Abstract
Littoral cell angioma is an uncommon primary vascular neoplasm of the spleen. It frequently follows a benign course, but cases with aggressive behavior have been described. We present a case of this rare disease highlighting radiological examinations showing a rapid increase in the size of the spleen as well as an increase in the number and size of existing nodules. We also discuss the relationship of this disorder to chronic infections and its possible association with Epstein Barr virus.
Key words: littoral cell angioma, spleen, infections
Introduction
Littoral cell angiomas (LCA) are primary vascular neoplasms of the spleen with welldescribed morphologic and immunologic characteristics.1–3 However, there is uncertainty regarding their pathogenesis and malignant potential. Classically, the cells of these tumors are CD34−/CD68+/CD21+/CD8− reflecting the reticuloendothelial origin of the littoral cells that normally line the red pulp sinuses of the spleen.4 An association of LCA with chronic infections and autoimmunity has been observed and suggested to play a role in the development of this lesion and its possible progression to malignancy.1 An association with second cancers has also been identified.5 We present a case of this rare disease, highlighting the prior history of chronic infections, extensive immunohistochemical studies, and serial radiological examinations.
Case Report
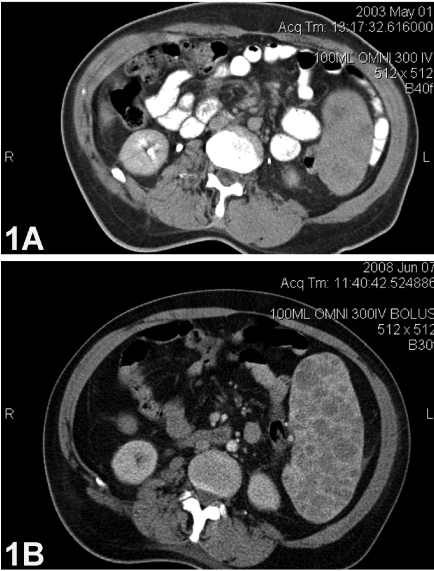
A 60-year-old Caucasian man presented with abdominal pain and early satiety for the last five years. Past medical history was remarkable for poliomyelitis (at age 4 years), subacute bacterial endocarditis requiring repair of the mitral valve (four years previously), and bilateral pneumonia several months prior to splenectomy. The physical examination was remarkable for splenomegaly extending almost to the anterior iliac crest and atrophy of both lower extremities. Laboratory findings included Hb=10.9 g/dL (range: 13.5–17.0 g/dL), platelet count=105 k/cmm (rage:140–450 k/cmm), WBC =7.5 k/cmm (range: 4.0–10.5 k/cmm), routine serum chemistries within normal limits, normal levels of immunoglobulins, and no serum monoclonal protein. A bone marrow examination was histologically normal. Radiographic images were obtained and compared to those from five years earlier (Figure 1). A computerized tomography (CT) scan performed with oral contrast and intravenous omnipaque revealed a spleen size of approximately 25 cm in the cranial-caudal dimension and 16 cm in the transverse dimension. This represented a 5×5 cm increase over the earlier examination. Heterogeneous enhancement related to confluence of multiple hypodensities was present on both radiographic studies, but markedly increased on the second study. The differential diagnosis was primarily a low-grade lymphoma.
Figure 1.
(A) Initial computerized tomography (CT) scan of the abdomen showing a slightly enlarged spleen with several poorly defined masses. (B) Second CT scan of the abdomen showing a markedly enlarged spleen with increased numbers and size of intra-splenic nodules.
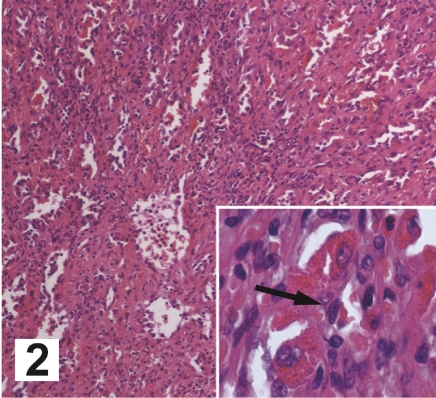
Our patient's symptom of early satiety plus progressive anemia and thrombocytopenia led to a strong recommendation for an elective splenectomy; therefore, he underwent no other imaging studies or needle biopsy. The large size of the spleen precluded a laparoscopic procedure, and the patient underwent an open splenectomy without complications. The spleen weighed 1212 g, approximately six times normal. The external surface had a diffuse cystic appearance with solid nodules ranging in size from 0.2 to 1.8 cm. The cut surface revealed multiple blood-filled cavities and multiple solid nodules that were firm, gray–tan, and non-necrotic (Figure 2). There were three tan lymph nodes each measuring 0.5 cm at the hilar region.
