Abstract
Nuclear import is a critical step in the life cycle of HIV-1. During the early (pre-integration) stages of infection, HIV-1 has to transport its pre-integration complex into the nucleus for integration into the host cell chromatin, while at the later (post-integration) stages viral regulatory proteins Tat and Rev need to get into the nucleus to stimulate transcription and regulate splicing and nuclear export of subgenomic and genomic RNAs. Given such important role of nuclear import in HIV-1 life cycle, this step presents an attractive target for anti-viral therapeutic intervention. In this review, we describe the current state of our understanding of the interactions regulating nuclear import of the HIV-1 pre-integration complex and describe current approaches to inhibit it.
1. Introduction
Viruses are intracellular parasites that commandeer cellular processes, such as nuclear import and export machineries, to perform virus-specific functions. For this purpose, many viral proteins and nucleoprotein complexes shuttle between the nuclear and cytoplasmic compartments, and this process is especially critical for viruses with a distinct replication step occurring in the nucleus. Among many different families of these viruses, retroviruses, and lentiviruses in particular, are of major interest both because of their clinical importance (HIV-1 is the most notable lentiviral pathogenic agent [103]) and their potential relevance as vectors for gene therapy [17]. While replication in the host cell’s nucleus provides clear benefits for the virus, such as ready access to cellular transcription and splicing apparatus, it imposes the barrier of the nuclear envelope that has to be overcome. During the early (pre-integration) stages of infection, lentiviruses have to transport their genome from the site of penetration to the nucleus and then through the nuclear membrane, while at the later (post-integration) stages they need to import regulatory proteins (Tat and Rev in the case of HIV-1) to stimulate transcription and regulate splicing and nuclear export of subgenomic and genomic RNAs. Given such important role that nuclear import plays in HIV-1 life cycle, it presents an attractive target for anti-viral therapeutic intervention. In this review, we will describe the current state of our understanding of the interactions regulating nuclear import of the HIV-1 pre-integration complex (PIC) and HIV-1 proteins critical for viral replication, focusing on potential targets for therapeutic interventions. In contrast to a recent article by Zhan et al. [111] covering similar topics, we will attempt to provide a critical analysis of the published data.
2. Nuclear import in the HIV-1 life cycle
The idea that nuclear import may play a special role in lentiviral life cycle [13] was prompted by the finding that HIV-1 can infect non-dividing cells such as terminally differentiated macrophages [67,100]. This ability implied that HIV-1, unlike gammaretroviruses which critically depend on nuclear envelope disassembly during mitosis for nuclear entry [87], acquired the ability to pass through the intact nuclear envelope. Because mitosis constitutes only a small part of the cell cycle, the ability to enter nuclear compartment during the interphase provides a huge benefit to HIV-1 and may account for its very high replication rate observed in HIV-infected patients [53,99]. Initially, it was presumed that HIV can get into the nucleus both during the interphase (through the nuclear envelope) and during the mitosis (by-passing the nuclear envelope), prompting the idea that infection of dividing and non-dividing cells by HIV occurs through different pathways and thus should involve different mechanisms [105]. However, a number of studies demonstrated that passage through the nuclear envelope is a necessary step in HIV infection of both dividing and non-proliferating cells [54,57,85], underscored by the requirement of the nuclear import factors transportin-SR2 (TNPO3/TRN-SR2) [22,39,61] and importin 7 [108] for infection of both cell types. Such solution appears evolutionary sensible, as evolution usually leads to development of the new pathways at the expense of the old ones, and it would be wasteful for a virus to preserve two replication mechanisms (one for dividing and one for non-dividing cells), when one is so much superior to the other. The requirement of active nuclear import for HIV infection of both dividing and non-dividing cells would imply that differences between these cell types in HIV replication cannot be used for conclusions regarding the role of HIV nuclear import in the viral life cycle [61,105,106]. Indeed, it is very difficult to make reliable conclusions from comparison of non-dividing cells growth arrested by potent anti-mitotic agents such as aphidicolin to untreated controls. In addition, recent studies demonstrated profound differences between dividng and non-dividing, post-mitotic cells in the genesis of nuclear pore complexes (NPC), the multiprotein assemblies that regulate exchange between the nucleoplasm and cytoplasm. In dividing cells, NPCs disassemble during mitosis and reassemble into the newly forming nuclei. In contrast, the renewal of NPCs is absent in post-mitotic cells, and certain nucleoporins, such as Nup107/160 complex, remain incorporated in the nuclear membrane during the entire lifespan of a cell [24,27]. Lack of NPC turnover in post-mitotic cells was associated with aging-related deterioration of NPCs, a loss of the nuclear permeability barrier and the leaking of cytoplasmic proteins into the nuclear compartment [52]. Such functional changes may greatly affect the NPC activity during nuclear import of viral PICs in non-dividing cells.
Being cellular parasites, viruses hijack cellular machinery to accomplish the necessary tasks in their replication. In the case of nuclear import, this paradigm implies that the HIV-1 PIC and the viral proteins needed in the nucleus (Tat and Rev) interact with the cellular nuclear import machinery that normally mediates transport of certain cellular proteins carrying specific nuclear localization signals (NLSs) from the cytoplasm into the nucleus. This machinery involves a number of nuclear transport proteins, importins, that bind and translocate NLS-containing proteins through the NPC via a complex series of interactions with NPC proteins nucleoporins (reviewed in [92]).
To use this machinery, the HIV-1 PIC and the viral proteins needed in the nucleus (Tat and Rev) have to express NLSs that could be recognized by importins. The questions concerning the nature of these NLSs, the PIC proteins expressing them, and the importins involved in translocation of the HIV-1 PIC remain the most controversial issue in HIV research. HIV-1 PIC contains at least four karyophilic proteins of both viral (MA, IN, Vpr) and host cell (LEDGF/p75) origin which have been shown to interact with importins. Some of the existing controversy comes from this unusually high number of potential factors regulating HIV-1 nuclear import. Indeed, nuclear import of the HIV-1 PIC cannot be completely suppressed by inactivation of NLSs in any one, or even simultaneously in several, karyophilic proteins within the PIC [104]. However, this finding can simply indicate that the nuclear import of the HIV PIC results from a cumulative activity of several karyophilic factors, or that different factors work in different circumstances (e.g., different cell types, different activation conditions, etc.). This would be a logical solution on the part of the virus to maximize efficiency of its nuclear import. Consistent with this interpretation, deletion or inactivation of known nuclear import signals in the HIV-1 PIC leads to about a 4 log decrease in infectivity [104]. Importantly, consistent with the critical role that nuclear import plays in HIV-1 infection of dividing and non-dividing cells, inactivation of NLSs equally affected viral replication in both cell types [105].
Similar considerations concern importins. A number of these nuclear import receptors, including importins α, β, 7 and TNPO3/TRN-SR2 have been proposed as the key components of the HIV nuclear import system in some reports, while other studies questioned their role. For example, using an in vitro model of nuclear import, Fassati and colleagues identified importin 7 as the critical component of PIC nuclear import and showed that RNAi-mediated knockdown of Imp7 in macrophages inhibits viral nuclear import and replication [31]. However, later study by Zielske and Stevenson [113] questioned this result by showing that knocking down Imp7 in macrophages did not impair infection by HIV. Potential resolution of this controversy may come from a recent report by Zaitseva and co-authors [108] which suggested that inactivation of Imp7 affects kinetics of the PIC nuclear import but not the final accumulation of PICs in the nuclear compartment. This scenario is similar to the situation with PIC NLSs (see below), whose role might be to accelerate the process of PIC nuclear import.
