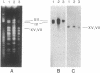
Abstract
The biased reptation theory has been applied to the pulsed-field electrophoresis of DNA in agarose gels. A computer simulation of the theoretical model that calculates the mobility of large DNA molecules as a function of agarose pore size, DNA chain properties, and electric field conditions has been used to generate mobility curves for DNA molecules in the size range of the larger yeast chromosomes. Pulsed-field electrophoresis experiments resulting in the establishment of an electrophoretic karyotype for yeast, where the mobility of the DNA fragments is a monotonic function of molecular size for the entire size range that is resolved (200-2200 kilobase pairs), has been compared to the theoretical mobility curves generated by the computer model. The various physical mechanisms and experimental conditions responsible for band inversion and improved electrophoretic separation are identified and discussed in the framework of the model.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards A., Kooter J. M., Michels P. A., Moberts R. M., Borst P. Pulsed field gradient electrophoresis of DNA digested in agarose allows the sizing of the large duplication unit of a surface antigen gene in trypanosomes. Gene. 1986;42(3):313–322. doi: 10.1016/0378-1119(86)90235-0. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Bird A. P. Long-range restriction site mapping of mammalian genomic DNA. 1986 Jul 31-Aug 6Nature. 322(6078):477–481. doi: 10.1038/322477a0. [DOI] [PubMed] [Google Scholar]
- Burmeister M., Lehrach H. Long-range restriction map around the Duchenne muscular dystrophy gene. Nature. 1986 Dec 11;324(6097):582–585. doi: 10.1038/324582a0. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Frank M., Olson M. V. Electrophoretic separations of large DNA molecules by periodic inversion of the electric field. Science. 1986 Apr 4;232(4746):65–68. doi: 10.1126/science.3952500. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Gardiner K., Laas W., Patterson D. Fractionation of large mammalian DNA restriction fragments using vertical pulsed-field gradient gel electrophoresis. Somat Cell Mol Genet. 1986 Mar;12(2):185–195. doi: 10.1007/BF01560665. [DOI] [PubMed] [Google Scholar]
- Giannini S. H., Schittini M., Keithly J. S., Warburton P. W., Cantor C. R., Van der Ploeg L. H. Karyotype analysis of Leishmania species and its use in classification and clinical diagnosis. Science. 1986 May 9;232(4751):762–765. doi: 10.1126/science.3961502. [DOI] [PubMed] [Google Scholar]
- Hardy D. A., Bell J. I., Long E. O., Lindsten T., McDevitt H. O. Mapping of the class II region of the human major histocompatibility complex by pulsed-field gel electrophoresis. Nature. 1986 Oct 2;323(6087):453–455. doi: 10.1038/323453a0. [DOI] [PubMed] [Google Scholar]
- Hurley I. DNA orientation during gel electrophoresis and its relation to electrophoretic mobility. Biopolymers. 1986 Apr;25(4):539–554. doi: 10.1002/bip.360250403. [DOI] [PubMed] [Google Scholar]
- Kenwrick S., Patterson M., Speer A., Fischbeck K., Davies K. Molecular analysis of the Duchenne muscular dystrophy region using pulsed field gel electrophoresis. Cell. 1987 Jan 30;48(2):351–357. doi: 10.1016/0092-8674(87)90438-7. [DOI] [PubMed] [Google Scholar]
- Lalande M., Kunkel L. M., Flint A., Latt S. A. Development and use of metaphase chromosome flow-sorting methodology to obtain recombinant phage libraries enriched for parts of the human X chromosome. Cytometry. 1984 Mar;5(2):101–107. doi: 10.1002/cyto.990050202. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Frisch H. L. Why does the electrophoretic mobility of DNA in gels vary with the length of the molecule? Biopolymers. 1982 May;21(5):995–997. doi: 10.1002/bip.360210511. [DOI] [PubMed] [Google Scholar]
- Lumpkin O. J., Déjardin P., Zimm B. H. Theory of gel electrophoresis of DNA. Biopolymers. 1985 Aug;24(8):1573–1593. doi: 10.1002/bip.360240812. [DOI] [PubMed] [Google Scholar]
- McPeek F. D., Jr, Coyle-Morris J. F., Gemmill R. M. Separation of large DNA molecules by modified pulsed field gradient gel electrophoresis. Anal Biochem. 1986 Aug 1;156(2):274–285. doi: 10.1016/0003-2697(86)90254-x. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae, edition 9. Microbiol Rev. 1985 Sep;49(3):181–213. doi: 10.1128/mr.49.3.181-213.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noolandi J, Rousseau J, Slater GW, Turmel C, Lalande M. Self-trapping and anomalous dispersion of DNA in electrophoresis. Phys Rev Lett. 1987 Jun 8;58(23):2428–2431. doi: 10.1103/PhysRevLett.58.2428. [DOI] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. 1986 Jul 31-Aug 6Nature. 322(6078):474–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Slater G. W., Rousseau J., Noolandi J. On the stretching of DNA in the reptation theories of gel electrophoresis. Biopolymers. 1987 Jun;26(6):863–872. doi: 10.1002/bip.360260607. [DOI] [PubMed] [Google Scholar]
- Slater GW, Noolandi J. New biased-reptation model for charged polymers. Phys Rev Lett. 1985 Oct 7;55(15):1579–1582. doi: 10.1103/PhysRevLett.55.1579. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Van der Velde-Koerts T., Ling V., Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1986 May;6(5):1671–1678. doi: 10.1128/mcb.6.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Smits M., Ponnudurai T., Vermeulen A., Meuwissen J. H., Langsley G. Chromosome-sized DNA molecules of Plasmodium falciparum. Science. 1985 Aug 16;229(4714):658–661. doi: 10.1126/science.3895435. [DOI] [PubMed] [Google Scholar]
- de Jonge P., de Jongh F. C., Meijers R., Steensma H. Y., Scheffers W. A. Orthogonal-field-alternation gel electrophoresis banding patterns of DNA from yeasts. Yeast. 1986 Sep;2(3):193–204. doi: 10.1002/yea.320020307. [DOI] [PubMed] [Google Scholar]
- van Ommen G. J., Verkerk J. M., Hofker M. H., Monaco A. P., Kunkel L. M., Ray P., Worton R., Wieringa B., Bakker E., Pearson P. L. A physical map of 4 million bp around the Duchenne muscular dystrophy gene on the human X-chromosome. Cell. 1986 Nov 21;47(4):499–504. doi: 10.1016/0092-8674(86)90614-8. [DOI] [PubMed] [Google Scholar]