Abstract
Since the first human embryonic stem cell (hESC) line was generated by Thomson et al. (in Science 282:1145–1147, 1998), hundreds of hESC lines have been reported by different labs, providing resources for basic research and regenerative medicine as well. However it has been widely recognized that hESC lines varied on their properties, in terms of gene expression profile, epigenetic modify profile, and differentiation tendency. Generation of more hESC lines will largely enhance our knowledge of hESCs innate character. In this current work, we reported the generation of HN4, a hESC line derived from grade III IVF human embryo by using a mixture of human foreskin fibroblast (HFF) and mouse embryonic fibroblast (MEF) as feeder layers, and a whole-mechanical method in inner cell mass (ICM) isolation. HN4 satisfied the criteria of hESCs pluripotency, with high expression of hESC surface markers (SSEA-3, SSEA-4, TRA-1-60, TRA-1-81), transcription factors (OCT-4, NANOG, REX-1), and alkaline phosphatase. It is able to differentiate to three germ layer derivatives when cultured in vitro, or in teratoma formation. Moreover, it displayed promising potential in neural differentiation under a proper culture condition, suggesting the advantage of HN4 in further investigation. Additionally, the whole-mechanical protocol for ICM isolation facilitates hESC line generation for its ease to handle.
Keywords: Human stem cells, ICM mechanical isolation, Mixed feeder layer, Neural differentiation
Introduction
With the properties of self-renewing and pluripotent differentiation potential, human embryonic stem cells (hESCs) derived from the inner cell mass of blastocyst (Familari and Selwood 2006; Findikli et al. 2005; Reubinoff et al. 2000) are valuable tools for human development biology study in vitro, sources for cellular replacement therapy and materials of drug screening (Thomson et al. 1998; Dvash et al. 2006; Srivastava and Ivey 2006; Urban et al. 2006; Zhang et al. 2001; Lerou and Daley 2005). Numerous hESC lines have been generated by immunosurgery protocol for inner cell mass isolation since the first successful attempt in 1998 (Thomson et al. 1998). Later generation of hESC lines by mechanical isolation of ICMs were reported (Mummery 2004; Van de Stolpe et al. 2005; Ström et al. 2007). However, the number of hESC lines available still does not satisfy the need of researches for hESC variation in gene expression profile, modified epigenetic profile, and differentiation tendency. Hence, more hESC lines are expected to be established, while the method to derivate cell lines should be further advanced.
Here, the hESC line HN4, which was generated through whole mechanical isolation of inner cell mass (ICM) from discarded embryos, and cultured on mixed feeders, is reported. To derive hESCs from ICM, only 1 ml-syringe was used, thus made the procedure time economical and chemical free.
The ability of hESCs to generate neural cell types in vitro offers a powerful tool to study human neurogenesis, and give therapeutical light on Parkinson’s disease or spinal cord injury (Keirstead et al. 2005). In this report, we demonstrated that HN4 could be induced to differentiate to neurons in a high percentage, suggesting HN4 is a promising cell resource on neural degeneration research.
Materials and methods
Blastocyst culture
Approved by the Ethics Committee of the Hainan Provincial Reproductive Medical Center and under the Guidelines for Research on Human Embryonic Stem Cells issued by the Ministry of Science and Technology and Ministry of Health of P. R. China, Human grade III embryos (Balaban et al. 2000) were obtained in the Hainan Provincial Reproductive Medical center with informed consent. These embryos were not suitable for immediate embryo transfer or cryopreservation and going to be discarded. They were cultured to the blastocyst stage according to our IVF laboratory standard protocol in IVC-Three blastocyst medium under mineral oil (InVitroCare, Frederick, MD).
Preparation of mixed feeder layer
Mouse embryonic fibroblasts (MEF) were isolated from 12.5 to 14.5-day-old fetal KM strain mice (Robertson 1987), and cultured in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal calf serum (Invitrogen, Carlsbad, CA).
Human foreskin fibroblasts (HFF) were isolated (Richards et al. 2002; Hovatta et al. 2003) from a child who underwent a circumcision with informed consent after approval by the Ethics Committee of the Hainan Provincial Reproductive Medical Center.
