Abstract
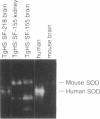
Down syndrome, the phenotypic expression of human trisomy 21, is presumed to result from a 1.5-fold increase in the expression of the genes on human chromosome 21. As an approach to the development of an animal model for Down syndrome, several strains of transgenic mice that carry the human Cu/Zn-superoxide dismutase gene have been prepared. These animals express the transgene in a manner similar to that of humans, with 0.9- and 0.7-kilobase transcripts in a 1:4 ratio, and synthesize the human enzyme in an active form capable of forming human-mouse enzyme heterodimers. Cu/Zn-superoxide superoxide dismutase activity is increased from 1.6- to 6.0-fold in the brains of four transgenic strains and to an equal or lesser extent in several other tissues. These animals provide a unique system for studying the consequences of increased dosage of the Cu/Zn-superoxide dismutase gene in Down syndrome and the role of this enzyme in a variety of other pathological processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annerén K. G., Epstein C. J. Lipid peroxidation and superoxide dismutase-1 and glutathione peroxidase activities in trisomy 16 fetal mice and human trisomy 21 fibroblasts. Pediatr Res. 1987 Jan;21(1):88–92. doi: 10.1203/00006450-198701000-00019. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Bagley A. C., Krall J., Lynch R. E. Superoxide mediates the toxicity of paraquat for Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3189–3193. doi: 10.1073/pnas.83.10.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C., Troll W. Modifiers of free radicals inhibit in vitro the oncogenic actions of x-rays, bleomycin, and the tumor promoter 12-O-tetradecanoylphorbol 13-acetate. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1304–1307. doi: 10.1073/pnas.80.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. H., Longar S., Fishman R. A. Protective effects of liposome-entrapped superoxide dismutase on posttraumatic brain edema. Ann Neurol. 1987 Jun;21(6):540–547. doi: 10.1002/ana.410210604. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cox D. R., Epstein L. B., Epstein C. J. Genes coding for sensitivity to interferon (IfRec) and soluble superoxide dismutase (SOD-1) are linked in mouse and man and map to mouse chromosome 16. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2168–2172. doi: 10.1073/pnas.77.4.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. R., Smith S. A., Epstein L. B., Epstein C. J. Mouse trisomy 16 as an animal model of human trisomy 21 (Down syndrome): production of viable trisomy 16 diploid mouse chimeras. Dev Biol. 1984 Feb;101(2):416–424. doi: 10.1016/0012-1606(84)90156-8. [DOI] [PubMed] [Google Scholar]
- Delabar J. M., Goldgaber D., Lamour Y., Nicole A., Huret J. L., de Grouchy J., Brown P., Gajdusek D. C., Sinet P. M. Beta amyloid gene duplication in Alzheimer's disease and karyotypically normal Down syndrome. Science. 1987 Mar 13;235(4794):1390–1392. doi: 10.1126/science.2950593. [DOI] [PubMed] [Google Scholar]
- Doroshow J. H. Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4514–4518. doi: 10.1073/pnas.83.12.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Bernstein Y., Groner Y. Overproduction of human Cu/Zn-superoxide dismutase in transfected cells: extenuation of paraquat-mediated cytotoxicity and enhancement of lipid peroxidation. EMBO J. 1986 Mar;5(3):615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. J., Cox D. R., Epstein L. B. Mouse trisomy 16: an animal model of human trisomy 21 (Down syndrome). Ann N Y Acad Sci. 1985;450:157–168. doi: 10.1111/j.1749-6632.1985.tb21490.x. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Young S. L., Crapo J. D. Liposome-mediated augmentation of superoxide dismutase in endothelial cells prevents oxygen injury. J Biol Chem. 1983 Oct 25;258(20):12534–12542. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Grankvist K., Marklund S., Täljedal I. B. Superoxide dismutase is a prophylactic against alloxan diabetes. Nature. 1981 Nov 12;294(5837):158–160. doi: 10.1038/294158a0. [DOI] [PubMed] [Google Scholar]