Interestingly, different lentiviruses appear to use alternative nuclear import pathways to access the nucleus. For example, Imp7 is required for rapid nuclear import of HIV-1, but not for HIV-2 or SIV [108]; TNPO3 is required for infection by HIV, SIV and BIV, but is dispensable for FIV [59]; nuclear import of primate lentiviruses can be inhibited by a fragment of cleavage and polyadenylation factor 6, CPSF6-358, whereas FIV is insensitive to this factor [61]. The reason for such diversity may lie in differences between the host species in the level of immune cell activation associated with lentiviral infection or the predominance of a certain target cell type. Indeed, hyperactivation of the immune system accompanies HIV infection of humans, whereas no such prolonged activation is observed in sooty mangabeys or African green monkeys naturally infected with SIV [89]. The primary target and reservoir of HIV infection are CD4+ T lymphocytes, whereas maedi-visna virus targets preferentially macrophages and does not infect T lymphocytes [93].
In addition to classical NLS-dependent pathways, HIV may use alternative nuclear import pathways that appear to be NLS-independent. One such pathway was identified by analyzing HeLa cytosolic fractions for the ability to support nuclear import of purified HIV-1 PICs into permeabilized nuclei. Surprisingly, the functional component of the active fraction turned out to be enriched in tRNA molecules, mostly with defective 3’ CCA ends [109]. Synthetic tRNAs also promoted nuclear import of the PIC. Intriguingly, retrograde tRNA transport in Saccharomyces cerevisiae is mediated by MTR10, a yeast orthologue of TNPO3/Transportin-SR2 which has been implicated in HIV nuclear import [22]. Association of tRNAs with PIC appears to depend on the Gag protein, at least replacement of HIV gag with gag of MLV blocked incorporation of tRNA species into the PIC. It remains to be determined whether this unusual pathway contributes to nuclear import of HIV in natural target cells.
Historically, MA was the first protein implicated in HIV-1 nuclear import [12,98]. While most of MA in the virion localizes between the viral core and the envelope, some MA molecules are found in tight association with the HIV core and the PIC [14,76]. Two NLSs, 26KKKYK and 110KSKKK, were identified in MA and shown to contribute to the nuclear localization of MA and PIC via interaction with importin α [29,43,78]. Interestingly, the basic aminoacids in the aminoterminal portion of MA, together with the N-terminal myristoyl moiety, also function as the membranotropic signal during viral assembly [40,112]. Switch of the MA tropism from membrane during viral assembly to nucleus during the early steps of infection remains poorly understood but is accomplished most likely by posttranslational modifications such as phosphorylation [10]. Such dual tropism of MA may be a reason that nuclear import activity of MA has not been detected in some reports [25,50]. Alternatively, the MA basic domain may function at a post-nuclear import pre-integration step, as reported by Mannioui and colleagues who found that mutation of MA residues Lys26Lys27 impaired viral infection and 2-LTR circle formation but did not reduce HIV-1 DNA docking to chromatin [73]. Overall, MA, while contributing to PIC nuclear import, appears to be non-essential as it is likely only one of several factors regulating this process (see below). This consideration explains reports showing that the virus with MA deletions was still capable of replication, albeit with a greatly reduced efficiency [32,84]. Nevertheless, NLSs within the MA may provide a good target for therapeutic approaches aimed at reducing HIV replication.
Vpr is another HIV-derived protein that has been implicated in nuclear import. Vpr significantly stimulates HIV-1 replication, in particular in macrophages, presumably by enhancing the nuclear import of the PIC [23,51,79]. Vpr has been shown to interact with importin α [83,97] using amino acid residues 17–34 [80], providing a mechanism for its nuclear localization. The increased activity of Vpr in macrophages versus CD4+ T cells was initially explained by the non-dividing state of the former, but in view of the requirement for nuclear import in both cell types it appears more likely that the PIC nuclear import pathway in macrophages is different from that in T cells and needs Vpr. Such flexibility of nuclear import pathways is consistent with a recent report showing that HIV-1 can use different nucleoporins and importins for PIC import [61]. Similar to MA, the role of Vpr is not strictly essential, as viruses lacking Vpr can still replicate in macrophages, albeit with reduced efficiency [51]. The importin-interacting domain of Vpr presents another target for potential therapeutic interventions (see below).
A role for IN in HIV-1 nuclear import was originally proposed by Gallay and co-workers [36], who demonstrated that IN interacts with importin α and can target a fusion GST-IN protein into the nucleus of microinjected cells. They also identified a basic-type NLS spanning amino acids 186–189, but this claim was later refuted by Tsurutani and colleagues [95] who demonstrated that this region is involved in reverse transcription rather than nuclear import. A more recent report [9] identified an unusual NLS spanning residues 161–173 within the central core domain of IN. IN is highly karyophilic and has been shown to interact with different importins using different domains, including importin α1 via the central core domain [5,9,36], importin α3 via the C-terminal domain (amino acids 250–270) [3], importin 7 via two regions, 235WKGPAKLLWKG and 262RRKAK, in the C-terminal domain [2] and TNPO3/transportin-SR2 via the catalytic core domain close to its N terminus [22]. These importin-interacting domains of IN present an attractive target for therapeutic interventions.
In addition to being karyophilic itself, IN was shown to interact with a host cell karyophilic protein lens epithelium-derived growth factor/transcriptional co-activator p75 (LEDGF/p75) [20,71]. LEDGF/p75 protein markedly stimulated HIV-1 IN activity in vitro and was reported to be associated with functional HIV-1 PICs [68]. The normal cellular function of LEDGF/p75 is still poorly understood, but appears to involve chromatin tethering of various cellular proteins; a similar function was proposed for LEDGF/p75 in HIV replication [74]. Interestingly, despite the presence of a canonical basic-type NLS responsible for nuclear localization of LEDGF/p75, disruption of this NLS did not abolish chromatin association of ectopically expressed IN and LEDGF/p75 [96]. This result suggests that LEDGF/p75, in an NLS-independent manner, may tether IN to chromatin during mitosis and prevent nuclear exclusion of this complex after completion of mitosis. This interpretation does not negate the possibility that NLS of LEDGF/p75 may facilitate the nuclear import of HIV-1 PIC [70], although solid experimental support for such prospect is lacking. Interaction between IN and LEDGF/p75 is determined by Trp131/132 and residues 161–170 of the HIV-1 IN [15,30]. These regions of IN present another target for potential interventions.
Recent reports suggested that HIV-1 nuclear import is regulated by the capsid protein CA p24 [59,61,107]. Indeed, mutations in CA changed the importins and nucleoporins used for PIC nuclear import, and C-terminally truncated cleavage and polyadenylation factor 6 (CPSF6-358) bound CA and inhibited HIV-1 nuclear import [61]. However, no interaction between CA and importins or nucleoporins has been reported, suggesting that the role of CA may simply reflect dependence of the PIC nuclear import on uncoating [26]. CA associated with the incompletely uncoated PIC may alter accessibility of NLSs to importins and/or interaction between the PIC and nucleoporins (Fig. 1), and by targeting CA CPSF6-358 may affect uncoating similar to the effect of TRIM5α. Blocking uncoating of HIV-1 by targeting CA may be another approach to indirectly inhibit HIV nuclear import. A similar consideration applies to another factor associated with HIV-1 nuclear import, the HIV-1 DNA Flap. This structure formed during HIV-1 reverse transcription has been implicated in HIV-1 nuclear import [110]. The mechanism of DNA Flap activity is most likely associated with facilitation of PIC uncoating at the nuclear pore [4]. Another possibility is that Flap provides a proper structure to the PIC allowing accurate exposure of the NLSs. Again, drugs targeting Flap may inhibit PIC nuclear import by affecting uncoating or disturbing the PIC conformation. A model presenting the currently known factors playing role in HIV-1 PIC nuclear import is shown in Fig. 1. In addition to nuclear import of PIC, successful HIV replication also depends on nuclear import of regulatory proteins Tat and Rev. Tat and Rev possess arginine-rich NLSs (49RKKRRQRRR57 in Tat and 35RQARRNRRRRWR46 in Rev), which, unlike most basic-type NLSs, interact with importin β but not with importin α [94]. Drugs inhibiting interaction between the HIV-1 regulatory proteins and importin β, presumably by targeting the NLS sequences, are expected to curtail viral replication.