Preparation of mixed feeder layer was carried out as follows: HFF and MEF cells were inactivated with 10 μg/ml mitomycin C (Sigma–Aldrich, St. Louis, MO) for 2.5–3 h, respectively, and were counted and mixed at a ratio of 1:1. Mixture of cells was incubated at a density of 4.5 × 104/cm2 in a gelatin-coated dish the day before use.
Mechanical isolation of ICM and the primary culture of hESCs
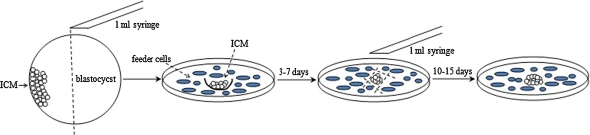
ICM were isolated from blastocysts mechanically with a 1 ml-syringe (25 gauge, BD Biosciences, Bedford, MA). Briefly, blastocysts were cut into two parts in the middle, and the ICM containing parts were then plated on dishes seeded with inactivated HFF and MEF mixed feeder layers. Cells were cultured in 5% CO2, 37 °C, 95% humidity, with medium replaced every day. About 3–7 days later, cell mass from ICM was isolated mechanically with 1 ml-syringe, re-seeded on dishes with mixed feeder cells and cultured. The hESC primary colonies were observed after 10–15 days (Fig. 1). Culture medium used was based on Knock-out DMEM with 20% Knock-out serum replacement, 2 mM L-glutamine, 0.1 mM non-essential amino Acid (NEAA), 0.1 mM β-mercaptoethanol (Invitrogen) and 4 μg/ml human basic fibroblast growth factor (bFGF, R&D Systems, Minneapolis, MN).
Fig. 1.
Schematic diagram of the mechanical isolation of ICM
HN4 culture
For passage, HN4 primary colony was mechanically cut into several pieces using a 1 ml-syringe. Each piece, containing about 50–100 cells, was seeded on dishes with inactivated mixed feeder cells and cultured. HN4 was passaged every 6–7 days.
Immunostaining
Undifferentiation markers of the HN4 cell line in its 15th–22nd passages were enzymatically or immunocytochemically analysed. Alkaline phosphatase (AKP) activity was assessed with the ELF phosphatise detection kit (ATCC, Manassas, VA) according to the manufacturer’s instruction. For immunostainning, cells were fixed with 4% paraformaldehyde (PFA, Sigma) at room temperature for 20 min, and then blocked with 10% donkey serum (Jackson Immuno Research, West Grove, PA), 1% bovine serum albumin (BSA, Genview, Houston, TX), and 0.1% Triton X-100 (Carl Roth, Karlsruhe) in phosphate buffer saline (PBS) at room temperature for 45 min. After blocking, cells were incubated with primary antibodies at 4 °C overnight. Primary antibodies were diluted to appropriate concentration: SSEA-3 (R&D Systems) was 1/50 diluted, SSEA-4 (R&D Systems), TRA-1-60 (Chemicon, Billerica, MA) and TRA-1-81 (Chemicon) were 1/100 diluted. OCT-4 (Santa Cruz Biotechnology, CA) was 1/500 diluted for use. The next day, cells were incubated with cy3- or cy2-conjugated secondary antibody (Jackson) for 1 h at room temperature, and followed by 1 μg/ml DAPI (Sigma) staining of cell nuclei for 5 min. Between each step, cells were fully washed with PBS containing 1% BSA (Genview). Cells were imaged under an inverted microscope (Olympus IX51) with a fluorescence imaging device (Olympus DP70).
RT-PCR
Expressions of OCT-4, REX-1 and NANOG were detected by reverse transcription PCR (RT-PCR). Total RNA was extracted with Trizol Reagent (Invitrogen), respectively from HN4 and mixed feeder cells. About 1 μg RNA was used for reverse transcription and cDNA was synthesized from total RNA with Revert Aid First Strand cDNA Synthesis Kit (MBI Fermentas, St Leon-Rot, Germany). For all genes, PCR was performed for 29 cycles using the following parameters: initial de-naturation at 94 °C for 5 min, then 94 °C for 30 s, 58 °C (55 °C for NANOG) for 30 s, 72 °C for 45 s, and terminated by final extention at 72 °C for 10 min. The sequences of primers used were as follows:
OCT-4 (forward, GAGAACAATGAGAACCTTCAGGA; reverse, TTCTGGCGCCGGTT ACAGAACCA);
RXE-1 (forward, CTGAAGAAACGGGCAAAGAC; reverse, GAACATTCAAGGGAGC TTGC);
Nanog (forward, AGTCCCAAAGGCAAACAACCCACTTC; reverse, TGCTGGAGGCTGAGGTATTTCTGTCTC);
β-Actin (forward, AGAAAATCTGGCACCACACC; reverse, CCATCTCTTGCTCGAAGTCC).
