inflammation was graded semiquantitatively on a scale from 0 to 6 in a blinded fashion as described previously (38). The inflammation score was combined of inflammatory cell infiltration ranging from 0 to 3 and tissue damage ranging from 0 to 3. In some experiments, longitudinally opened colons were stained for 5 min with methylene blue solution (1%) for macroscopical analysis and evaluation of aberrant crypt foci.
Detection of apoptosis
For detection of AOM-induced apoptosis, mice were injected with 10 mg/kg AOM in PBS. Forty-eight hours later, colons were removed, flushed with PBS, fixed in 10% neutral buffered formalin overnight, embedded in paraffin and sectioned at 5 μm thickness. Apoptotic cells were detected by terminal deoxynucleotidyl transferase-mediated deoyuridine triphosphate nick end labeling (TUNEL) assay using the fluorescein in situ cell death detection kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions.
Statistics
Assays were performed as outlined in the legend of the figures.
Results
Initially, mice WT and knockout for Mgmt, Aag and Mgmt/Aag were treated with a single dose of AOM (10 mg/kg), which was insufficient to induce tumors on its own, followed by two cycles with DSS (2% in the drinking water) (for the experimental protocol see Figure 1A). As shown in Figure 1B, nearly all WT mice survived the treatment, whereas the knockout individuals died to different extent. Aag−/− mice were not significantly more susceptible than WT mice showing >70% survival after 14 days, whereas Mgmt−/− mice display high mortality showing ∼15% survival (Figure 1B). Mgmt−/− and Mgmt−/−/Aag−/− (designated as DKO) were not significantly different in their toxic response suggesting that Mgmt is particularly important for protection against AOM/DSS-induced toxicity. To obtain information about the pathological events leading to high mortality in Mgmt-deficient mice, we analyzed the weight development and mucosal alterations in AOM/DSS-treated animals. As a result, both Mgmt−/− and DKO mice showed a rapid weight loss that was significantly different from WT and Aag−/− mice, which lost only moderate weight (Figure 2A). Endoscopic analysis at day 6 of the experiment clearly demonstrated that this severe weight loss after AOM/DSS treatment was associated with strong intestinal damage in Mgmt−/− and DKO mice (Figure 2B). Most notably, intestinal pathology was characterized by multiple deep ulcerations in Mgmt−/− and DKO mice (an example is shown in Figure 2C) indicating that severe intestinal damage is essential for wasting disease and lethality in these mice. Overall, the data show that Mgmt−/− mice are more sensitive than Aag−/− mice, and mice deficient in both Mgmt and Aag exhibit sensitivity similar to Mgmt−/− mice as to the toxic effect induced by AOM followed by DSS.
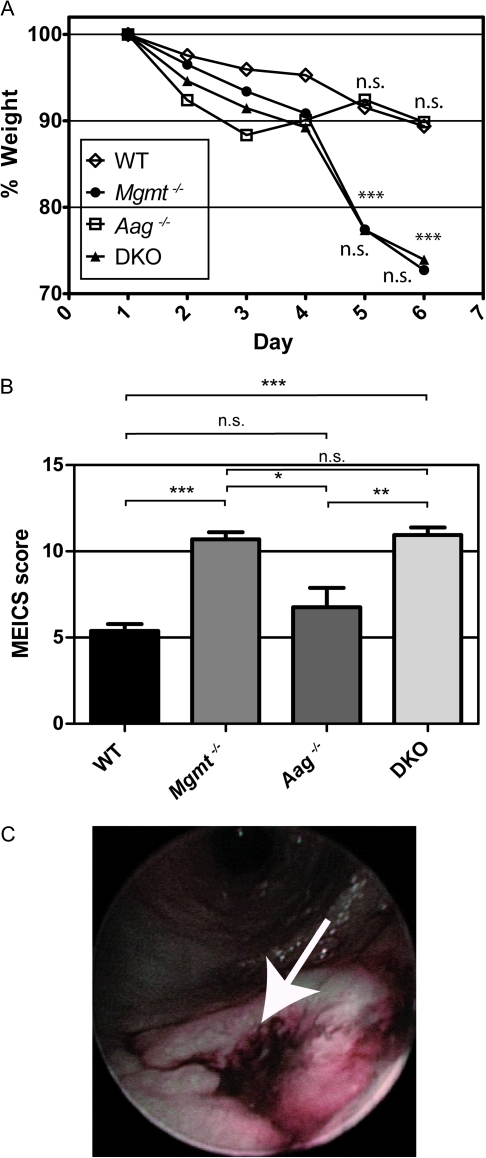
Fig. 2.
