Abstract
Cholera toxin (CT) represents a class of ligands that binds preferentially to noncoated pits on the cell surface. In the present study, we have investigated the mechanism of endocytosis of this class of ligand and compared it to the classic coated pit mechanism. When either CT coupled to colloidal gold particles (CT-gold) or 125I-labeled CT were incubated with 3T3 L1 fibroblasts at 4 degrees C, both ligands bound in a preferential fashion to small noncoated pits on the cell surface. CT-gold surface-labeled cells were then incubated at 22 degrees C. The labeled ligand progressively moved into noncoated vesicles and a tubulovesicular compartment composed of a network of tubules and vesicles closely associated with multivesicular bodies but distinct from the Golgi complexes. The ligand next passed into multivesicular bodies. By contrast, alpha 2-macroglobulin (alpha 2m)-gold initially localized preferentially to coated pits and subsequently to coated vesicles and tubulovesicular structures before associating with multivesicular bodies. To directly compare the intracellular pathway followed by CT-gold to that followed by alpha 2m-gold, CT-gold (7 nm) was coincubated with alpha 2m-gold (15 nm). By 10 min of incubation at 22 degrees C, up to 66% of tubulovesicular units contained both ligands when analyzed in serial sections. Subsequently, both ligands were colocalized in multivesicular bodies. We conclude that CT-gold endocytosed via noncoated vesicles and alpha 2m-gold endocytosed through coated vesicles subsequently associate with the same tubulovesicular units, multivesicular bodies, and lysosomes.
Full text
PDF


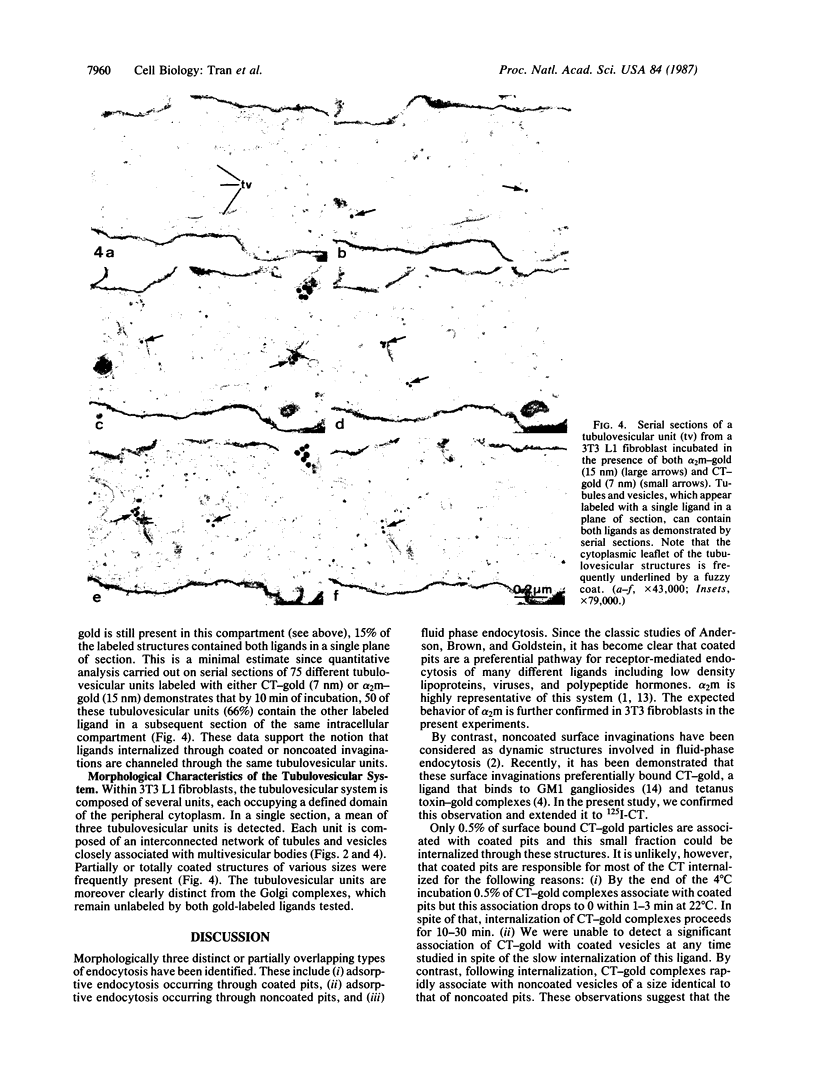

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Cuatrecasas P. Mechanism of activation of adenylate cyclase by Vibrio cholerae enterotoxin. J Membr Biol. 1975 Jun 3;22(1):29–52. doi: 10.1007/BF01868162. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Amherdt M., Van Obberghen E., Kahn C. R., Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978 Apr;61(4):1057–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982 Oct;95(1):73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Rees A. R., Gregoriou M., Kris R., Schlessinger J., Orci L. Subcellular distribution of the external and internal domains of the EGF receptor in A-431 cells. Exp Cell Res. 1986 Oct;166(2):312–326. doi: 10.1016/0014-4827(86)90479-9. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Willingham M. C., Pastan I. alpha 2-macroglobulin adsorbed to colloidal gold: a new probe in the study of receptor-mediated endocytosis. J Cell Biol. 1981 Apr;89(1):29–34. doi: 10.1083/jcb.89.