PCR products underwent 2% agarose electrophoresis and were photographed under an ultraviolet lamp.
Genetic characterization of HN4
Karyotype analysis was performed on cells in their exponential growth stage, after colcemid arrest G-banding technology was carried out (Mandal et al. 2006).
Differentiation of HN4 in vitro and in vivo
The in vitro differentiation potential of HN4 at its 28th and 33rd passage was assayed via embryoid body (EB) formation (Itskovitz-Eldor et al. 2000; Carpenter et al. 2003).
Briefly, hESCs were harvested and transferred to plastic Petri dishes for non-adherent growth (Corning, NY) to form EBs in knock-out DMEM containing 20% knock-out serum replacement, 2 mM L-glutamine, 1× NEAA, and 0.1 mM β-mercaptoethanol (Invitrogen) with medium changed every other day. After 10 days’ suspension culture, EBs were transferred to 0.2% gelatin (Sigma) coated cell culture plates (for adherent cultures) and cultured in the same medium for 6 days for further differentiation. Differentiated hES cells were identified by immunocytochemical staining against markers of endoderm (Foxa2, specific for posterior epiblast cell; Santa Cruz), mesoderm (Bry, specific marker for mesoderm; Abcam Inc, Cambridge, MA), and ectoderm (Nestin, specific for progenitor of neuron; Chemicon) according to manufacturer ‘s instruction.
For teratoma formation, undifferentiated cells in passage 20 were resuspended in PBS and injected subcutaneously into severe combined immunodeficient (SCID) mice of 5 weeks old. Mere mixed feeder cells were injected as negative control. Ten weeks after injection, teratomas were surgically removed and histological sections were analyzed (Heins et al. 2006).
Neural differentiation of HN4
HN4 neural differentiation was performed according to Ma et al. (2008). Cell clusters in passage 28 were harvested and suspended in Petri dishes for non-adherent growth (Corning) in hESC medium (without bFGF) renewed every day. Five days later, EBs were transferred into neural differentiation medium (65% DMEM, 32.5% F12/Glutamax, 0.1mM NEAA, 1× N2 (Invitrogen) and 5 ng/ml bFGF (R&D Systems)), and cultured for 10 days. After 10 days of induction, EBs were plated on Poly-D-Lysine (PDL) and Laminin (Sigma) coated dishes, and cultured for 15 days in neural differentiation medium. Medium was changed every day during the whole differentiation process. Immunostaining for β-III-tubulin (Covance, Princeton, NJ) and Nestin (Chemicon) were carried out to examine the neural differentiation potential of HN4.
Results
Human blastocysts culture and generation of hESC line
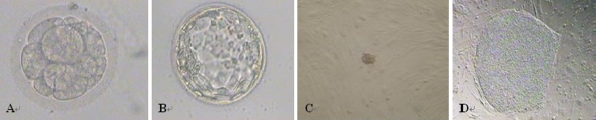
Nine 3-day embryos with poor quality (evaluated as Grade III in accordance with Balaban et al. 2000, Fig. 2a) and thereby considered improper for embryo transfer or cryopreservation were used for ICM isolation. After cultured for 2 days in IVC-Three blastocyst medium, two of them got to blasocyst stage (Fig. 2b). The two blastocysts were cut into two semispheres with a 1 ml-syringe, with one part including ICM and the other part not. The ICM parts were then seeded on HFF and MEF mixed feeder layers as described in materials and methods. About 3–7 days later, clumps of small, tightly packed cells proliferated from the ICM. These homogeneous cells were mechanically dissected from the adjacent trophoblasts and transferred to prepared feeder layers (Fig. 2c). After 10 days culturing, one distinguishable primary colony appeared in one of the two blastocysts. It was then passaged for further identification, and finally identified as hESC line, named HN4.