Increased acute mucosal inflammation in Mgmt−/− and Mgmt−/−/Aag−/− versus Aag−/− and WT mice after administration of AOM/DSS. (A) Weight analysis of WT (n = 8), Mgmt−/− (n = 6), Aag−/− (n = 8) and Mgmt−/−Aag−/− (n = 8) mice treated with AOM (10 mg/kg) and 2% DSS. Weight differences between Mgmt−/− and DKO versus WT and Aag−/− groups were highly significant at day 5 and 6. Differences of Mgmt−/− versus DKO and WT versus Aag−/− mice were not significant. (B) Analysis of mucosal inflammation by mini-colonoscopy at day 6 after administration of DSS. Data represent mean ± SEM. Statistical analysis was performed using unpaired, two-tailed Student’s t-test. ***P < 0.001, **P < 0.01, *P < 0.05; n.s. not significant. (C) Endoscopic image of an area with severe ulcerative inflammation (labeled by arrow) in DKO mice.
Since the use of 2% DSS resulted in 90–100% mortality in the Mgmt−/− and DKO group in the period between 6 and 10 days after treatment (Fig. 1B), we reduced the dose of the promoter and used 1% DSS, which caused only mild colon inflammation and complete survival in all experimental groups (data not shown). We should note that the first treatment cycle with DSS occurred 2 days after AOM injection in order to avoid any possible interference of the tumor promoter DSS with AOM damage fixation. Animals were weighed twice per week, and at day 60 and 120 following AOM treatment, they were inspected by mini-colonoscopy (36) to determine non-invasively the frequency of neoplastic lesions (adenocarcinomas in situ) in the colon (Figure 1A). Examples of colon inspection by mini-colonoscopy at day 60 are shown in Figure 3. The number of tumors per mice after treatment with AOM alone was between 0 and 0.4 in the DKO group and for DSS alone between 0 and 0.2 in the Mgmt−/− group. This shows that DSS alone (treatment over two cycles) was ineffective in increasing the spontaneous tumor yield significantly, even in the DKO group. Similarly, a single treatment with AOM alone was not sufficient to induce a significant tumor yield both in the WT and the repair knockout mice strains. Only the combination of AOM plus DSS was effective in colon cancer induction. The tumor yield after treatment with a low and a high dose of AOM of 3 and 10 mg/kg, respectively, followed by 1% DSS is presented in Figure 4A and B, respectively. The low dose AOM treatment followed by 1% DSS did not induce colon carcinomas in Mgmt−/− mice at higher level than in the WT, whereas Aag−/− mice displayed a significantly higher tumor incidence. The DKO individuals exhibited the highest tumor incidence, which was, however, not significantly different from Aag−/− mice (Figure 4A). For the 10 mg/kg AOM dose Mgmt−/− mice clearly responded with a tumor yield that was significantly higher than in the WT and similar to Aag−/− and DKO mice (Figure 4B). Obviously, with a tumor yield of four to six tumors per individual the saturation level of tumor incidence was reached.
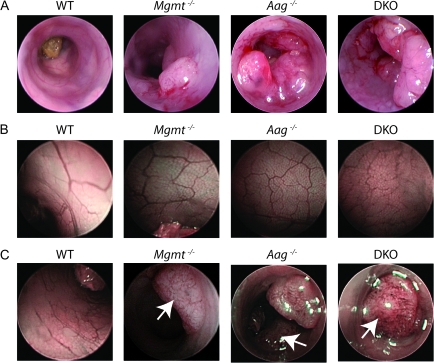
Fig. 3.