- Groner Y., Lieman-Hurwitz J., Dafni N., Sherman L., Levanon D., Bernstein Y., Danciger E., Elroy-Stein O. Molecular structure and expression of the gene locus on chromosome 21 encoding the Cu/Zn superoxide dismutase and its relevance to Down syndrome. Ann N Y Acad Sci. 1985;450:133–156. doi: 10.1111/j.1749-6632.1985.tb21489.x. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Levanon D., Lieman-Hurwitz J., Dafni N., Wigderson M., Sherman L., Bernstein Y., Laver-Rudich Z., Danciger E., Stein O., Groner Y. Architecture and anatomy of the chromosomal locus in human chromosome 21 encoding the Cu/Zn superoxide dismutase. EMBO J. 1985 Jan;4(1):77–84. doi: 10.1002/j.1460-2075.1985.tb02320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieman-Hurwitz J., Dafni N., Lavie V., Groner Y. Human cytoplasmic superoxide dismutase cDNA clone: a probe for studying the molecular biology of Down syndrome. Proc Natl Acad Sci U S A. 1982 May;79(9):2808–2811. doi: 10.1073/pnas.79.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Petkau A., Chelack W. S., Pleskach S. D., Meeker B. E., Brady C. M. Radioprotection of mice by superoxide dismutase. Biochem Biophys Res Commun. 1975 Aug 4;65(3):886–893. doi: 10.1016/s0006-291x(75)80468-2. [DOI] [PubMed] [Google Scholar]
- Robakis N. K., Wisniewski H. M., Jenkins E. C., Devine-Gage E. A., Houck G. E., Yao X. L., Ramakrishna N., Wolfe G., Silverman W. P., Brown W. T. Chromosome 21q21 sublocalisation of gene encoding beta-amyloid peptide in cerebral vessels and neuritic (senile) plaques of people with Alzheimer disease and Down syndrome. Lancet. 1987 Feb 14;1(8529):384–385. doi: 10.1016/s0140-6736(87)91754-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld W., Evans H., Concepcion L., Jhaveri R., Schaeffer H., Friedman A. Prevention of bronchopulmonary dysplasia by administration of bovine superoxide dismutase in preterm infants with respiratory distress syndrome. J Pediatr. 1984 Nov;105(5):781–785. doi: 10.1016/s0022-3476(84)80307-8. [DOI] [PubMed] [Google Scholar]
- Shaffer S. G., O'Neill D. H., Thibeault D. W. Administration of bovine superoxide dismutase fails to prevent chronic pulmonary sequelae of neonatal oxygen exposure in the rat. J Pediatr. 1987 Jun;110(6):942–946. doi: 10.1016/s0022-3476(87)80419-5. [DOI] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L., Levanon D., Lieman-Hurwitz J., Dafni N., Groner Y. Human Cu/Zn superoxide dismutase gene: molecular characterization of its two mRNA species. Nucleic Acids Res. 1984 Dec 21;12(24):9349–9365. doi: 10.1093/nar/12.24.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinet P. M., Lejeune J., Jerome H. Trisomy 21 (Down's syndrome). Glutathione peroxidase, hexose monophoshate shunt and I.Q. Life Sci. 1979 Jan 1;24(1):29–33. doi: 10.1016/0024-3205(79)90276-5. [DOI] [PubMed] [Google Scholar]
- Sinet P. M. Metabolism of oxygen derivatives in down's syndrome. Ann N Y Acad Sci. 1982;396:83–94. doi: 10.1111/j.1749-6632.1982.tb26845.x. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Kessler J. A., Bennett M. V., Spray D. C. Superoxide dismutase protects cultured neurons against death by starvation. Proc Natl Acad Sci U S A. 1987 May;84(9):3056–3059. doi: 10.1073/pnas.84.9.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tippett P., Kaplan J. C. Report of the Committee on the Genetic Constitution of Chromosomes 20, 21, and 22. Cytogenet Cell Genet. 1985;40(1-4):268–295. doi: 10.1159/000132177. [DOI] [PubMed] [Google Scholar]
- Weisfeldt M. L. Reperfusion and reperfusion injury. Clin Res. 1987 Jan;35(1):13–20. [PubMed] [Google Scholar]