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. Y., Carpentier J. L., Gorden P., Van Obberghen E., Blackett N. M., Grunfeld C., Orci L. Receptor-mediated endocytosis of insulin: role of microvilli, coated pits, and coated vesicles. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7788–7791. doi: 10.1073/pnas.79.24.7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P. H. Internalization and degradation of cholera toxin by cultured cells: relationship to toxin action. J Cell Biol. 1982 Jun;93(3):860–865. doi: 10.1083/jcb.93.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Gonatas J., Stieber A., Olsnes S., Gonatas N. K. Pathways involved in fluid phase and adsorptive endocytosis in neuroblastoma. J Cell Biol. 1980 Dec;87(3 Pt 1):579–588. doi: 10.1083/jcb.87.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Stieber A., Hickey W. F., Herbert S. H., Gonatas J. O. Endosomes and Golgi vesicles in adsorptive and fluid phase endocytosis. J Cell Biol. 1984 Oct;99(4 Pt 1):1379–1390. doi: 10.1083/jcb.99.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover J. A., Willingham M. C., Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984 Dec;39(2 Pt 1):283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- Huet C., Ash J. F., Singer S. J. The antibody-induced clustering and endocytosis of HLA antigens on cultured human fibroblasts. Cell. 1980 Sep;21(2):429–438. doi: 10.1016/0092-8674(80)90479-1. [DOI] [PubMed] [Google Scholar]
- Joseph K. C., Stieber A., Gonatas N. K. Endocytosis of cholera toxin in GERL-like structures of murine neuroblastoma cells pretreated with GM1 ganglioside. Cholera toxin internalization into Neuroblastoma GERL. J Cell Biol. 1979 Jun;81(3):543–554. doi: 10.1083/jcb.81.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Roth J., Robert A., Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982 Apr 15;296(5858):651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Neutra M. R., Ciechanover A., Owen L. S., Lodish H. F. Intracellular transport of transferrin- and asialoorosomucoid-colloidal gold conjugates to lysosomes after receptor-mediated endocytosis. J Histochem Cytochem. 1985 Nov;33(11):1134–1144. doi: 10.1177/33.11.2997327. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B., Tønnessen T. I., Petersen O. W., Sandvig K., Olsnes S. Routing of internalized ricin and ricin conjugates to the Golgi complex. J Cell Biol. 1986 Jan;102(1):37–47. doi: 10.1083/jcb.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]