Fig. 2.
Generation of HN4. a Morphology of the Grade III 3-day embryo, b morphology of the blastocyst, c morphology of ICM on the mixed feeder layer, and d morphology of hESC line HN4 after 17 passages on the mixed feeder layer. (a,b,d 100× and c 60×)
Maintenance of HN4
For the first 3–5 passages, spontaneously differentiated cells were observed in the centre or at the edge of the colonies, which were removed with a 1 ml-syringe while passaging. After several mechanical passages, less and less spontaneously differentiated cells were observed. HN4 colonies appeared homogeneous with typical hESC morphology: flat and compacted colony structure, well-defined colony edge, and the cells had a high nucleus to cytoplasm ratio (Fig. 2d). After 5 passages, when a considerable amount of hES cells was reached and they were perfectly homogenous, enzymatic passage procedure was used to ease the time-consuming work of mechanical passage (Lerou et al. 2008). HN4 was split every 6–7 days, and has been cultured for more than 33 passages in vitro.
Colonies grown in the MEF and HFF mixed feeders showed a more obvious clustering of cells and were thicker than those on either MEF or HFF (compare Fig. 3c to a and b).
Fig. 3.
Growth of human embryonic stem cells in different feeder layers. a Human embryonic stem cells on an MEF feeder layer have a clear colony border, but the colony does not show a clear clustering of cells and is flat (20×), b human embryonic stem cells on a HFF feeder layer have a clear colony border, but the center of colony differentiates easily (20×), and c human embryonic stem cells in a mixed feeder layer (MEF: HFF = 1:1) have a clear colony border and apparent limitation to surrounding cells, the colony is thick (20×)
Colonies on MEF feeders were flat (Fig. 3a) and the center of the colonies on HFF feeders had a tendency to differentiate (Fig. 3b). Mixed feeder layer was used to support HN4 growth, which proved to be superior to either mere MEF or HFF as feeder layer (Li et al. 2008). Generally, MEF cells used for hESCs culture should have less than five passages, while HFF cells have supportive capability for up to fifteen passages. However we found that in the long-term culture mixed feeder layers could well support hESCs growing even in the 17th passage of MEF and 18th passage of HFF, thereby greatly reducing labor work.
Pluripotency of HN4
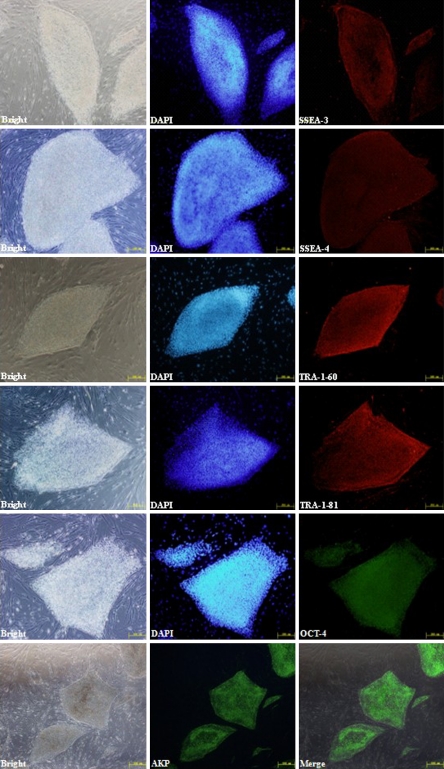
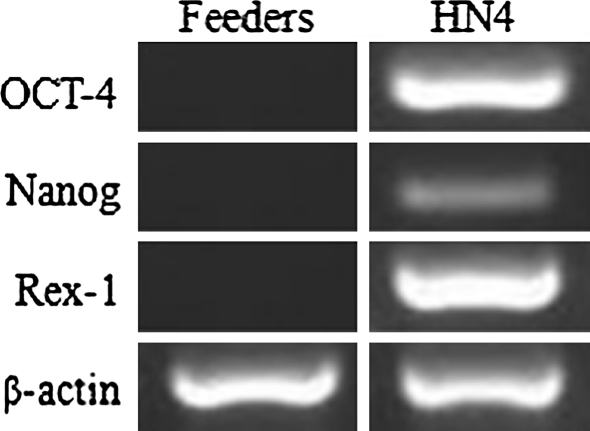
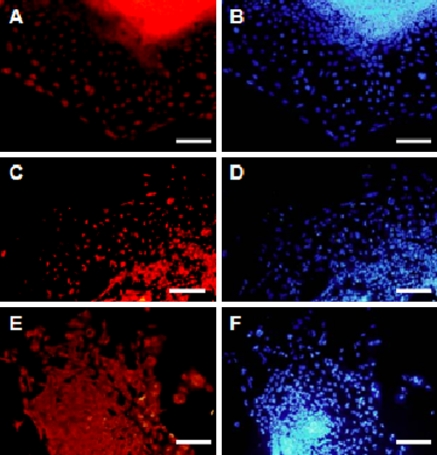
