Abstract
The ubiquitous inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R) channel, localized primarily in the endoplasmic reticulum (ER) membrane, releases Ca2+ into the cytoplasm upon binding InsP3, generating and modulating intracellular Ca2+ signals that regulate numerous physiological processes. Together with the number of channels activated and the open probability of the active channels, the size of the unitary Ca2+ current (iCa) passing through an open InsP3R channel determines the amount of Ca2+ released from the ER store, and thus the amplitude and the spatial and temporal nature of Ca2+ signals generated in response to extracellular stimuli. Despite its significance, iCa for InsP3R channels in physiological ionic conditions has not been directly measured. Here, we report the first measurement of iCa through an InsP3R channel in its native membrane environment under physiological ionic conditions. Nuclear patch clamp electrophysiology with rapid perfusion solution exchanges was used to study the conductance properties of recombinant homotetrameric rat type 3 InsP3R channels. Within physiological ranges of free Ca2+ concentrations in the ER lumen ([Ca2+]ER), free cytoplasmic [Ca2+] ([Ca2+]i), and symmetric free [Mg2+] ([Mg2+]f), the iCa–[Ca2+]ER relation was linear, with no detectable dependence on [Mg2+]f. iCa was 0.15 ± 0.01 pA for a filled ER store with 500 µM [Ca2+]ER. The iCa–[Ca2+]ER relation suggests that Ca2+ released by an InsP3R channel raises [Ca2+]i near the open channel to ∼13–70 µM, depending on [Ca2+]ER. These measurements have implications for the activities of nearby InsP3-liganded InsP3R channels, and they confirm that Ca2+ released by an open InsP3R channel is sufficient to activate neighboring channels at appropriate distances away, promoting Ca2+-induced Ca2+ release.
INTRODUCTION
Modulating cytoplasmic free Ca2+ concentration ([Ca2+]i) is a ubiquitous intracellular signaling pathway that regulates numerous cellular physiological processes, including apoptosis, gene expression, bioenergetics, secretion, immune responses, fertilization, muscle contraction, synaptic transmission, and learning and memory (Clapham, 1995; Berridge et al., 2000; Bootman et al., 2001; Braet et al., 2004; Randriamampita and Trautmann, 2004; Cárdenas et al., 2010b). The inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R), a transmembrane protein localized mainly at the ER in all animal cell types, plays a central role in this [Ca2+]i signaling pathway (Taylor and Richardson, 1991; Bezprozvanny and Ehrlich, 1995; Furuichi and Mikoshiba, 1995; Patterson et al., 2004; Foskett et al., 2007; Joseph and Hajnóczky, 2007). In response to extracellular stimuli, phosphatidylinositol 4,5-bisphosphate in the plasma membrane is hydrolyzed to generate InsP3 (Berridge, 1993). InsP3 rapidly diffuses through the cytoplasm to bind to the InsP3R and activates it as an intracellular Ca2+ channel to release Ca2+ stored inside the lumen of the ER into the cytoplasm, generating diverse local and global [Ca2+]i signals (Berridge, 1997).
The InsP3R-mediated Ca2+ flux from the ER store in response to various extracellular stimuli is ∝ NA iCa Po, where NA is the number of InsP3R channels activated, iCa is the unitary calcium ion current passing through an individual open InsP3R channel, and Po is the open probability of the active InsP3R channels. The amount of Ca2+ released, and therefore the amplitude and spatial and temporal nature of the [Ca2+]i signals generated, is directly dependent on iCa (Berridge, 1997; Bootman et al., 1997). Furthermore, the Po of InsP3R channels is regulated by [Ca2+]i with a biphasic dependence: at low concentrations, Ca2+ activates the channel and increases its Po, whereas at higher concentrations, Ca2+ inhibits the channel (Foskett et al., 2007). Consequently, iCa also affects indirectly the amount of Ca2+ released by regulating the Po of the activated channel itself, as well as that of nearby surrounding channels. Therefore, the measurement of iCa in ionic conditions similar to those that exist physiologically in cells is critical for the understanding of the mechanisms regulating this important signaling pathway.
Although InsP3R channel activity level (Po) and the number of channels activated (NA) under various physiological conditions have been studied previously by electrophysiological methods, especially single-channel nuclear patch clamp experiments in various configurations (Foskett et al., 2007), the unitary Ca2+ current (iCa) passing through an open InsP3R channel has not been characterized, primarily as a consequence of technical difficulties. Here, we measured the iCa of recombinant homotetrameric rat type 3 InsP3R channels under physiological ionic conditions and studied its single-channel conductance properties.