Figure 1. Nuclear import of the HIV-1 PIC.
CA protein covers PIC NLSs preventing their interaction with importins. Partial uncoating in the perinuclear area frees some NLSs. Availability of a particular NLS determines binding of an importin (one or several). The figure shows PICs binding only one of importins (importin α/β, importin 7 or TNPO3). The importins, probably via interaction with an importin-specific nucleoporin, target the PIC to the nuclear pore complex and into the nucleus.
3. Peptides as a tool to inhibit nuclear import
The process of HIV-1 nuclear import depends on protein-protein interactions between the PIC components or individual HIV-1 proteins and the cellular nuclear import factors. In many cases, the domains responsible for these interactions have been characterized (see above). Although some small molecules have been discovered that can modulate such interactions (see below), the design of these agents purely from the knowledge of the details of a given protein-protein interaction, or through screening, remains highly difficult [101]. Therefore, using peptides derived from either protein binding partner directly, or after modification to improve affinity and physicochemical properties, continues to be very attractive and resulted in obtaining peptides that can be used to interfere with protein-protein interactions as well as to obtain anti-viral drugs [60].
Short peptides have a low metabolic stability since linear peptides are rapidly degraded by intracellular proteolytic activities [8,102]. Structural modifications such as cyclization have been shown to increase metabolic stability by making the modified peptide un-available to intracellular proteolysis [38]. Side chain to side chain cyclization (SC) utilizes functional groups like amine, carboxylic acid or thiol, that naturally exist on the side chains or at the amino or carboxy ends of the peptide, to form the cyclization through amide or disulfide bonds. Another type of cyclization is the backbone cyclization (BC) that is preformed by a covalent interconnection of two backbone amides by artificial spacer. This strategy significantly increases metabolic stability of the resulted BC peptides [16,37]. Below, we provide a short overview of the use of synthetic peptides to inhibit nuclear import of the HIV-1 PIC and its karyophilic proteins.
There are two possible mechanisms by which peptides can inhibit NLS-dependent nuclear import: they can either specifically interact with the NLS domain of a karyophilic protein masking this domain and thus preventing its interaction with the appropriate importin, or they can mimic the domain competing with the NLS-carrying protein for interaction with importin (Fig.2). So far, most nuclear import-inhibiting peptides were designed to function through the latter mechanism. Peptides derived from SV40 large T-antigen NLS, which is similar to the NLS of MA and mediates interaction with importin α, were shown to inhibit nuclear import of the HIV-1 in CD4+ MT4 cells [41]. Furthermore, screening of the library of BC peptides bearing the NLS of the MA protein identified a BC peptide, designated as BCvir, which inhibited infection of HIV-1 [35,45].
Figure 2. Peptides targeting NLS of IN.

NLS bearing peptides—such as NLS-IN—compete with IN for binding to nuclear import receptor (like importin α) and thus block nuclear import. On the other hand anti-NLS peptides—such as LEDGF 361–370—bind the NLS of the karyophilic protein (IN) thus preventing its binding to the nuclear import receptor (importin α). See text for details.
One of importins shown to interact with HIV-1 IN is importin α [36,49], so peptides mimicking importin α-interacting NLS on IN were expected to inhibit HIV-1 infection. This assumption was confirmed by studies using the peptide containing residues 161–173 of IN designated as NLS-IN [5,65]. Both a cell permeable peptide bearing the SV40 NLS and the NLS-IN peptide inhibited nuclear accumulation of IN in transfected cells and integration of viral cDNA as well as HIV-1 replication in infected cells [65]. As expected, both peptides disrupted the IN-importin α complex. A similar inhibitory effect was observed with IN-derived peptide inhibiting IN-TNPO3 interaction (unpublished observation), consistent with proposed flexible use of nuclear import pathways by HIV-1 PIC [61].
The cellular protein LEDGF/p75, a binding partner of IN, interacts with IN primarily through a domain termed the Integrase Binding Domain (IBD) [19]. Based on the crystal structure and solution structure analysis of the complex between the LEDGF/p75 IBD and a dimer of the IN catalytic core domain it can be inferred that residues 361–370 of the IBD interact with the putative IN-NLS (residues 161–173) [18,21]. Support for this view comes from results showing that interaction between a nonkaryophilic version of LEDGF/p75, mutated at its NLS, and IN blocked nuclear import of the IN [70]. Therefore, a peptide bearing residues 361–370 of LEDGF/p75 was a good candidate as an inhibitor of IN NLS. This notion was tested by Hayouka et.al. who demonstrated that a cell permeable peptide bearing LEDGF/p75 residues 361–370 inhibits HIV-1 replication and infection of cultured cells [46,47]. The LEDGF/p75 361–370 peptide may function as an “anti IN-NLS” (Fig. 2), at the same time preventing interaction of IN with LEDGF/p75 through direct competition with IBD. Interestingly, the LEDGF/p75-derived peptide was found to interact also with the NLS sequence of the HIV-1 Rev protein, but this interaction did not lead to inhibition of the Rev nuclear import [66].
Recently, an interaction between the HIV-1 Rev and IN proteins has been reported [66,88]. Surprisingly, this interaction inhibited IN nuclear import [63]. Two Rev-derived peptides, comprising Rev residues 13–23 and 53–67, were found to specifically interact with IN and to promote dissociation of the Rev-IN complex in HIV-infected cells, supposedly via competition with Rev for binding to IN [66,88] (Fig. 3). Binding to IN of both peptides blocked nuclear import of the viral IN and inhibited viral replication ([63,72,86,88] and Fig. 3). Interestingly, in contrast to the SV40 and NLS-IN peptides, the Rev-derived peptides did not disrupt the intracellular IN-importin α complex in virus-infected cells, but caused dissociation of the IN-TNPO3 complex (A.L., unpublished observation). In addition, the peptides promoted a shift in the oligomerization state of IN to predominantly tetrameric form [48]. It remains to be determined whether inhibition of the IN nuclear import by these peptides is related to their ability to disrupt the IN-TNPO3 interaction or to promote IN tetramerization. A similar nuclear import inhibitory effect and promotion of IN tetramerization was observed with another IN interacting peptide selected from a peptide library using a yeast 2 hybrid (Y2H) screen [72,86].
Figure 3. Peptides that disrupt Rev-IN interaction.
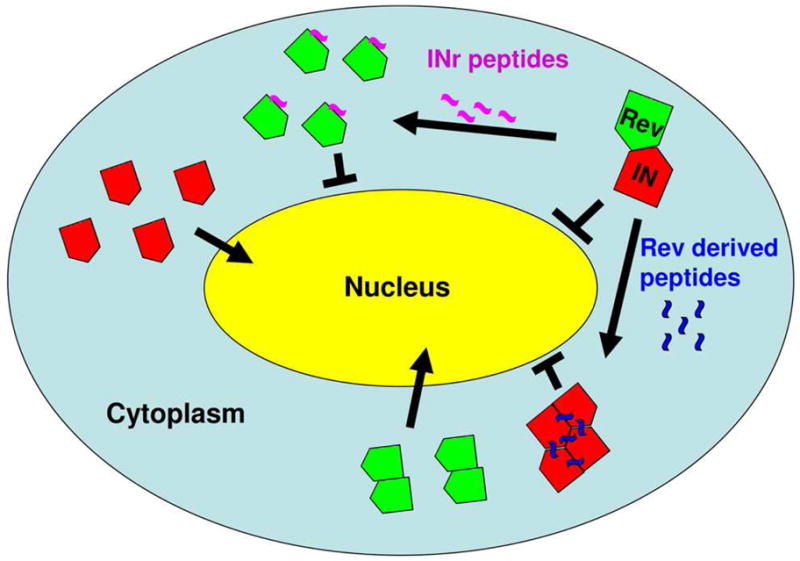
Rev derived peptides (Rev 13–23 and Rev 53–67) promote dissociation of the Rev-IN complex allowing nuclear import of Rev oligomers while shifting the IN oligomeric state to tetramer and consequently blocking its nuclear import. On the other hand, the INr peptides (INr-1 and INr-2), which also dissociate the Rev-IN complex, allow nuclear import of IN but block Rev nuclear import, probably by preventing Rev from forming the oligomeric state required for its nuclear import. See text for details.