Representative endoscopic images of the distal colon following treatment with AOM/DSS. Mice received treatment with AOM (10 mg/kg) and 1% DSS as outlined in Figure 1A. (A) Representative images of neoplastic colon mucosa obtained by mini-colonoscopy. (B) Optical contrast enhanced mini-colonoscopy of normal non-neoplastic colonic mucosa and (C) tumors (indicated by arrow) at day 120 after treatment with AOM/DSS.
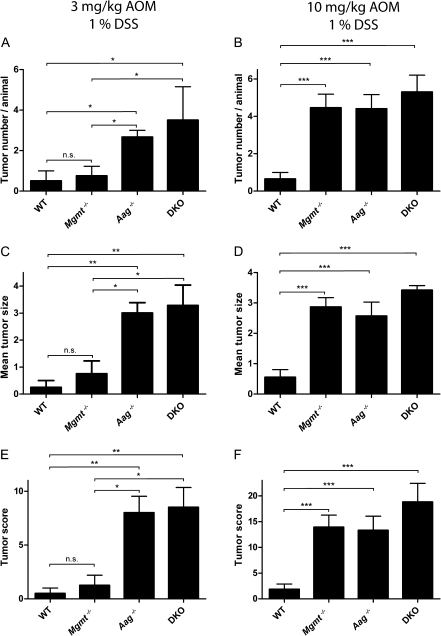
Fig. 4.
Both AAG and MGMT protect from AOM/DSS induced colon carcinogenesis. Mice received a single does of 3 mg/kg or 10 mg/kg AOM followed by repeated treatment with 1% DSS as outlined in Figure 1A. The number of animals treated are as follows: WT (n = 20), Mgmt−/− (n = 11), Aag−/− (n = 10), Mgmt−/−Aag−/− (n = 10). Tumor numbers and their size were evaluated by mini-endoscopy. The tumor size was graded from 1 to 5 as described in materials and methods. (A and B) Tumor number per animal, (C and D) mean tumor size and (E and F) combined tumor score (sum of all size scores/animal) at day 120. Similar data were obtained by inspecting the animals at day 60, although tumors had a smaller size. Statistical analysis was performed using unpaired, two-tailed Student’s t-test. ***P < 0.001, **P < 0.01, *P < 0.05; n.s. not significant.
Another end point we used is tumor size, which was again determined by mini-endoscopy. As shown in Figure 4C and D, the tumor size clearly mirrored the tumor yield shown in Figure 4A and B, respectively. Thus, with 3 mg/kg AOM, the average size of tumors was significantly higher in Aag−/− and DKO mice than in Mgmt−/− and the WT. With the high dose of 10 mg/kg AOM, tumors in Mgmt−/−, Aag−/− and DKO mice had about the same size, indicating again a saturation effect at the maximum tolerable dose. The tumor score (taking into account number and size of tumors per animal; see Materials and Methods) is given in Figure 4E and F. The data shows the same responses for WT and the DNA repair-defective knockout strains as described above. At the end of the experiment, tumors were inspected and histologically defined as carcinoma in situ, an example of which is shown in Figure 5A. Overall, for all end points determined and at low AOM dose level, Aag−/− mice were more susceptible to colon cancer formation than Mgmt−/− mice.
Fig. 5.
