Abstract
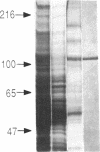
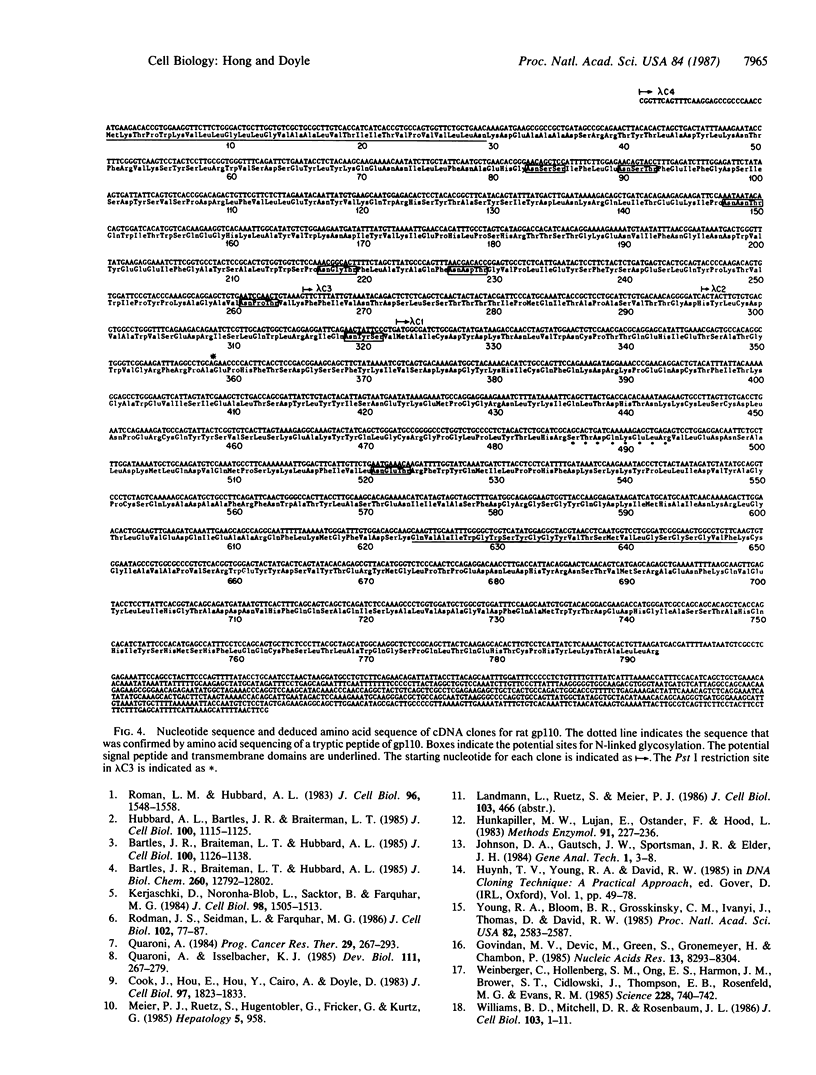
Hepatocytes are polarized cells with distinct sinusoidal, bile canalicular, and basolateral plasma membrane domains. Each domain contains proteins that are specific for it. We have isolated three cDNA clones encoding a rat liver bile canaliculus domain-specific glycoprotein with Mr 110,000 (gp110) by immunologically screening a rat kidney lambda gt11 cDNA library with a rabbit polyclonal antiserum directed against purified gp110. The authenticity of these clones was verified as follows. (i) The antiserum recognizes specifically isopropyl beta-D-thiogalactoside-induced fusion proteins on electrophoretic transfer blots of total lysogen lysates containing these cDNA clones. (ii) Antibodies epitope-selected by these clones are able to interact with gp110 on electrophoretic transfer blots. (iii) The amino acid sequencing derived from the DNA sequence was confirmed by amino acid sequencing of a tryptic peptide of gp110. Rescreening of the same library with the cDNA clones identified a full-length cDNA clone for this glycoprotein. Sequence analysis indicates that the N-linked carbohydrate chains are concentrated on the N-terminal part of this highly glycosylated protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartles J. R., Braiterman L. T., Hubbard A. L. Biochemical characterization of domain-specific glycoproteins of the rat hepatocyte plasma membrane. J Biol Chem. 1985 Oct 15;260(23):12792–12802. [PubMed] [Google Scholar]
- Bartles J. R., Braiterman L. T., Hubbard A. L. Endogenous and exogenous domain markers of the rat hepatocyte plasma membrane. J Cell Biol. 1985 Apr;100(4):1126–1138. doi: 10.1083/jcb.100.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan M. J., Anderson H. C., Palade G. E., Jamieson J. D. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell. 1986 Aug 15;46(4):623–631. doi: 10.1016/0092-8674(86)90888-3. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fuller S. D., Bravo R., Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985 Feb;4(2):297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland E. C., Leung J. O., Drickamer K. Rat liver asialoglycoprotein receptor lacks a cleavable NH2-terminal signal sequence. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7338–7342. doi: 10.1073/pnas.81.23.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Spiess M., Semenza G., Lodish H. F. The sucrase-isomaltase complex: primary structure, membrane-orientation, and evolution of a stalked, intrinsic brush border protein. Cell. 1986 Jul 18;46(2):227–234. doi: 10.1016/0092-8674(86)90739-7. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D., Noronha-Blob L., Sacktor B., Farquhar M. G. Microdomains of distinctive glycoprotein composition in the kidney proximal tubule brush border. J Cell Biol. 1984 Apr;98(4):1505–1513. doi: 10.1083/jcb.98.