MATERIALS AND METHODS
Nucleus isolation and nuclear patch clamp electrophysiology
The generation and maintenance of DT40-KO-r-InsP3R-3 cells (mutant cells derived from chicken B cells with the endogenous genes for all three InsP3R isoforms knocked out and then stably transfected to express recombinant rat type 3 InsP3R) were described in Mak et al. (2005). Nuclear patch clamp experiments were performed using nuclei isolated from DT40-KO-r-InsP3R-3 cells as described previously (Mak et al., 2005). Excised nuclear membrane patches in the luminal side–out (lum-out) or cytoplasmic side–out (cyto-out) configuration were obtained from isolated nuclei (Mak et al., 2007) using protocols analogous to those used to obtain inside-out or outside-out excised patches in plasma membrane patch clamp experiments. The solution around the excised nuclear membrane patch was rapidly switched multiple times using a solution-switching setup described in Mak et al. (2007).
InsP3R channel current traces were acquired at room temperature as described previously (Mak et al., 1998), digitized at 5 kHz, and anti-aliasing filtered at 1 kHz. Data analysis and the fitting of channel current–voltage curves were performed using Igor-Pro software. All electrical potentials were measured relative to the bath electrode.
Experimental solution composition
In cyto-out experiments, pipette solutions contained 140 mM KCl, 10 mM HEPES, pH to 7.3 with KOH, 0.5 mM Na2ATP, and 10 µM InsP3, with free [Ca2+] ([Ca2+]f) buffered to 3 µM by 0.5 mM 5,5′-dibromo 1,2-bis(o-aminophenoxy) ethane-N,N,N’,N’-tetraacetic acid (dibromo BAPTA) and 0.2 mM CaCl2. Perfusion solutions on the cytoplasmic side of the channel contained 10 mM HEPES, pH to 7.3 with KOH, 0.5 mM Na2ATP, and either 140 mM KCl with no InsP3 or 70 mM KCl with 10 µM InsP3, with [Ca2+]f buffered to 3 µM by 0.5 mM (2-hydroxyethyl) ethylenediaminetriacetate and 0.22 mM CaCl2 (Mak et al., 2005). InsP3 and Na2ATP were included in the pipette solution to confirm that the cyto-out configuration was properly achieved. Because of the presence of InsP3 and ATP in the pipette solution, observation of InsP3R channel activity before solution switching would be evidence that the lum-out configuration was erroneously obtained.
The same pipette solution was used in lum-out experiments to determine ion permeability ratios of the InsP3R channel. The perfusion solution used to determine PK: PCl contained 30 mM KCl, 110 mM NMDG chloride, and 10 mM HEPES, pH to 7.3 with NMDG, with [Ca2+]f buffered to 300 nM by 0.5 mM 1,2-bis(o-aminophenoxy) ethane-N,N,N’,N’-tetraacetic acid (BAPTA) and 0.2 mM CaCl2. The perfusion solution used to determine PK: PNa contained 140 mM NaCl and 10 mM HEPES, pH to 7.3 with NaOH. The perfusion solution used to determine PX: PK (X = Mg2+, Ca2+, Sr2+, or Ba2+) contained 140 mM KCl, 10 mM HEPES, pH to 7.3 with KOH, and 10 mM XCl2. The perfusion solutions contained no Na2ATP or Ca2+ chelator. No CaCl2 was added to the perfusion solutions used to determine permeability ratios of Mg2+, Sr2+, and Ba2+ versus K+, so free Ca2+ in those solutions were from contaminants in the water and salts used to make the solutions. Based on the purity assays of the salts (Sigma-Aldrich) and induction-coupled plasma mass spectrometry assay (Mayo Medical Laboratory) of deionized water samples, [Ca2+]f in those solutions were ∼5–8 µM.
In lum-out experiments to determine the iCa through the InsP3R-3 channel, all solutions contained 10 mM HEPES, pH to 7.3 with KOH. Unless stated otherwise, all solutions used in the same experiment contained the same concentrations of MgCl2 (0, 0.5, or 1 mM), KCl (140 or 40 mM), and potassium methanesulfonate (KCH3SO3; 0 or 100 mM). Pipette solutions also contained 0.5 mM Na2ATP and 2 or 10 µM InsP3 to activate the channel to Po of ∼0.5 (Mak et al., 2001a,b; Vais et al., 2010), with [Ca2+]f buffered to either 70 nM by 0.5 mM BAPTA and 0.06 mM CaCl2, or to 3 µM by 0.5 mM dibromo BAPTA and 0.2 mM CaCl2. The same solution (without Na2ATP or InsP3) was used as perfusion solution for symmetric ionic conditions. Although [Na+] was not symmetric because of the presence of 0.5 mM Na2ATP only in the pipette solutions, the extra Na+ is <1% of the amount of monovalent cations, and its contribution to channel current is negligible. Perfusion solutions used for asymmetric ionic conditions contained 0.3, 1, or 2 mM CaCl2, with no Ca2+ chelator, Na2ATP, or InsP3.