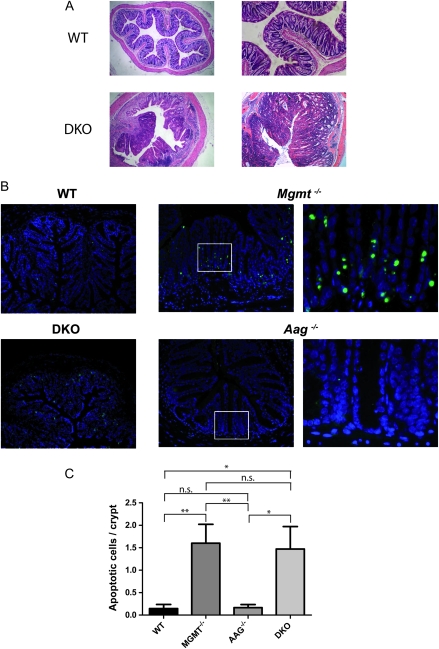
Tumor histology and intestinal epithelial cell apoptosis following AOM administration. (A) Haematoxylin/eosin-stained colonic cross sections at day 120. Whereas WT mice show normal gut architecture, DKO mice developed high-grade dysplasia consisting of well to moderately differentiated tubular adenocarcinoma or mucinous carcinoma invading into lamina propria and sometimes also into muscularis propria. (B) Mice received 10 mg/kg AOM. Forty-eight hours later, apoptosis was analyzed in colon cross sections by TUNEL staining. Pictures at the right panel demonstrate a magnification of the area labeled in the low magnification picture for Mgmt−/− -and Aag−/− individuals. Green labeled spots demonstrate nuclei of cells undergoing apoptosis. (C) Quantification of apoptotic cells in a crypt. Five mice per group were analyzed. Statistical analysis was performed using unpaired, two-tailed Student’s t-test. **P < 0.01, *P < 0.05; n.s. not significant.
It is striking that Mgmt−/− mice displayed at 3 mg/kg AOM a lower tumor response than Aag−/− mice. A reasonable explanation might rest on the finding that O6MeG is a powerful apoptotic DNA lesion. Thus, it might be surmised that notably in Mgmt−/− mice premalignant tumor cells become eliminated by apoptosis triggered by non-repaired O6MeG adducts. This elimination mechanism is probably not operative in the WT and Aag−/− mice, which are proficient for the repair of O6MeG adducts. To check this hypothesis, we inspected the colon of AOM-treated individuals for apoptotic cells. Indeed, Mgmt−/− mice exhibited a clearly higher level of apoptotic cells in the colon crypts than Aag−/− mice and the WT, a representative example is shown in Figure 5B. The quantification shown in Figure 5C demonstrates that Mgmt−/− and DKO mice exhibit upon AOM treatment a dramatically higher level of apoptotic cells per crypt than the WT and Aag−/− mice, which supports the hypothesis noted above.
Discussion
This study was aimed at elucidating the role of Mgmt and BER in the defense against colon cancer formation. We applied the AOM–DSS protocol, administering a single dose of the initiator AOM followed by two cycles of treatment with the colon-specific tumor promoter DSS. Colon cancer formation was monitored by mini-colonoscopy (36), which has the advantage that individuals need not be killed for colon inspection and neoplastic changes can be detected at an early stage.
Using a high dose of DSS (2% in the drinking water for two treatment cycles together with AOM) Mgmt−/− mice responded more sensitively than Aag−/− mice as to survival. Background experiments showed that repeated cycles of DSS administered at >2% in the drinking water leads to massive epithelial cell apoptosis and, therefore, very probably to a disruption of the colon epithelial barrier. This leads to infiltration of bacteria into the mucosa causing severe intestinal inflammation that clearly contributes to animal death (37,39). A low-dose DSS (1%), which was used in our experiments, was not toxic, not carcinogenic and caused only mild inflammation, but nevertheless was able to drive the process of colon cancer formation if applied following AOM. We should note that, as shown in a previous study, at very high concentration (2.5%) and long-term exposure (seven cycles), DSS alone can already be active in inducing colon cancer in mice (40). This was taken to indicate that inflammation provoked DNA damage (e.g. by free radical formation) may cause colon carcinogenesis per se. Under these conditions, Aag may exert protection presumably by repairing oxidative DNA lesions (40). As noted above, in the experiments reported here, the DSS concentration in the drinking water (1%) and short-term exposure (two cycles) did not cause severe inflammation in the colon and was ineffective in increasing the frequency of colon carcinomas above the background. Therefore, under the experimental conditions applied the tumorigenic effects in the repair-deficient mice cannot be attributed to DSS. We infer that the lack of repair of methylation lesions induced by AOM is responsible for colon cancer formation. The mechanism of tumor promotion by low concentration of DSS is not entirely known, but inflammation associated increase in cryptal cell proliferation and angiogenesis (41) might represent critical driving components.