Interaction between IN and Rev implies a possibility that IN-derived peptides may be used to block nuclear import of Rev. To identify the IN domains responsible for interaction with Rev, screening of an IN-derived peptide library was performed and revealed two specific peptides bearing IN residues 66–80 and 118–128 (designated INr-1 and INr-2, respectively [64]). Binding of the INr peptides to Rev in HIV-infected cells disrupted the Rev-IN complex, similar to the Rev-derived peptides, and thus released the IN from the Rev inhibitory effect [62,64] (Fig. 3). At the same time, the INr peptides blocked Rev nuclear import [63]. An ELISA based screening assay indicated that the INr peptides interact with the oligomerization domains of the Rev protein [55,58,64] and thus may inhibit Rev nuclear import by blocking Rev oligomerization required for this step [91].
Tat is translocated into nuclei of infected cells by arginine rich motif (ARM) sequences interacting with importin β [94]. Import of Tat-NLS-BSA conjugate into nuclei of permeabilized cells was inhibited by several BC peptides bearing the Tat ARM but not—as expected—by a peptide containing the SV40 NLS [34].
Taken together, the studies described above provide a strong rationale for further development of peptide-based inhibitors of HIV-1 nuclear import.
4. Small molecular weight inhibitors of HIV-1 nuclear import
MA was the first HIV-1 protein implicated in PIC nuclear import, and the first efforts to identify inhibitors of HIV-1 nuclear translocation focused on MA NLS. We approached this goal by looking for compounds that could neutralize the positive charge on the basic type NLS of MA. The first such compound was ITI-002 {2-amino-4-(3,5,diacetylphenyl)-amino-1,6-dimethyl pyrimidine}, which reduced HIV-1 nuclear import and inhibited viral replication [28,44]. Biochemical analysis showed that ITI-002 associated with HIV-1 MA protein via the carbonyl residues on the phenol ring of the compound by forming Schiff base adducts with lysine residues, likely in the first NLS of MA [1]. Such mechanism suggests that the compound may interact with all lysine-rich sequences and thus may be very toxic. However, this was not the case. The reason for the ITI-002 specificity for MA lies in the initial interaction between the pyrimidine side chain of ITI-002 and the HIV-1 RT, which is required for accumulation of the drug in the PIC and shifting the Schiff reaction towards the adducts [82]. The interaction with RT appears to be outside the enzyme active site since generation of the full-length nascent viral cDNA was not affected by the drug. This interaction with RT is critical for activity of ITI-002, as the drug did not inhibit nuclear import of model substrates carrying basic-type NLS, and modified drugs unable to bind RT did not inhibit HIV-1 replication [82]. It is likely that interaction with RT is required to create high concentration of the drug in the vicinity of MA NLS, thus shifting the equilibrium of the reaction towards Schiff base formation. This interesting property of arylene bis(methyl ketone) compounds explains their low toxicity and high specificity. ITI-002 inhibited HIV-1 replication in both macrophages and CD4+ T cells, as well as in ex vivo cultured lymphoid tissue [44]. Given that MA appears to be only one of several interchangeable factors mediating HIV-1 nuclear import, such potent inhibition of HIV-1 replication by MA NLS-targeting compound came as a surprise. A likely explanation is that ITI-002 may inhibit also later (post-integration) steps of viral replication which are dependent on the basic region of MA. One such step is viral assembly, as the basic region of MA within the Gag precursor is a key membranotropic signal [81].
Another class of compounds targeting the MA NLS, oxadiazols, was selected using computer aided drug design (CADD) by modeling a 250,000 compound library on the crystal structure of the HIV-1 MA [42]. These compounds were predicted to bind to MA within a structural groove in the N-terminal NLS generated by the inward orientation of the tyrosine residue (amino acid #29). ITI-367 [3-(2-methoxyphenyl)-4-[3-(trifluoromethyl)phenyl-1,2,4-oxadiazol-5(4H)-one], a lead compound from this group, inhibited the binding of the PIC to importin α, PIC nuclear import, and HIV replication in macrophages and CD4+ T cells [42]. Importantly, ITI-367 demonstrated at least an additive inhibitory effect when combined in vitro with nucleoside reverse transcriptase inhibitors (AZT, d4T), non-nucleoside reverse transcriptase inhibitors (efavirenz), or protease inhibitors (saquinavir, nelfinavir). Following culture of HIV-infected cells in the presence of increasing concentrations of ITI-367, a drug-resistant mutant was produced. Surprisingly, mutations identified in this virus localized to the cytoplasmic domain of gp41, but not to MA (unpublished results). This result suggests that the main step of HIV replication affected by ITI-367 is the step of virus assembly, rather than PIC nuclear import. Indeed, interaction between the MA-corresponding part of Gag and the cytoplasmic tail of gp41 is critical for production of infectious virions [6,7,69], and the L30E mutation located within the MA NLS impairs this interaction [7]. This result, consistent with flexible use of nuclear import pathways by HIV-1 PIC, underscores attractiveness of the MA protein as a target for therapeutic interventions.
Vpr is another HIV-1 protein implicated in PIC nuclear import. Although a number of mechanisms were proposed to explain nuclear localization of Vpr [11], association of Vpr with importin α has been reported by several independent groups [56,80,83,97]. Searching for compounds that can specifically inhibit the interaction between importin α and amino acids 17–34 of Vpr, shown to be indispensable for nuclear import of Vpr and for HIV-1 replication in macrophages [80], identified hematoxylin [90]. Hematoxilin inhibited specific binding of Vpr to importin α in a dose-dependent manner, it also inhibited HIV-1 replication in macrophages and nuclear import of HIV-1 PIC at concentrations 20–40 μM [90]. Importantly, hematoxylin did not impair the ability of Vpr to induce G2 cell cycle arrest, suggesting a specific effect on Vpr-mediated nuclear import. Surprisingly, the authors reported that the drug did not inhibit HIV-1 replication in PBMC. This result may reflect different requirements for PIC nuclear import in different cell types; nuclear import in PBMC may not require Vpr.
Styrylquinolines have been recognized as potent HIV-1 IN inhibitors that block HIV-1 replication in cell-based assays [75]. This activity may be determined, at least in part, by a specific inhibition of IN nuclear import [77]. It should be noted here that the effect on IN nuclear import was only seen at relatively high concentrations of the drug (30 μM). However, nuclear import of karyophiles carrying basic-type NLSs similar to the NLS of SV40 T antigen was not affected by these concentrations of styrylquinolines, suggesting that the drug inhibited IN interaction with importins that are not involved in transport of SV40 T antigen-type NLSs, e.g., importin 7 or TNPO3. It seems likely that binding of the compounds to IN impair both the integration activity and nuclear import of the protein. These functions appear to be regulated by adjacent or even overlapping sites on IN.