[Ca2+]f of <100 µM (buffered by various Ca2+ chelators) was confirmed by fluorimetry. [Ca2+]f in solutions without Ca2+ chelators was calculated using activity coefficients (see next section).
Evaluating ion permeability ratios
According to the general Goldman-Hodgkin-Katz current equation (Lewis, 1979), the permeability ratio PY/PK for Y, one of the charge-carrying permeant ion species, can be evaluated from the reversal potential (Vrev) of the channel as:
| (1) |
where X represents other permeant ion species present; PX is the permeability of X through the channel; zX and zY are the valence of X and Y, respectively; [X]i, [X]o, [Y]i, and [Y]o are the activities of X and Y in the pipette and bath solutions, respectively; F and R are the Faraday and gas constants, respectively; and T is the absolute temperature, provided that all of the PX/PK values are known. The activities of all ion species were calculated from activity coefficients, except for [Ca2+] < 100 µM, which was determined by fluorimetry.
The activity coefficients of KCl and NaCl were calculated using the Debye-Hückel equation based on data reported by Hamer and Wu (1972). The activity coefficients of CaCl2 in a 140-mM KCl solution (0.546–0.534 for 0.3–10 mM CaCl2) were derived by interpolation using data reported by Butler (1968), assuming that CaCl2 activity coefficients are similar in 140-mM KCl and NaCl solutions. The activity coefficients of CaCl2 were used for other alkaline earth metal halides (MgCl2, BaCl2, and SrCl2) in solutions with the same concentrations because activity coefficients of the alkaline earth metal halides differ by <5% in concentrations when activity coefficients are ≈0.54 (Goldberg and Nuttall, 1978).
RESULTS
Conductance properties of homotetrameric recombinant rat InsP3R-3 channels
Although it has been a well-established approach to study the single-channel properties of the InsP3R channel in its native membrane environment by performing patch clamp electrophysiology on isolated nuclei (Foskett et al., 2007), the molecular composition of the channels observed in many of those studies could not be definitively ascertained. This is because the channels studied were either the endogenous channels (Mak and Foskett, 1994, 1997; Marchenko et al., 2005; Ionescu et al., 2006) with the possibility of different channel isoforms expressed and with possible alternative splicing (Foskett et al., 2007), or recombinant channels expressed in cells with a nonzero level of endogenous InsP3R expression (Mak et al., 2000; Boehning et al., 2001a), so that heteroligomeric channels of recombinant and endogenous InsP3Rs could have been formed (Joseph et al., 1995; Mak et al., 2000).
To avoid possible variability that such heterogeneity might introduce, we ensured that only homotetrameric InsP3R channels with known identical amino acid sequences were studied by using a stably transfected cell line (DT40-KO-r-InsP3R-3) derived from mutant DT40-InsP3R-KO cells (Sugawara et al., 1997) that have all endogenous genes for the three InsP3R isoforms knocked out, with only recombinant rat type 3 InsP3R (InsP3R-3) expressed (Mak et al., 2005; Li et al., 2007). During our extensive experience working with this cell line (Mak et al., 2005; Foskett and Mak, 2010), and in hundreds of nuclear membrane patches (in both cyto-out and lum-out configurations), only one kind of channel with a conductance >100 pS in symmetric 140 mM KCl was detected. The identity of these channels as recombinant rat InsP3R-3 channels was confirmed by their sensitivity to activation by cytoplasmic InsP3 (Fig. 1 A).
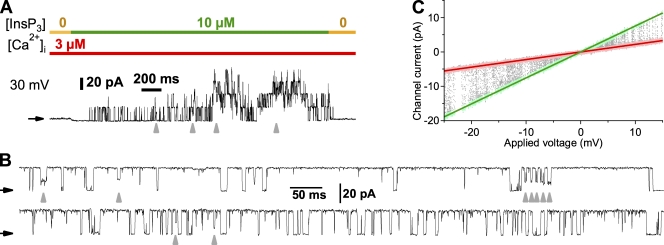
Figure 1.