To confirm the stemness of HN4, various markers were examined by immunostaining or RT-PCR. As shown in Fig. 4, SSEA-3, SSEA-4, TRA-1-60, TRA-1-81, OCT-4, as well as high levels of alkaline phosphatase activity were expressed in HN4. Expression of transcription factors OCT-4, REX-1 and NANOG was confirmed by RT-PCR analysis. Comparing with the mixed feeder cells as negative control, HN4 expressed high levels of the three markers (Fig. 5).
Fig. 4.
Immunocytochemical analysis of HN4. Immunofluorescence staining of undifferentiated HN4 cells using SSEA-3 (red), SSEA-4 (red), Tra-1-60 (red), Tra-1-81 (red); Oct-4 (green) antibodies and AKP (Alkaline phosphatase activity)(green). In each panel nuclei are blue (DAPI). The bar of AP is 500 μm and other bars are 200 μm
Fig. 5.
RT-PCR analysis of HN4. RT-PCR analysis of undifferentiated HN4 cells using primers specific for Oct-4, Nanog, Rex-1, and β-actin. Feeder cells are used as negative control
Genetic characterization of HN4
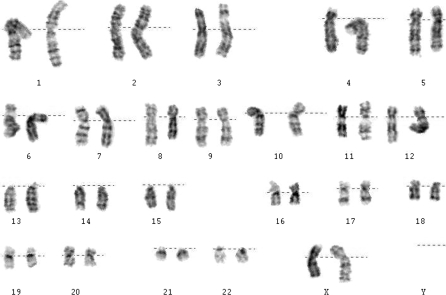
To examine the genetic stability of HN4 over long-term culture, karyotype analysis was carried out at passages 3 and 27 by G-banding technique. In both cases, cells were found to retain stable 46 X X karyotype (Fig. 6).
Fig. 6.
Karyotype of HN4. The chromosomes from HN4 are diploid (46, XX)
Differentiation potential of HN4
HESC spontaneously form three-dimensional aggregates of differentiated cells known as EBs in suspention culture. HN4 EB was replaced on gelatin-covered slides for marker gene expression analysis. BRY+ cells (Fig. 7a), Foxa2+ cells (Fig. 7c), and Nestin+ cells (Fig. 7e) indicated that HN4 could differentiate into derivatives of all three embryonic germ layers in vitro.
Fig. 7.
Pluripotent analysis of HN4 in vitro. Confirmation of pluripotency of the hESC line HN4 in vitro. a–b Bry positive cells (mesoderm), c–d Foxa2 positive cells (endoderm), e–f nestin positive cells (ectoderm). b, d, and f nuclei were stained with DAPI (blue). Scale bar = 200 μm
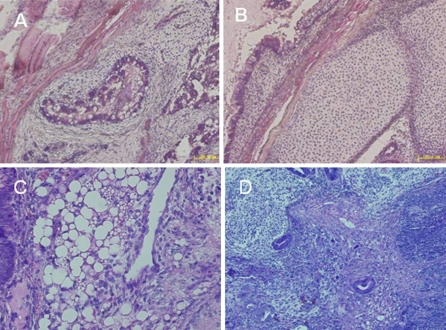
To explore the pluripotency of HN4 in vivo, cell clumps of HN4 were injected into SCID mice subcutaneously. After 10 weeks, teratoma formation was observed and examined histologically. The appearances of endodermal (gut-like epithelium, Fig. 8a), mesodermal (cartilage, Fig. 8b and fat cells Fig. 8c), and ectodermal (neuroectoderm, Fig. 8d) tissues within the teratomas demonstrated that HN4 cells exhibited the characteristic differentiation capacity of pluripotent hESC in vivo.