5. Conclusions
Development of HIV-specific nuclear import inhibitors critically depends on the progress of basic research aimed at understanding the molecular mechanisms of this process. This field is rapidly evolving. At present, it appears that nuclear import of HIV-1 PIC is a highly flexible process involving a number of interchangeable pathways. It is possible that these pathways are specific for certain cell types (e.g., macrophages or CD4+ T cells). Alternatively, several pathways may be used by the virus in each cell type, with selection depending on a cell status, such as activation or cell cycle progression. The cell status parameters may influence HIV nuclear import through an effect on viral uncoating: accelerated or delayed uncoating may change the import pathway used by the virus through altering accessibility of NLSs on PIC proteins. Therefore, the optimal approach to designing a universal inhibitor of HIV-1 PIC nuclear import is to target a step in viral replication preceding the nuclear import pathway bifurcation (Fig. 1). A good candidate for this approach is the step of HIV uncoating regulated by the CA protein. Although uncoating is not formally a step of nuclear import, CA appears to determine the nuclear import pathway used by the virus [61], so drugs blocking or modifying uncoating may also block viral nuclear import. Targeting nuclear import of individual viral proteins, Tat and Rev, may be an easier task, although such approach has the danger of allowing viral integration.
It can be expected that new findings will provide the basis for the design of compounds targeting not only viral proteins, but also their cellular counterparts, such as importins and nucleoporins. While targeting cellular proteins with drugs carries a danger of cytotoxicity, some compromises may be worked out. For example, multiple isoforms of proteins involved in nuclear import are expressed in eukaryotic cells [33]. These isoforms are specific for transporting certain substrates, and it is possible that isoforms may be identified that are essential for survival of the virus but not of the host cell.
A major concern with any new anti-HIV therapy is the development of drug-resistant mutants. Future studies will likely identify mutations that provide some resistance to nuclear import inhibitors. It would be interesting to find out whether resistant virus can emerge when drugs targeting several viral nuclear import factors are used simultaneously. Given that viral proteins need to interact with very conserved cellular nuclear import factors, it can be expected that the fitness of such resistant virus will be greatly compromised. This would be good news for anti-HIV therapy, as unfit HIV may be well controlled by the immune system and other drugs in the HAART cocktail.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Al Abed Y, Dubrovsky L, Ruzsicska B, Seepersaud M, Bukrinsky M. Inhibition of HIV-1 nuclear import via schiff base formation with arylene bis(methylketone) compounds. Bioorg Med Chem Lett. 2002;12:3117–3119. doi: 10.1016/s0960-894x(02)00642-x. [DOI] [PubMed] [Google Scholar]
- 2.Ao Z, Huang G, Yao H, Xu Z, Labine M, Cochrane AW, Yao X. Interaction of human immunodeficiency virus type 1 integrase with cellular nuclear import receptor importin 7 and its impact on viral replication. J Biol Chem. 2007;282:13456–13467. doi: 10.1074/jbc.M610546200. [DOI] [PubMed] [Google Scholar]
- 3.Ao Z, Jayappa KD, Wang B, Zheng Y, Kung S, Rassart E, Depping R, Kohler M, Cohen EA, Yao X. Importin {alpha}3 interacts with HIV-1 integrase and contributes to HIV-1 nuclear import and replication. J Virol. 2010 doi: 10.1128/JVI.00508-10. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arhel NJ, Souquere-Besse S, Munier S, Souque P, Guadagnini S, Rutherford S, Prevost MC, Allen TD, Charneau P. HIV-1 DNA Flap formation promotes uncoating of the pre-integration complex at the nuclear pore. EMBO J. 2007;26:3025–3037. doi: 10.1038/sj.emboj.7601740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armon-Omer A, Graessmann A, Loyter A. A synthetic peptide bearing the HIV-1 integrase 161–173 amino acid residues mediates active nuclear import and binding to importin alpha: characterization of a functional nuclear localization signal. J Mol Biol. 2004;336:1117–1128. doi: 10.1016/j.jmb.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia AK, Kaushik R, Campbell NA, Pontow SE, Ratner L. Mutation of critical serine residues in HIV-1 matrix result in an envelope incorporation defect which can be rescued by truncation of the gp41 cytoplasmic tail. Virology. 2009;384:233–241. doi: 10.1016/j.virol.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya J, Repik A, Clapham PR. Gag regulates association of human immunodeficiency virus type 1 envelope with detergent-resistant membranes. J Virol. 2006;80:5292–5300. doi: 10.1128/JVI.01469-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biron E, Chatterjee J, Ovadia O, Langenegger D, Brueggen J, Hoyer D, Schmid HA, Jelinek R, Gilon C, Hoffman A, Kessler H. Improving oral bioavailability of peptides by multiple N-methylation: somatostatin analogues. Angew Chem Int Ed Engl. 2008;47:2595–2599. doi: 10.1002/anie.200705797. [DOI] [PubMed] [Google Scholar]
- 9.Bouyac-Bertoia M, Dvorin JD, Fouchier RA, Jenkins Y, Meyer BE, Wu LI, Emerman M, Malim MH. Hiv-1 infection requires a functional integrase nls. Mol Cell. 2001;7:1025–1035. doi: 10.1016/s1097-2765(01)00240-4. [DOI] [PubMed] [Google Scholar]
- 10.Bukrinskaya AG, Ghorpade A, Heinzinger NK, Smithgall TE, Lewis RE, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci U S A. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukrinsky M. A hard way to the nucleus. Mol Med. 2004;10:1–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Bukrinsky MI, Haggerty S, Dempsey MP, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci U S A. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bukrinsky MI, Sharova N, McDonald TL, Pushkarskaya T, Tarpley WG, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci U S A. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busschots K, Voet A, De MM, Rain JC, Emiliani S, Benarous R, Desender L, Debyser Z, Christ F. Identification of the LEDGF/p75 binding site in HIV-1 integrase. J Mol Biol. 2007;365:1480–1492. doi: 10.1016/j.jmb.2006.10.094. [DOI] [PubMed] [Google Scholar]
- 16.Byk G, Halle D, Zeltser I, Bitan G, Selinger Z, Gilon C. Synthesis and biological activity of NK-1 selective, N-backbone cyclic analogs of the C-terminal hexapeptide of substance P. J Med Chem. 1996;39:3174–3178. doi: 10.1021/jm960154i. [DOI] [PubMed] [Google Scholar]
- 17.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrere F, Blanche S, Audit M, Payen E, Leboulch P, l’Homme B, Bougneres P, Von KC, Fischer A, Cavazzana-Calvo M, Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 18.Cherepanov P, Ambrosio AL, Rahman S, Ellenberger T, Engelman A. Structural basis for the recognition between HIV-1 integrase and transcriptional coactivator p75. Proc Natl Acad Sci U S A. 2005;102:17308–17313. doi: 10.1073/pnas.0506924102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherepanov P, Devroe E, Silver PA, Engelman A. Identification of an evolutionarily conserved domain in human lens epithelium-derived growth factor/transcriptional co-activator p75 (LEDGF/p75) that binds HIV-1 integrase. J Biol Chem. 2004;279:48883–48892. doi: 10.1074/jbc.M406307200. [DOI] [PubMed] [Google Scholar]