The data reported here also show that at the low AOM dose (3 mg/kg) Aag−/− mice had a significantly higher tumor response than Mgmt−/− mice, which was indistinguishable from the WT. The Mgmt−/−/Aag−/− mice exhibited a tumor response, which was similar to the Aag−/− mice. In contrast, at the high-dose level of AOM (10 mg/kg), tumors were induced at a similar high frequency in the Mgmt−/−, Aag−/− and Mgmt−/−/Aag−/−-double knockout individuals. The finding that Aag-deficient animals are even more sensitive than Mgmt-deficient mice to tumor induction at the low AOM dose level indicates that repair of N-alkylated base lesions is highly important for protecting against methylation-induced colon cancer.
Why were Mgmt−/− mice not responding to colon cancer formation at low AOM dose level? Colon inspection and TUNEL staining revealed the induction of apoptotic cells in the colon tissue following treatment. It was striking that in Mgmt-lacking mice and in the DKOs, significant more apoptotic cells were found in the crypts than in the WT and Aag lacking individuals. Since O6MeG is a powerful apoptotic DNA damage in proliferating cells (42,43) causing death at levels of <5000 lesions per cell (44), we posit that the low cancer incidence in Mgmt−/− mice at the low AOM dose level is probably due to the elimination of a large proportion of cells harboring the lesion. At a higher dose level, elimination is not anymore perfect and an increasing frequency of cells with a high amount of critical DNA damage escape apoptosis. Under these conditions, elimination of genetically damaged cells and mutation fixation might reach an equilibrium, which might explain why the tumor incidence did not exceed four to six carcinomas per treated mice. We should note that a single dose of 15 mg/kg AOM is toxic even in WT mice indicating that the defense brought about by constitutive expression of Mgmt and Aag is overloaded, causing massive cell death and, as a final consequence, systemic toxicity.
Non-repaired N-methylpurines, such as N3-methyladenine and N3-methylguanine, may interfere with replication giving rise to DNA breaks and chromosomal changes in the proliferating colon epithelium and thus may contribute to tumor initiation in colon cells. Also, these adducts are subject to error-prone translesion synthesis that contributes to mutagenesis (45). Non-repaired N7-methylguanine, which is not a replication-blocking lesion, may also contribute to mutagenesis since spontaneous hydrolysis of the adduct leads to apurinic sites that, if not repaired in time, block replication and generate DNA breaks as well (46). Overall, the data presented here demonstrate for the first time that N-methylpurines contribute to colon cancer formation and stress the importance of the BER system in colon cancer protection. This conclusion supports findings obtained in chronic inflammatory disease of the colon where upregulation of Aag was found to be accompanied by microsatellite instability (47). This is consistent with in vitro studies demonstrating that transfection-mediated overexpression of Aag causes genomic instability upon methylating agent exposure, which was explained by imbalance in the BER pathway (48). Therefore, either lack or overexpression of Aag may be deleterious, increasing genomic instability that drives the process of cancer formation. Thus, proper expression of BER proteins in the colon appears to be more important than hitherto thought.
Colon cancer is the second most frequent cancer and a number of nutritional and genetic factors are known to be causally involved. Much interest has been drawn to polycyclic aromatic hydrocarbons, food-borne heterocyclic amines and heme iron in red and processed meat (49–51). Our study indicates that carcinogens with methylating properties (together with inflammatory stimuli) might play a very important role in colon cancer. While SN1 agents have been considered to be powerful carcinogens because they target the O6-position of guanine, this study shows that N-alkylated bases induced by SN1 agents also bear carcinogenic potential. Furthermore, they indicate that SN2-type agents producing mainly N-methylations in the DNA might also bear carcinogenic potency in the colon. Overall, the data illuminate the importance of the BER system that, together with Mgmt, constitutes an effective barrier against colon cancer formation and suggest further studies on BER in colon cancer patients.