Fig. 8.
Pluripotent analysis of HN4 in vivo. Confirmation of pluripotency of the hESC line HN4 in vivo. Histological analysis of teratomas from HN4 at passage 20: a secretory epithelium (endoderm), b cartilage and c fat cells (mesoderm), d neuroectoderm (ectoderm). Scale bar = 100 μm
Neural differentiation of HN4
In order to explore the differentiation tendency, HN4 cells were treated for neural differentiation (Ma et al. 2008). Colonies of HN4 were detached from the feeder cells and suspended in low-attachment dishes to get spontaneously differentiating EBs.
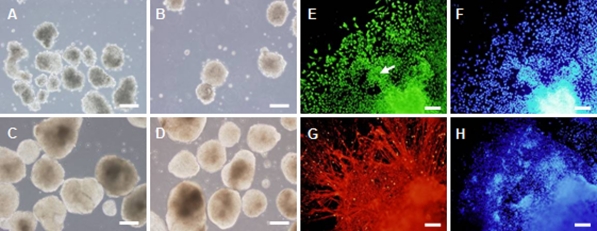
During the 15 days in suspension, the EBs got gradually larger, and the rim of the aggregates was changing from rough to smooth (Fig. 9a–d). After plating, the differentiated cells migrated radially away from the aggregates, resulting in a rim of cells around the EB sphere. Two days postplating, almost all differentiated cells were nestin+ neural progenitors. In the following days, great numbers of neurites of neurons were observed. By counting the number of cells positive for DAPI and cells positive for Nestin or β-III-tubulin, we found 99.2 ± 1.3% cells expressed nestin and 25.2 ± 5.7% cells expressed β-III-tubulin. Radially organized neuroectodermal cells in rosettes expressed nestin, and a great number of fiber-like neurite outgrowths from neurons expressed β-III-tubulin (Fig. 9).
Fig. 9.
Neual differentiation of HN4 in vitro. a–d Floating EBs of HN4 hES cells at 6, 24 h, 4 and 14 days. e–f Differentiated cells at day 2 after plating. e Nestin positive cells. Nestin stains neural tube—like structures (pointed by white arrow). g–h Differentiated cells at day 18 after plating. g β-III-tubulin positive cells. f and h Nuclei were stained with DAPI (blue). Scale bar = 200 μm
Discussion
Most embyos used to establish hES cell lines are surplus from clinical in vitro fertilization (IVF) treatment, which always have a poor morphology. The low-grade embryos with ill-defined ICM and some irregular and fragmented blastomeres may adversely affect the success rate of the hES cell line derivation. In this regard, it is essential to develop a derivation technique, which is of the slightest effect on hESCs, thus enhance the success rate of cell line establishment as well as the quality of the cell line.
Conventionally, antibodies, pronase or Tyrode’s acid are used to remove the zona pellucid when isolation of ICM. The whole-mechanical method which we used here avoids using any of them, thus avoids potential effects caused by exotic components.
We applied this whole-mechanical method to generate human embryonic stem cell lines several times. Three hESC lines were obtained from ten blastocysts, with one of which named HN4. Compared to widely used immunosurgery method, the whole-mechanical method is characterized by a high yield of hESC line generation.
HESCs are routinely maintained on feeder layers such as MEF cells (Thomson et al. 1998; Reubinoff et al. 2000), STO (SIM mouse embryo-derived thioquanine and ouabain resistant) cells (Park et al. 2003), or a variety of human fetal, neonatal, and adult cells (Richards et al. 2002; Hovatta et al. 2003; Richards et al.2003; Amit et al. 2003; Cheng et al. 2003), as well as some specific kinds of cells such as immortalized MEF cells (Choo et al. 2006) and hESC-derived fibroblasts (Stojkovic et al. 2005). In our study, the HFF and MEF mixed feeder layers were used to support growth of HN4 in vitro to passage 33, and maintain HN4 in undifferentiated state. Generally, MEFs are recommended only to be used within five generations as feeder layer due to their loosing capability in maintaining the undifferentiation state of hESCs (Qian et al. 2006). But our study revealed that the passages could be remarkably prolonged.