- 20.Cherepanov P, Maertens G, Proost P, Devreese B, Van Beeumen J, Engelborghs Y, De Clercq E, Debyser Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J Biol Chem. 2003;278:372–381. doi: 10.1074/jbc.M209278200. [DOI] [PubMed] [Google Scholar]
- 21.Cherepanov P, Sun ZY, Rahman S, Maertens G, Wagner G, Engelman A. Solution structure of the HIV-1 integrase-binding domain in LEDGF/p75. Nat Struct Mol Biol. 2005;12:526–532. doi: 10.1038/nsmb937. [DOI] [PubMed] [Google Scholar]
- 22.Christ F, Thys W, De RJ, Gijsbers R, Albanese A, Arosio D, Emiliani S, Rain JC, Benarous R, Cereseto A, Debyser Z. Transportin-SR2 imports HIV into the nucleus. Curr Biol. 2008;18:1192–1202. doi: 10.1016/j.cub.2008.07.079. [DOI] [PubMed] [Google Scholar]
- 23.Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 24.D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Depienne C, Roques P, Creminon C, Fritsch L, Casseron R, Dormont D, Dargemont C, Benichou S. Cellular distribution and karyophilic properties of matrix, integrase, and Vpr proteins from the human and simian immunodeficiency viruses. Exp Cell Res. 2000;260:387–395. doi: 10.1006/excr.2000.5016. [DOI] [PubMed] [Google Scholar]
- 26.Dismuke DJ, Aiken C. Evidence for a functional link between uncoating of the human immunodeficiency virus type 1 core and nuclear import of the viral preintegration complex. J Virol. 2006;80:3712–3720. doi: 10.1128/JVI.80.8.3712-3720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doucet CM, Talamas JA, Hetzer MW. Cell cycle-dependent differences in nuclear pore complex assembly in metazoa. Cell. 2010;141:1030–1041. doi: 10.1016/j.cell.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubrovsky L, Ulrich P, Nuovo GJ, Manogue KR, Cerami A, Bukrinsky M. Nuclear localization signal of HIV-1 as a novel target for therapeutic intervention. Mol Med. 1995;1:217–230. [PMC free article] [PubMed] [Google Scholar]
- 29.Dupont S, Sharova N, DeHoratius C, Virbasius CM, Zhu X, Bukrinskaya AG, Stevenson M, Green MR. A novel nuclear export activity in HIV-1 matrix protein required for viral replication. Nature. 1999;402:681–685. doi: 10.1038/45272. [DOI] [PubMed] [Google Scholar]
- 30.Emiliani S, Mousnier A, Busschots K, Maroun M, Van MB, Tempe D, Vandekerckhove L, Moisant F, Ben-Slama L, Witvrouw M, Christ F, Rain JC, Dargemont C, Debyser Z, Benarous R. Integrase mutants defective for interaction with LEDGF/p75 are impaired in chromosome tethering and HIV-1 replication. J Biol Chem. 2005;280:25517–25523. doi: 10.1074/jbc.M501378200. [DOI] [PubMed] [Google Scholar]
- 31.Fassati A, Gorlich D, Harrison I, Zaytseva L, Mingot JM. Nuclear import of HIV-1 intracellular reverse transcription complexes is mediated by importin 7. EMBO J. 2003;22:3675–3685. doi: 10.1093/emboj/cdg357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fouchier RA, Meyer BE, Simon JH, Fischer U, Malim MH. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried H, Kutay U. Nucleocytoplasmic transport: taking an inventory. Cell Mol Life Sci. 2003;60:1659–1688. doi: 10.1007/s00018-003-3070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedler A, Friedler D, Luedtke NW, Tor Y, Loyter A, Gilon C. Development of a functional backbone cyclic mimetic of the HIV-1 Tat arginine-rich motif. J Biol Chem. 2000;275:23783–23789. doi: 10.1074/jbc.M002200200. [DOI] [PubMed] [Google Scholar]
- 35.Friedler A, Zakai N, Karni O, Broder YC, Baraz L, Kotler M, Loyter A, Gilon C. Backbone cyclic peptide, which mimics the nuclear localization signal of human immunodeficiency virus type 1 matrix protein, inhibits nuclear import and virus production in nondividing cells. Biochemistry. 1998;37:5616–5622. doi: 10.1021/bi972878h. [DOI] [PubMed] [Google Scholar]
- 36.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci U S A. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gazal S, Gelerman G, Ziv O, Karpov O, Litman P, Bracha M, Afargan M, Gilon C. Human somatostatin receptor specificity of backbone-cyclic analogues containing novel sulfur building units. J Med Chem. 2002;45:1665–1671. doi: 10.1021/jm0100281. [DOI] [PubMed] [Google Scholar]
- 38.Gilon C, Halle D, Chorev M, Selinger Z, Byk G. Backbone cyclization: A new method for conferring conformational constraint on peptides. Biopolymers. 1991;31:745–750. doi: 10.1002/bip.360310619. [DOI] [PubMed] [Google Scholar]
- 39.Goff SP. Knockdown screens to knockout HIV-1. Cell. 2008;135:417–420. doi: 10.1016/j.cell.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gottlinger HG, Sodroski JG, Haseltine WA. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulizia J, Dempsey MP, Sharova N, Bukrinsky MI, Spitz L, Goldfarb D, Stevenson M. Reduced nuclear import of human immunodeficiency virus type 1 preintegration complexes in the presence of a prototypic nuclear targeting signal. J Virol. 1994;68:2021–2025. doi: 10.1128/jvi.68.3.2021-2025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haffar O, Dubrovsky L, Lowe R, Berro R, Kashanchi F, Godden J, Vanpouille C, Bajorath J, Bukrinsky M. Oxadiazols: a new class of rationally designed anti-human immunodeficiency virus compounds targeting the nuclear localization signal of the viral matrix protein. J Virol. 2005;79:13028–13036. doi: 10.1128/JVI.79.20.13028-13036.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haffar OK, Popov S, Dubrovsky L, Agostini I, Tang H, Pushkarsky T, Nadler SG, Bukrinsky M. Two nuclear localization signals in the HIV-1 matrix protein regulate nuclear import of the HIV-1 pre-integration complex. J Mol Biol. 2000;299:359–368. doi: 10.1006/jmbi.2000.3768. [DOI] [PubMed] [Google Scholar]
- 44.Haffar OK, Smithgall MD, Popov S, Ulrich P, Bruce AG, Nadler SG, Cerami A, Bukrinsky MI. CNI-H0294, a nuclear importation inhibitor of the human immunodeficiency virus type 1 genome, abrogates virus replication in infected activated peripheral blood mononuclear cells. Antimicrob Agents Chemother. 1998;42:1133–1138. doi: 10.1128/aac.42.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hariton-Gazal E, Friedler D, Friedler A, Zakai N, Gilon C, Loyter A. Inhibition of nuclear import by backbone cyclic peptidomimetics derived from the HIV-1 MA NLS sequence. Biochim Biophys Acta. 2002;1594:234–242. doi: 10.1016/s0167-4838(01)00306-5. [DOI] [PubMed] [Google Scholar]
- 46.Hayouka Z, Levin A, Maes M, Hadas E, Shalev DE, Volsky DJ, Loyter A, Friedler A. Mechanism of action of the HIV-1 integrase inhibitory peptide LEDGF 361–370. Biochem Biophys Res Commun. 2010;394:260–265. doi: 10.1016/j.bbrc.2010.02.100. [DOI] [PubMed] [Google Scholar]
- 47.Hayouka Z, Rosenbluh J, Levin A, Loya S, Lebendiker M, Veprintsev D, Kotler M, Hizi A, Loyter A, Friedler A. Inhibiting HIV-1 integrase by shifting its oligomerization equilibrium. Proc Natl Acad Sci U S A. 2007;104:8316–8321. doi: 10.1073/pnas.0700781104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayouka Z, Rosenbluh J, Levin A, Maes M, Loyter A, Friedler A. Peptides derived from HIV-1 Rev inhibit HIV-1 integrase in a shiftide mechanism. Biopolymers. 2008;90:481–487. doi: 10.1002/bip.20930. [DOI] [PubMed] [Google Scholar]