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. M., Gilula N. B. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol. 1986 Sep;103(3):767–776. doi: 10.1083/jcb.103.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Villasante A., Sherline P., Cowan N. J. Brain-specific expression of MAP2 detected using a cloned cDNA probe. J Cell Biol. 1986 Jun;102(6):2098–2105. doi: 10.1083/jcb.102.6.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Misek D. E., Bard E., Rodriguez-Boulan E. Biogenesis of epithelial cell polarity: intracellular sorting and vectorial exocytosis of an apical plasma membrane glycoprotein. Cell. 1984 Dec;39(3 Pt 2):537–546. doi: 10.1016/0092-8674(84)90460-4. [DOI] [PubMed] [Google Scholar]
- Paul D. L. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986 Jul;103(1):123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Isselbacher K. J. Study of intestinal cell differentiation with monoclonal antibodies to intestinal cell surface components. Dev Biol. 1985 Oct;111(2):267–279. doi: 10.1016/0012-1606(85)90482-8. [DOI] [PubMed] [Google Scholar]
- Rodman J. S., Seidman L., Farquhar M. G. The membrane composition of coated pits, microvilli, endosomes, and lysosomes is distinctive in the rat kidney proximal tubule cell. J Cell Biol. 1986 Jan;102(1):77–87. doi: 10.1083/jcb.102.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman L. M., Hubbard A. L. A domain-specific marker for the hepatocyte plasma membrane: localization of leucine aminopeptidase to the bile canalicular domain. J Cell Biol. 1983 Jun;96(6):1548–1558. doi: 10.1083/jcb.96.6.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. Sequence of a second human asialoglycoprotein receptor: conservation of two receptor genes during evolution. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6465–6469. doi: 10.1073/pnas.82.19.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Schwartz A. L., Lodish H. F. Sequence of human asialoglycoprotein receptor cDNA. An internal signal sequence for membrane insertion. J Biol Chem. 1985 Feb 25;260(4):1979–1982. [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W., Earl P., Moss B. Surface expression of viral glycoproteins is polarized in epithelial cells infected with recombinant vaccinia viral vectors. EMBO J. 1986 Feb;5(2):237–245. doi: 10.1002/j.1460-2075.1986.tb04204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Ong E. S., Harmon J. M., Brower S. T., Cidlowski J., Thompson E. B., Rosenfeld M. G., Evans R. M. Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science. 1985 May 10;228(4700):740–742. doi: 10.1126/science.2581314. [DOI] [PubMed] [Google Scholar]
- Williams B. D., Mitchell D. R., Rosenbaum J. L. Molecular cloning and expression of flagellar radial spoke and dynein genes of Chlamydomonas. J Cell Biol. 1986 Jul;103(1):1–11. doi: 10.1083/jcb.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]