Funding
Deutsche Forschungsgemeinschaft (DFG) FOR 527, DFG KA 724/13-3, WI 3304/1-1.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AOM
azoxymethane
- BER
base excision repair
- DKO
double knockout
- DSS
dextran sulfate sodium
- Mgmt
O6-methylguanine-DNA methyltransferase
- MNU
N-methyl-N-nitrosourea
- O6MeG
O6-methylguanine
- PBS
phosphate-buffered saline
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoyuridine triphosphate nick end labeling
- WT
wild-type
References
- 1.Sedgwick B. Nitrosated peptides and polyamines as endogenous mutagens in O6-alkylguanine-DNA alkyltransferase deficient cells. Carcinogenesis. 1997;18:1561–1567. doi: 10.1093/carcin/18.8.1561. [DOI] [PubMed] [Google Scholar]
- 2.Peterson LA, et al. 06-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 1991;51:5557–5564. [PubMed] [Google Scholar]
- 3.Hecht SS. DNA adduct formation from tobacco-specific N-nitrosamines. Mutat. Res. 1999;424:127–142. doi: 10.1016/s0027-5107(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 4.Mirvish SS, et al. Total N-nitroso compounds and their precursors in hot dogs and in the gastrointestinal tract and feces of rats and mice: possible etiologic agents for colon cancer. J. Nutr. 2002;132:3526S–3529S. doi: 10.1093/jn/132.11.3526S. [DOI] [PubMed] [Google Scholar]
- 5.Villano JL, et al. Temozolomide in malignant gliomas: current use and future targets. Cancer Chemother. Pharmacol. 2009;64:647–655. doi: 10.1007/s00280-009-1050-5. [DOI] [PubMed] [Google Scholar]
- 6.Eggermont AM, et al. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur. J. Cancer. 2004;40:1825–1836. doi: 10.1016/j.ejca.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Beranek DT. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat. Res. 1990;231:11–30. doi: 10.1016/0027-5107(90)90173-2. [DOI] [PubMed] [Google Scholar]
- 8.Swann PF. Why do O6-alkylguanine and O4-alkylthymine miscode? The relationship between the structure of DNA containing O6-alkylguanine and O4-alkylthymine and the mutagenic properties of these bases. Mutat. Res. 1990;233:81–94. doi: 10.1016/0027-5107(90)90153-u. [DOI] [PubMed] [Google Scholar]
- 9.Fang Q, et al. Function of domains of human O6-alkylguanine-DNA alkyltransferase. Biochemistry. 2005;44:15396–15405. doi: 10.1021/bi051460d. [DOI] [PubMed] [Google Scholar]
- 10.Kaina B, et al. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair. 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Dumenco LL, et al. The prevention of thymic lymphomas in transgenic mice by human O6-alkylguanine-DNA alkyltransferase. Science. 1993;259:219–222. doi: 10.1126/science.8421782. [DOI] [PubMed] [Google Scholar]
- 12.Nakatsuru Y, et al. O6-methylguanine-DNA methyltransferase protects against nitrosamine-induced hepatocarcinogenesis. Proc. Natl Acad. Sci. USA. 1993;90:6468–6472. doi: 10.1073/pnas.90.14.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, et al. Reduced lung tumorigenesis in human methylguanine DNA-methyltransferase transgenic mice achieved by expression of transgene within the target cell. Carcinogenesis. 1999;20:279–284. doi: 10.1093/carcin/20.2.279. [DOI] [PubMed] [Google Scholar]
- 14.Zaidi NH, et al. Transgenic expression of human MGMT protects against azoxymethane-induced aberrant crypt foci and G to A mutations in the K-ras oncogene of mouse colon. Carcinogenesis. 1995;16:451–456. doi: 10.1093/carcin/16.3.451. [DOI] [PubMed] [Google Scholar]
- 15.Becker K, et al. Targeted expression of human O(6)-methylguanine-DNA methyltransferase (MGMT) in transgenic mice protects against tumor initiation in two-stage skin carcinogenesis. Cancer Res. 1996;56:3244–3249. [PubMed] [Google Scholar]