- 49.Hearps AC, Jans DA. HIV-1 integrase is capable of targeting DNA to the nucleus via an Importin alpha/beta-dependent mechanism. Biochem J. 2006;398:475–484. doi: 10.1042/BJ20060466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hearps AC, Wagstaff KM, Piller SC, Jans DA. The N-terminal basic domain of the HIV-1 matrix protein does not contain a conventional nuclear localization sequence but is required for DNA binding and protein self-association. Biochemistry. 2008;47:2199–2210. doi: 10.1021/bi701360j. [DOI] [PubMed] [Google Scholar]
- 51.Heinzinger NK, Bukrinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, Gendelman HE, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci U S A. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hetzer MW. The role of the nuclear pore complex in aging of post-mitotic cells. Aging (Albany, NY) 2010;2:74–75. doi: 10.18632/aging.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 54.Iordanskiy S, Berro R, Altieri M, Kashanchi F, Bukrinsky M. Intracytoplasmic maturation of the human immunodeficiency virus type 1 reverse transcription complexes determines their capacity to integrate into chromatin. Retrovirology. 2006;3(4):4. doi: 10.1186/1742-4690-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jain C, Belasco JG. Structural model for the cooperative assembly of HIV-1 Rev multimers on the RRE as deduced from analysis of assembly-defective mutants. Mol Cell. 2001;7:603–614. doi: 10.1016/s1097-2765(01)00207-6. [DOI] [PubMed] [Google Scholar]
- 56.Kamata M, Nitahara-Kasahara Y, Miyamoto Y, Yoneda Y, Aida Y. Importin-{alpha} Promotes Passage through the Nuclear Pore Complex of Human Immunodeficiency Virus Type 1 Vpr. J Virol. 2005;79:3557–3564. doi: 10.1128/JVI.79.6.3557-3564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katz RA, Greger JG, Boimel P, Skalka AM. Human immunodeficiency virus type 1 DNA nuclear import and integration are mitosis independent in cycling cells. J Virol. 2003;77:13412–13417. doi: 10.1128/JVI.77.24.13412-13417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kjems J, Askjaer P. Rev protein and its cellular partners. Adv Pharmacol. 2000;48:251–298. doi: 10.1016/s1054-3589(00)48009-9. [DOI] [PubMed] [Google Scholar]
- 59.Krishnan L, Matreyek KA, Oztop I, Lee K, Tipper CH, Li X, Dar MJ, Kewalramani VN, Engelman A. The requirement for cellular transportin 3 (TNPO3 or TRN-SR2) during infection maps to human immunodeficiency virus type 1 capsid and not integrase. J Virol. 2010;84:397–406. doi: 10.1128/JVI.01899-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Latham PW. Therapeutic peptides revisited. Nat Biotechnol. 1999;17:755–757. doi: 10.1038/11686. [DOI] [PubMed] [Google Scholar]
- 61.Lee K, Ambrose Z, Martin TD, Oztop I, Mulky A, Julias JG, Vandegraaff N, Baumann JG, Wang R, Yuen W, Takemura T, Shelton K, Taniuchi I, Li Y, Sodroski J, Littman DR, Coffin JM, Hughes SH, Unutmaz D, Engelman A, Kewalramani VN. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe. 2010;7:221–233. doi: 10.1016/j.chom.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levin A, Hayouka Z, Friedler A, Brack-Werner R, Volsky D, Loyter A. A novel role for the viral Rev protein in promoting resistance to super-infection by Human Immunodeficiency Virus type 1. J Gen Virol. 2010;91:1503–1513. doi: 10.1099/vir.0.019760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levin A, Hayouka Z, Friedler A, Loyter A. Nucleocytoplasmic Shuttling of HIV-1 Integrase Is Controlled by the Viral Rev Protein. Nucleus. 2010 doi: 10.4161/nucl.1.2.11300. available at http://www.landesbioscience.com/journals/nucleus/article/11300/ [DOI] [PMC free article] [PubMed]
- 64.Levin A, Hayouka Z, Helfer M, Brack-Werner R, Friedler A, Loyter A. Peptides derived from HIV-1 integrase that bind Rev stimulate viral genome integration. PLoS One. 2009;4:e4155. doi: 10.1371/journal.pone.0004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levin A, Armon-Omer, Rosenbluh J, Melamed-Book N, Graessmann A, Waigmann E, Loyter A. Inhibition of HIV-1 integrase nuclear import and replication by a peptide bearing integrase putative nuclear localization signal. Retrovirology. 2009;6:112. doi: 10.1186/1742-4690-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levin A, Rosenbluh J, Hayouka Z, Friedler A, Loyter A. Integration of HIV-1 DNA is regulated by interplay between viral rev and cellular LEDGF/p75 proteins. Mol Med. 2010;16:34–44. doi: 10.2119/molmed.2009.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Llano M, Vanegas M, Fregoso O, Saenz D, Chung S, Peretz M, Poeschla EM. LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral preintegration complexes. J Virol. 2004;78:9524–9537. doi: 10.1128/JVI.78.17.9524-9537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez-Verges S, Camus G, Blot G, Beauvoir R, Benarous R, Berlioz-Torrent C. Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions. Proc Natl Acad Sci U S A. 2006;103:14947–14952. doi: 10.1073/pnas.0602941103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maertens G, Cherepanov P, Debyser Z, Engelborghs Y, Engelman A. Identification and characterization of a functional nuclear localization signal in the HIV-1 integrase interactor LEDGF/p75. J Biol Chem. 2004;279:33421–33429. doi: 10.1074/jbc.M404700200. [DOI] [PubMed] [Google Scholar]
- 71.Maertens G, Cherepanov P, Pluymers W, Busschots K, De CE, Debyser Z, Engelborghs Y. LEDGF/p75 is essential for nuclear and chromosomal targeting of HIV-1 integrase in human cells. J Biol Chem. 2003;278:33528–33539. doi: 10.1074/jbc.M303594200. [DOI] [PubMed] [Google Scholar]
- 72.Maes M, Levin A, Hayouka Z, Shalev DE, Loyter A, Friedler A. Peptide inhibitors of HIV-1 integrase: from mechanistic studies to improved lead compounds. Bioorg Med Chem. 2009;17:7635–7642. doi: 10.1016/j.bmc.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 73.Mannioui A, Nelson E, Schiffer C, Felix N, Le RE, Benichou S, Gluckman JC, Canque B. Human immunodeficiency virus type 1 KK26–27 matrix mutants display impaired infectivity, circularization and integration but not nuclear import. Virology. 2005;339:21–30. doi: 10.1016/j.virol.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 74.Meehan AM, Poeschla EM. Chromatin tethering and retroviral integration: recent discoveries and parallels with DNA viruses. Biochim Biophys Acta. 2010;1799:182–191. doi: 10.1016/j.bbagrm.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mekouar K, Mouscadet JF, Desmaele D, Subra F, Leh H, Savoure D, Auclair C, d’Angelo J. Styrylquinoline derivatives: a new class of potent HIV-1 integrase inhibitors that block HIV-1 replication in CEM cells. J Med Chem. 1998;41:2846–2857. doi: 10.1021/jm980043e. [DOI] [PubMed] [Google Scholar]
- 76.Miller MD, Farnet CM, Bushman FD. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mousnier A, Leh H, Mouscadet JF, Dargemont C. Nuclear Import of HIV-1 Integrase Is Inhibited in Vitro by Styrylquinoline Derivatives. Mol Pharmacol. 2004;66:783–788. doi: 10.1124/mol.104.001735. [DOI] [PubMed] [Google Scholar]
- 78.Nadler SG, Tritschler D, Haffar OK, Blake J, Bruce AG, Cleaveland JS. Differential expression and sequence-specific interaction of karyopherin alpha with nuclear localization sequences. J Biol Chem. 1997;272:4310–4315. doi: 10.1074/jbc.272.7.4310. [DOI] [PubMed] [Google Scholar]