Figure 2.
Hematoxylin and eosin stain of the cut surface of the spleen; magnification, 200×. Insert: higher magnification of a sinusoid with the arrow indicating a littoral cell; magnification, 400×.
Microscopic evaluation showed dilated vascular channels forming anastomoses, many with micro-thrombi. The channels had irregular lumina and papillary projections containing enlarged endothelial cells with vesicular nuclei and eosinophilic cytoplasm (Figure 2). Exfoliated histiocytes were present in the lumina. The lining cells showed no nuclear atypia or atypical mitoses. An iron stain showed extensive siderosis. Staining was positive with endothelial cell markers PAS, PAS+/- D, and Factor VIII-related antigen, as well as the histiocytic marker, CD68. In addition, there was intense expression of CD21 and Ki-67; however, Epstein Bar nuclear antigen (EBNA) was negative. An analysis of sinus-lining cells for the Ki-ras gene revealed only wild type. The lymph nodes were microscopically normal.
Discussion
The differential diagnosis of an enlarging multi-nodular spleen includes hematolymphoid tumors (e.g. lymphomas, either Hodgkin's or diffuse large B-cell), granulomatous lesions (e.g. mycobacteriosis, deep fungal infection, visceral Leishmaniasis), metastatic carcinoma, inflammatory myofibroblastic tumor, splenic hamartomas, and relatively uncommon vascular tumors such as angioma, hemangioendothelioma, and angiosarcoma. Littoral cell angioma is a rare, recently characterized, vascular neoplasm of the spleen with immunophenotypic features suggesting an origin from the littoral cells lining the sinuses of the splenic red pulp. There is no gender predilection and the median age at presentation is 49 years.1–2
Imaging studies utilized to evaluate splenic masses have included ultrasound, CT, magnetic resonance imaging (MRI), and nuclear medicine scans. Ultrasound characteristics of LCA are nonspecific. CT features of LCA are usually iso-attenuating on non-contrast images, while contrast enhancement reveals early hypo-attenuation with arterial and early portal phases. Technetium 99m red blood cell (RBC) scans have been suggested as a method to distinguish hemangiomas from other lesions including LCA, as the former are “hot” on RBC scan with persistent signal intensity on delayed images, while two reports have indicated LCA are “cold”. Recent reports have suggested that the MRI characteristics of LCA depend upon the amount of hemosiderin within the tumor cells. When there is significant hemosiderin, there is loss of intensity of the MRI signal. This is best demonstrated on Gradient Echo sequences. Hemangiomas also have a typical appearance on dynamic contrast MRI and can therefore be differentiated from other masses.6
The pathogenesis is unclear but immune system dysregulation may explain its frequent association with other malignancies and autoimmune disorders.3 Harmon et al. reviewed the literature and tabulated 74 cases of LCA with 23 (31.1%) having a second primary cancer; no site predominated.5 The high frequency of second cancers emphasizes the importance of continued surveillance of patients with LCA. It is of note that our patient has a past history of poliomyelitis and repeated bacterial infections, and we suggest that the development of LCA may be related to this history, although the association might be coincidental.
Epstein Barr virus (EBV) belongs to the human herpes virus family and is ubiquitously distributed all over the world. It is the causative agent of infectious mononucleosis and is associated with several malignancies in man, such as African endemic Burkitt's lymphoma, nasopharyngeal carcinoma of the undifferentiated form, B-cell lymphomas in immunosuppressed individuals, and Hodgkin's disease. The protein CD21 is an EBV receptor found on B-lymphocytes. Its expression in LCA has been described.1–2 We also were able to confirm this observation in our tumor. The presence of CD 21 on the tumor cells therefore may be a potential target of therapy. Nuclear antigen 1 (EBNA-1) is a key protein of the EBV, and it is the only viral protein consistently expressed in EBV-associated malignancies.7 Although our patient's splenic tissue was positive for CD 21, the EBNA-1 was negative. This could imply that the EB virus had no etiological role in this case, or it could reflect technical difficulties in detection. EBV also has been associated with immune dysregulation, and EBV infection could be the link between LCA and autoimmune conditions identified in patients with LCA.8 Further studies to clarify a relationship between EBV and LCA would benefit from a computerized registry to facilitate tissue acquisition.