- 16.Becker K, et al. The DNA repair protein O6-methylguanine-DNA methyltransferase protects against skin tumor formation induced by antineoplastic cloroethylnitrosourea. Cancer Res. 1997;57:3335–3338. [PubMed] [Google Scholar]
- 17.Becker K, et al. DNA repair protein MGMT protects against N-methyl-N-nitrosourea-induced conversion of benign into malignant tumors. Carcinogenesis. 2003;24:541–546. doi: 10.1093/carcin/24.3.541. [DOI] [PubMed] [Google Scholar]
- 18.Glassner BJ, et al. DNA repair methyltransferase (Mgmt) knockout mice are sensitive to the lethal effects of chemotherapeutic alkylating agents. Mutagenesis. 1999;14:339–347. doi: 10.1093/mutage/14.3.339. [DOI] [PubMed] [Google Scholar]
- 19.Iwakuma T, et al. High incidence of nitrosamine-induced tumorigenesis in mice lacking DNA repair methyltransferase. Carcinogenesis. 1997;18:1631–1635. doi: 10.1093/carcin/18.8.1631. [DOI] [PubMed] [Google Scholar]
- 20.Tsuzuki T, et al. Targeted disruption of the DNA repair methyltransferase gene renders mice hypersensitive to alkylating agent. Carcinogenesis. 1996;17:1215–1220. doi: 10.1093/carcin/17.6.1215. [DOI] [PubMed] [Google Scholar]
- 21.Sakumi K, et al. Methylnitrosourea-induced tumorigenesis in MGMT gene knockout mice. Cancer Res. 1997;57:2415–2418. [PubMed] [Google Scholar]
- 22.Bugni JM, et al. Alkylation-induced colon tumorigenesis in mice deficient in the Mgmt and Msh6 proteins. Oncogene. 2009;28:734–741. doi: 10.1038/onc.2008.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaina B, et al. Transfection and expression of human O6-methylguanine-DNA methyltransferase (MGMT) cDNA in Chinese hamster cells: the role of MGMT in protection against the genotoxic effects of alkylating agents. Carcinogenesis. 1991;12:1857–1867. doi: 10.1093/carcin/12.10.1857. [DOI] [PubMed] [Google Scholar]
- 24.Klapacz J, et al. O6-methylguanine-induced cell death involves exonuclease 1 as well as DNA mismatch recognition in vivo. Proc. Natl Acad. Sci. USA. 2009;106:576–581. doi: 10.1073/pnas.0811991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Wind N, et al. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell. 1995;82:321–330. doi: 10.1016/0092-8674(95)90319-4. [DOI] [PubMed] [Google Scholar]
- 26.Kawate H, et al. Separation of killing and tumorigenic effects of an alkylating agent in mice defective in two of the DNA repair genes. Proc. Natl Acad. Sci. USA. 1998;95:5116–5120. doi: 10.1073/pnas.95.9.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meira LB, et al. Base excision repair. Adv. Exp. Med. Biol. 2005;570:125–173. doi: 10.1007/1-4020-3764-3_5. [DOI] [PubMed] [Google Scholar]
- 28.Baute J, et al. Base excision repair and its role in maintaining genome stability. Crit. Rev. Biochem. Mol. Biol. 2008;43:239–276. doi: 10.1080/10409230802309905. [DOI] [PubMed] [Google Scholar]
- 29.Dianov GL, et al. Base excision repair in nuclear and mitochondrial DNA. Prog. Nucleic Acid Res. Mol. Biol. 2001;68:285–297. doi: 10.1016/s0079-6603(01)68107-8. [DOI] [PubMed] [Google Scholar]
- 30.Engelward BP, et al. Base excision repair deficient mice lacking the Aag alkyladenine DNA glycosylase. Proc. Natl Acad. Sci. USA. 1997;94:13087–13092. doi: 10.1073/pnas.94.24.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelward BP, et al. Repair-deficient 3-methyladenine DNA glycosylase homozygous mutant mouse cells have increased sensitivity to alkylation-induced chromosome damage and cell killing. EMBO J. 1996;15:945–952. [PMC free article] [PubMed] [Google Scholar]