- 79.Nie Z, Bergeron D, Subbramanian RA, Yao XJ, Checroune F, Rougeau N, Cohen EA. The putative alpha helix 2 of human immunodeficiency virus type 1 Vpr contains a determinant which is responsible for the nuclear translocation of proviral DNA in growth-arrested cells. J Virol. 1998;72:4104–4115. doi: 10.1128/jvi.72.5.4104-4115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nitahara-Kasahara Y, Kamata M, Yamamoto T, Zhang X, Miyamoto Y, Muneta K, Iijima S, Yoneda Y, Tsunetsugu-Yokota Y, Aida Y. Novel nuclear import of Vpr promoted by importin alpha is crucial for human immunodeficiency virus type 1 replication in macrophages. J Virol. 2007;81:5284–5293. doi: 10.1128/JVI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ono A, Freed EO. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J Virol. 1999;73:4136–4144. doi: 10.1128/jvi.73.5.4136-4144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Popov S, Dubrovsky L, Lee MA, Pennathur S, Haffar O, aL-Abed Y, Tonge P, Ulrich P, Rexach M, Blobel G, Cerami A, Bukrinsky M. Critical role of reverse transcriptase in the inhibitory mechanism of CNI-H0294 on HIV-1 nuclear translocation. Proc Natl Acad Sci U S A. 1996;93:11859–11864. doi: 10.1073/pnas.93.21.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Popov S, Rexach M, Zybarth G, Reiling N, Lee MA, Ratner L, Lane CM, Moore MS, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reil H, Bukovsky AA, Gelderblom HR, Gottlinger HG. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 1998;17:2699–2708. doi: 10.1093/emboj/17.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riviere L, Darlix JL, Cimarelli A. Analysis of the viral elements required in the nuclear import of HIV-1 DNA. J Virol. 2010;84:729–739. doi: 10.1128/JVI.01952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Armon-Omer, Levin A, Hayouka Z, Butz K, Hoppe-Seyler F, Loya S, Hizi A, Friedler A, Loyter A. Correlation between shiftide activity and HIV-1 integrase inhibition by a peptide selected from a combinatorial library. J Mol Biol. 2008;376:971–982. doi: 10.1016/j.jmb.2007.11.095. [DOI] [PubMed] [Google Scholar]
- 87.Roe T, Reynolds TC, Yu G, Brown PO. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosenbluh J, Hayouka Z, Loya S, Levin A, Armon-Omer, Britan E, Hizi A, Kotler M, Friedler A, Loyter A. Interaction between HIV-1 Rev and integrase proteins: a basis for the development of anti-HIV peptides. J Biol Chem. 2007;282:15743–15753. doi: 10.1074/jbc.M609864200. [DOI] [PubMed] [Google Scholar]
- 89.Silvestri G. AIDS pathogenesis: a tale of two monkeys. J Med Primatol. 2008;37(Suppl 2):6–12. doi: 10.1111/j.1600-0684.2008.00328.x. [DOI] [PubMed] [Google Scholar]
- 90.Suzuki T, Yamamoto N, Nonaka M, Hashimoto Y, Matsuda G, Takeshima SN, Matsuyama M, Igarashi T, Miura T, Tanaka R, Kato S, Aida Y. Inhibition of human immunodeficiency virus type 1 (HIV-1) nuclear import via Vpr-Importin alpha interactions as a novel HIV-1 therapy. Biochem Biophys Res Commun. 2009;380:838–843. doi: 10.1016/j.bbrc.2009.01.180. [DOI] [PubMed] [Google Scholar]
- 91.Szilvay AM, Brokstad KA, Boe SO, Haukenes G, Kalland KH. Oligomerization of HIV-1 Rev mutants in the cytoplasm and during nuclear import. Virology. 1997;235:73–81. doi: 10.1006/viro.1997.8671. [DOI] [PubMed] [Google Scholar]
- 92.Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- 93.Thormar H. Maedi-visna virus and its relationship to human immunodeficiency virus. AIDS Rev. 2005;7:233–245. [PubMed] [Google Scholar]
- 94.Truant R, Cullen BR. The arginine-rich domains present in human immunodeficiency virus type 1 Tat and Rev function as direct importin beta-dependent nuclear localization signals. Mol Cell Biol. 1999;19:1210–1217. doi: 10.1128/mcb.19.2.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsurutani N, Kubo M, Maeda Y, Ohashi T, Yamamoto N, Kannagi M, Masuda T. Identification of critical amino acid residues in human immunodeficiency virus type 1 IN required for efficient proviral DNA formation at steps prior to integration in dividing and nondividing cells. J Virol. 2000;74:4795–4806. doi: 10.1128/jvi.74.10.4795-4806.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vanegas M, Llano M, Delgado S, Thompson D, Peretz M, Poeschla E. Identification of the LEDGF/p75 HIV-1 integrase-interaction domain and NLS reveals NLS-independent chromatin tethering. J Cell Sci. 2005;118:1733–1743. doi: 10.1242/jcs.02299. [DOI] [PubMed] [Google Scholar]
- 97.Vodicka MA, Koepp DM, Silver PA, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.von Schwedler U, Kornbluth RS, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci U S A. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei X, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 100.Weinberg JB, Matthews TJ, Cullen BR, Malim MH. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J Exp Med. 1991;174:1477–1482. doi: 10.1084/jem.174.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 102.Woodley JF. Enzymatic barriers for GI peptide and protein delivery. Crit Rev Ther Drug Carrier Syst. 1994;11:61–95. [PubMed] [Google Scholar]
- 103.Woodman Z, Williamson C. HIV molecular epidemiology: transmission and adaptation to human populations. Curr Opin HIV AIDS. 2009;4:247–252. doi: 10.1097/COH.0b013e32832c0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamashita M, Emerman M. The cell cycle independence of HIV infections is not determined by known karyophilic viral elements. PLoS Pathog. 2005;1:e18. doi: 10.1371/journal.ppat.0010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamashita M, Emerman M. Retroviral infection of non-dividing cells: old and new perspectives. Virology. 2006;344:88–93. doi: 10.1016/j.virol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 106.Yamashita M, Perez O, Hope TJ, Emerman M. Evidence for direct involvement of the capsid protein in HIV infection of nondividing cells. PLoS Pathog. 2007;3:1502–1510. doi: 10.1371/journal.ppat.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamashita M, Emerman M. Capsid Is a Dominant Determinant of Retrovirus Infectivity in Nondividing Cells. J Virol. 2004;78:5670–5678. doi: 10.1128/JVI.78.11.5670-5678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zaitseva L, Cherepanov P, Leyens L, Wilson SJ, Rasaiyaah J, Fassati A. HIV-1 exploits importin 7 to maximize nuclear import of its DNA genome. Retrovirology. 2009;6:11. doi: 10.1186/1742-4690-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zaitseva L, Myers R, Fassati A. tRNAs promote nuclear import of HIV-1 intracellular reverse transcription complexes. PLoS Biol. 2006;4:e332. doi: 10.1371/journal.pbio.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 111.Zhan P, Liu X, De CE. Blocking nuclear import of pre-integration complex: an emerging anti-HIV-1 drug discovery paradigm. Curr Med Chem. 2010;17:495–503. doi: 10.2174/092986710790416335. [DOI] [PubMed] [Google Scholar]
- 112.Zhou W, Parent LJ, Wills JW, Resh MD. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zielske SP, Stevenson M. Importin 7 May Be Dispensable for Human Immunodeficiency Virus Type 1 and Simian Immunodeficiency Virus Infection of Primary Macrophages. J Virol. 2005;79:11541–11546. doi: 10.1128/JVI.79.17.11541-11546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]