A spleen with LCA grossly may contain multiple red pulp nodules of variable consistency and color depending on the degree of necrosis, cyst formation, thrombi, and fibrosis. The nodules are composed of irregular anastomosing vascular channels lined by tall endothelial cells with vesicular nuclei, open chromatin, and small nucleoli. Papillary fronds, prominent cellular vacuolization, and evidence of hemophagocytosis and focal aggregates of eosinophilic globules have been described. Immunohistochemical analysis reveals that the neoplastic cells in LCA express CD31, CD68, and CD21, whereas they are negative for CD34 and CD8.4,5 Our case showed similar findings.
Although LCA frequently follows a benign course, cases with aggressive behavior have been described, showing a more solid pattern with nuclear atypia, necrosis, and even distant metastases several months after splenectomy.9–10 Therefore, follow-up is recommended. It is interesting to note the rather rapid increase in the size of the spleen as well as the increase in the size of existing nodules in our case, as demonstrated by scans. One can postulate that this may have reflected a change in the biology of the neoplastic cells. Gene profiling of clinically benign LCA and those with metastatic disease could help identify poor prognostic markers.
Littoral cell angioma should be considered when evaluating a patient with splenomegaly. A rapidly increasing spleen size, particularly with cystic features noted on imaging should suggest the possibility of LCA and the need for splenectomy. In the future, molecular genetic studies may provide better understanding of the etiology and possible therapeutic options.
Acknowledgments
Acknowledgements: the authors thank the H. Nussbaum Foundation of Saint Barnabas Medical Center for financial support.
References
- 1.Falk S, Stutte HJ, Frizzera G. Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Path. 1991;15:1023–33. [PubMed] [Google Scholar]
- 2.Bisceglia M, Sickel JZ, Giangaspero F, et al. Littoral cell angioma of the spleen: an additional report of four cases with emphasis on the association with visceral organ cancers. Tumori. 1998;84:595–9. doi: 10.1177/030089169808400516. [DOI] [PubMed] [Google Scholar]
- 3.Tee M, Vos P, Zetler P, et al. Incidental littoral cell angioma of the spleen. World J Surg Oncol. 2008;6:87. doi: 10.1186/1477-7819-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosso R, Paulli M, Gianelli U, et al. Littoral cell angiosarcoma of the spleen. Case report with immunohistochemical and ultrastructural analysis. Am J Surg Pathol. 1995;19:1203–8. doi: 10.1097/00000478-199510000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Harmon RL, Cerruto CA, Scheckner A. Littoral cell angioma: a case report and review. Curr Surg. 2006;63:345–50. doi: 10.1016/j.cursur.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Johnson C, Goyal M, Kim B, et al. Littoral cell angioma. Clin Imaging. 2007;31:27–31. doi: 10.1016/j.clinimag.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Snudden DK, Hearing J, Smith PR, et al. EBNA-1, the major nuclear antigen of Epstein-Barr virus, resembles ‘RGG’ RNA binding proteins. EMBO J. 1994;13:4840–7. doi: 10.1002/j.1460-2075.1994.tb06810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glaser R, Litsky ML, Padgett DA, et al. EBV-encoded dUTPase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology. 2006;346:205–18. doi: 10.1016/j.virol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Izhak O, Bejar J, Ben-Eliezer S, et al. Splenic littoral cell haemangioendothelioma: a new low-grade variant of malignant littoral cell tumor. Histopathology. 2001;39:469–75. doi: 10.1046/j.1365-2559.2001.01242.x. [DOI] [PubMed] [Google Scholar]
- 10.Suto H, Imai H, Sato E, et al. Severe thrombocytopenia caused by littoral cell angioma. Int J Hematol. 2008;88:253–4. doi: 10.1007/s12185-008-0162-8. [DOI] [PubMed] [Google Scholar]