- 32.Meira LB, et al. Aag-initiated base excision repair drives alkylation-induced retinal degeneration in mice. Proc. Natl Acad. Sci. USA. 2009;106:888–893. doi: 10.1073/pnas.0807030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaina B, et al. Transgenic systems in studies on genotoxicity of alkylating agents: critical lesions, thresholds and defense mechanisms. Mutat. Res. 1998;405:179–191. doi: 10.1016/s0027-5107(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 34.Frei JV, et al. Chromosome demage in bone marrow of mice treated with the methylating agent methyl methane sulfonate and N-methyl-N-nitrosourea in the presence or absence of caffein and its relationship with thymoma induction. Mutat. Res. 1975;29:89–96. [PubMed] [Google Scholar]
- 35.Furstenberger G, et al. Tumor induction in initiated mouse skin by phorbol esters and methyl methanesulfonate: correlation between chromosomal damage and conversion ('stage I of tumor promotion') in vivo. Carcinogenesis. 1989;10:749–752. doi: 10.1093/carcin/10.4.749. [DOI] [PubMed] [Google Scholar]
- 36.Becker C, et al. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut. 2005;54:950–954. doi: 10.1136/gut.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirtz S, et al. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 38.Waldner MJ, et al. Perforin deficiency attenuates inflammation and tumor growth in colitis-associated cancer. Inflamm. Bowel Dis. 2010;16:559–567. doi: 10.1002/ibd.21107. [DOI] [PubMed] [Google Scholar]
- 39.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 40.Meira LB, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chidlow JH, et al. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:5–18. doi: 10.1152/ajpgi.00107.2007. [DOI] [PubMed] [Google Scholar]
- 42.Kaina B, et al. Chromosomal instability, reproductive cell death and apoptosis induced by O6-methylguanine in Mex-, Mex+ and methylation-tolerant mismatch repair compromised cells: facts and models. Mutat. Res. 1997;381:227–241. doi: 10.1016/s0027-5107(97)00187-5. [DOI] [PubMed] [Google Scholar]
- 43.Meikrantz W, et al. O6-alkylguanine DNA lesions trigger apoptosis. Carcinogenesis. 1998;19:369–372. doi: 10.1093/carcin/19.2.369. [DOI] [PubMed] [Google Scholar]
- 44.Roos WP, et al. Apoptosis triggered by DNA damage O6-methylguanine in human lymphocytes required DNA replication and is mediated by p53 and Fas/CD95/Apo-1. Oncogene. 2003;23:359–367. doi: 10.1038/sj.onc.1207080. [DOI] [PubMed] [Google Scholar]
- 45.Roos WP, et al. The translesion polymerase Rev3L in the tolerance of alkylating anticancer drugs. Mol. Pharmacol. 2009;76:927–934. doi: 10.1124/mol.109.058131. [DOI] [PubMed] [Google Scholar]
- 46.Gates KS. An overview of chemical processes that damage cellular DNA: spontaneous hydrolysis, alkylation, and reactions with radicals. Chem. Res. Toxicol. 2009;22:1747–1760. doi: 10.1021/tx900242k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hofseth LJ, et al. The adaptive imbalance in base excision-repair enzymes generates microsatellite instability in chronic inflammation. J. Clin. Invest. 2003;112:1887–1894. doi: 10.1172/JCI19757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coquerelle T, et al. Overexpression of N-methylpurine-DNA glycosylase in Chinese hamster ovary cells renders them more sensitive to the production of chromosomal aberrations by methylating agents - a case of imbalanced DNA repair. Mutat. Res. 1995;336:9–17. doi: 10.1016/0921-8777(94)00035-5. [DOI] [PubMed] [Google Scholar]
- 49.Santarelli RL, et al. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr. Cancer. 2008;60:131–144. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norat T, et al. Meat consumption and colorectal cancer: a review of epidemiologic evidence. Nutr. Rev. 2001;59:37–47. doi: 10.1111/j.1753-4887.2001.tb06974.x. [DOI] [PubMed] [Google Scholar]
- 51.Cross AJ, et al. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–2360. [PubMed] [Google Scholar]