When MEF and HFF were mixed, even in their 17th and 18th passage, respectively, the feeder layer could still support HN4 favourably. The underlying reason might be a combination of cytokine activity and extracellular matrix. Therefore, application of the mixed feeders could greatly reduce lab work, which is worth to be more widely used. Analysis of marker expression showed that HN4 uniformly expressed the expected markers of undifferentiated hESCs, and could differentiate into derivatives of the three embryonic germ layers in vitro and in vivo. HN4 had a stable diploid karyotype (46, XX). Successful establishment of HN4 indicated that the discarded Grade III 3-day embryos from clinical IVF treatment could be a valuable source for establishment of hESC lines, using the method described above.
HESCs are special with two unique properties, which are pluripotency and self-renewal ability (Thomson et al. 1998). HESCs can be induced into neural cells including functional neurons, oligodendrocytes and glial cells (Carpenter et al. 2001; Keirstead et al. 2005; Shin et al. 2006; Hong et al. 2008). However, one main challenge in this field is to generate a renewable and homogeneous neural cell population, using as a cell source for replacement therapy or for other studies. Thus, it is critical to optimally differentiate hESC to neural cell population and generate highly pure population of specified cell types. In this current study, we could efficiently differentiate HN4 into neurons on PDL/Laminin coated plates under chemically defined conditions. High percentage of nestin positive cells indicate that homogeneous neural progenitor cells were induced and along with further induction, mature neurons spreaded with long fiber-like neurites (Ma et al. 2008).
Successfully generating neurons from HN4 indicated that HN4 could serve as a stem cell source for the treatment of neural degeneration and for cell therapy research.
Footnotes
Lan Xu contributed equally to the work.
Contributor Information
Bin Li, Email: libinliccc@163.com.
Lan Xu, Email: xl_any@163.com.
Xiao-Yan Ding, Email: xyding@sunm.shcnc.ac.cn.
Yuan-Hua Huang, Email: huangyh4180@sohu.com.
References
- Amit M, Margulets V, Segev H, Shariki K, Laevsky I, Coleman R, et al. Human feeder layers for human embryonic stem cells. Biol Reprod. 2003;68:2150–2156. doi: 10.1095/biolreprod.102.012583. [DOI] [PubMed] [Google Scholar]
- Balaban B, Urman B, Sertac A, Alatas C, Aksoy S, Mercan R. Blastocyst quality affects the success of blastocyst-stage embryo transfer. Fertil Steril. 2000;74:282–287. doi: 10.1016/S0015-0282(00)00645-2. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Entrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Rosler E, Rao MS. Characterization and differentiation of human embryonic stem cells. Cloning Stem Cells. 2003;5:79–88. doi: 10.1089/153623003321512193. [DOI] [PubMed] [Google Scholar]
- Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- Choo A, Padmanabhan J, Chin A, Fong WJ, Oh SK. Immortalized feeders for the scale-up of human embryonic stem cells in feeder and feeder-free conditions. J Biotechnol. 2006;122:130–141. doi: 10.1016/j.jbiotec.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Dvash T, Ben-Yosef D, Eiges R. Human embryonic stem cells as a powerful tool for studying human embryogenesis. Pediatr Res. 2006;60:111–117. doi: 10.1203/01.pdr.0000228349.24676.17. [DOI] [PubMed] [Google Scholar]
- Familari M, Selwood L. The potential for derivation of embryonic stem cells in vertebrates. Mol Reprod Dev. 2006;73:123–131. doi: 10.1002/mrd.20376. [DOI] [PubMed] [Google Scholar]
- Findikli N, Kahraman S, Akcin O, Sertyel S, Candan Z. Establishment and characterization of new human embryonic stem cell lines. Reprod Biomed Online. 2005;10:617–627. doi: 10.1016/S1472-6483(10)61669-0. [DOI] [PubMed] [Google Scholar]
- Heins N, LindahI A, Karlsson U, Rehnström M, Caisander G, Emanuelsson K, et al. Clonal derivation and characterization of human embryonic stem cell lines. J Biotechnol. 2006;122:511–520. doi: 10.1016/j.jbiotec.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Hong S, Kang UJ, Isacson O, Kim KS. Neural precursors derived from human embryonic stem cells mainstain long-term proliferation without losing the potential to differentiate into all three neural lineages, including dopaminergic neurons. J Neurochem. 2008;104:316–324. doi: 10.1111/j.1471-4159.2007.04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovatta O, Mikkola M, Gertow K, Strömberg AM, Inzunza J, Hreinsson J, et al. A culture system using foreskin fibroblasts as a feeder cells allow production of human embryonic stem cells. Hum Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies comprising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerou PH, Daley GQ. Therapeutic potential of embryonic stem cells. Blood Rev. 2005;19:321–331. doi: 10.1016/j.blre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Lerou PH, Yabuuchi A, Huo H, Miller JD, Boyer LF, Schlaeger TM, et al. Derivation and maintenance of human embryonic stem cells from poor-quality in vitro fertilization embryos. Nat Protoc. 2008;3:923–933. doi: 10.1038/nprot.2008.60. [DOI] [PubMed] [Google Scholar]
- Li B, Peng QP, Lu WY, Xu W, Jin YX, Huang YH. Growth state of human embryonic stem cells on mixed feeder layers with mouse embryonic fibroblasts and human foreskin fibroblasts at different ratios. CRTER. 2008;12:424–428. [Google Scholar]
- Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A, Tipnis S, Pal R, Ravindran G, Bose B, Patki A, et al. Characterization and in vitro differentiation potential of a new human embryonic stem cell line, ReliCellhES1. Differentiation. 2006;74:81–90. doi: 10.1111/j.1432-0436.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Mummery C. Stem cell research: immortality or healthy old age? Eur J Endocrinol. 2004;151(Suppl. 3):U7–U12. doi: 10.1530/eje.0.151U007. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim SJ, Oh EJ, Moon SY, Roh SI, Kim CG, et al. Establishment and maintenance of human embryonic stem cells on STO, a permanently growing cell line. Biol Reprod. 2003;69:2007–2014. doi: 10.1095/biolreprod.103.017467. [DOI] [PubMed] [Google Scholar]
- Qian K, Chen H, Zhang SM, Zhu GJ. Human fibroblast cell supporting undifferentiated human embryonic stem cells proliferation in vitro. Acta Med Univ Sci Technol Huazhong. 2006;35:462–464. [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cells masses and embryonic stem cell lines. Nat Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- Robertson EJ. Embryo-derived stem cell lines. In: Robertson EJ, editor. Teratocarcinomas and embryonic stem cells. Oxford: IRL Press; 1987. pp. 71–112. [Google Scholar]
- Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao P, et al. Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells. 2006;24:125–138. doi: 10.1634/stemcells.2004-0150. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Ivey KN. Potential of stem cell-based therapies for heart disease. Nature. 2006;441:1097–1099. doi: 10.1038/nature04961. [DOI] [PubMed] [Google Scholar]
- Stojkovic P, Lako M, Stewart R, Przyborski S, Armstrong L, Evans J, et al. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306–314. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- Ström S, Inzunza J, Grinnemo KH, Holmberg K, Matilainen E, Strömberg AM, et al. Mechanical isolation of the inner cell mass is effective in derivation of new human embryonic stem cell lines. Human Reprod. 2007;22:3051–3058. doi: 10.1093/humrep/dem335. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Urban SV, Kiss J, Vas V, Kovacs J, Uher F. Stem cell therapy for diabetes mellitus: progress, prospects and challenges. Orv Hetil. 2006;147:791–797. [PubMed] [Google Scholar]
- Stolpe A, Brink S, Rooijen M, Ward-van Oostwaard D, Inzen W, Slaper-Cortenbach I, et al. Human embryonic stem cells: towards therapies for cardiac disease. Derivation of a Dutch human embryonic stem cell line. Reprod Biomed Online. 2005;11:476–485. doi: 10.1016/S1472-6483(10)61144-3. [DOI] [PubMed] [Google Scholar]
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]