Current records of homotetrameric recombinant type 3 InsP3R channels in membrane patches excised from nuclei isolated from DT40-KO-r-InsP3R-3 cells. (A) Activation by InsP3 of channels in a cyto-out nuclear membrane patch. Concentrations of InsP3 and free Ca2+ in the perfusion solution on the cytoplasmic side of the channel are indicated by color bars at top. Vapp = 30 mV. Channels opened only in the presence of cytoplasmic InsP3. Gray arrowheads indicate some of the occasions when one of the active channels entered a conductance substate. In this and subsequent current traces, black arrows indicate the membrane current levels when all InsP3R channels in the membrane patch were closed. (B) Single InsP3R-3 channel current records observed in different lum-out nuclear patches in symmetric 140 mM KCl and 3 µM [Ca2+]f. Pipette solution contained 10 µM InsP3. Vapp = 40 mV. Gray arrowheads indicate when the channels entered different conductance substates with different conductance values. (C) Representative I-Vapp plot for a single InsP3R-3 channel in the same experimental conditions as in B. Vapp was ramped from −25 to 15 mV in 1 s. 20 ramps were analyzed. Data points selected for Iopen and Iclosed are plotted in green and pink, respectively. Green and red lines represent linear fits to Iopen and Iclosed data points, respectively.
Averaged over reasonable intervals (>1 s), the rat InsP3R-3 channel dwells in a main open conductance state >95% of the time it is open, whereas it occasionally exhibits brief substates of lower conductances (Fig. 1, A and B). The substates are not a result of non-InsP3R channels because in current records showing only one active InsP3R channel gating, the channel current level dropped to substate levels from the main open state directly without channel closing (Fig. 1 B, arrowheads). Substates have also been observed for other InsP3R channels (endogenous or recombinant) in other cell systems (Watras et al., 1991; Mak and Foskett, 1997; Mak et al., 2000; Boehning et al., 2001a; Ionescu et al., 2006). To determine the conductance of the main open state of the channel in symmetric 140-mM KCl solutions ([K+]f = [Cl−]f ≈ 104 mM) in the absence of Mg2+, currents through excised lum-out membrane patches (I) were recorded as the applied potential (Vapp) was ramped (Fig. 1 C). Slope conductance of the channel (gch), evaluated as the difference between the slopes of the fits to open- and closed-channel current (Iopen and Iclosed, respectively) data, was 545 ± 7 pS (n = 16). This and all subsequent open-channel current measurements were not affected by the presence of substates because atypical current data arising from them were excluded when data points were selected for I-Vapp fits.
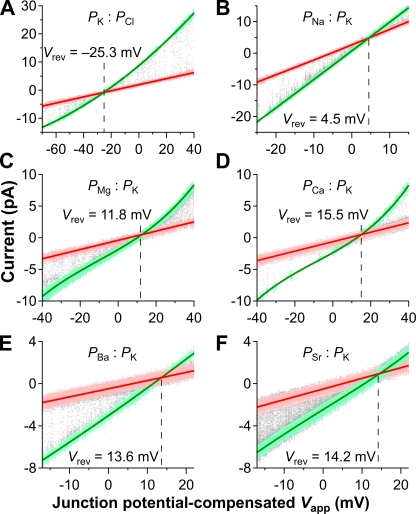
To properly design an experimental approach to measure the iCa passing through a single open InsP3R-3 channel under physiological ionic conditions, it was useful to obtain a maximum estimate of the size of the current using the Goldman-Hodgkin-Katz current equation (Hille, 2001). To obtain such an estimate requires knowledge of the permeability values of all permeant ionic species present. To evaluate the permeabilities of various ions through the InsP3R-3 channel, I-Vapp data were recorded as Vapp was ramped during a series of lum-out patch clamp experiments in which the excised membrane patches were exposed to asymmetric ionic conditions (Fig. 2). After correcting for liquid junction potentials (Neher, 1995), the reversal potential (Vrev) of the channel was determined as the Vapp at the intersection of the Iopen-Vapp and Iclosed-Vapp fits. Permeability ratios for various ions were calculated from Vrev using the general Goldman-Hodgkin-Katz current equation (Eq. 1). The permeability ratio for K+ versus Cl− (PK:PCl) was derived using asymmetric ionic solutions containing only two permeant ionic species: K+ and Cl− (Fig. 2 A). That value was then used to calculate the permeability ratios of other cations (Mg2+, Ca2+, as well as Ba2+, Sr2+, and Na+) versus K+ from Vrev measured in asymmetric ionic solutions containing three permeant ionic species (Fig. 2, B–F), assuming that PK:PCl is the same in all ionic conditions used. These measurements indicated that PCa:PSr:PBa:PMg:PNa:PK:PCl = (15.2 ± 0.6):(13.2 ± 0.7):(11.8 ± 0.5):(10.2 ± 0.3):(1.24 ± 0.003):1:(0.27 ± 0.01) (n = 3 for each ratio). This same InsP3R permeability ratio sequence was also observed in other nuclear patch clamp experiments (Mak and Foskett, 1998; Mak et al., 2000; Boehning et al., 2001a; Ionescu et al., 2006).
Figure 2.
Representative I-Vapp plots for single InsP3R-3 channels in excised lum-out nuclear membrane patches under asymmetric ionic conditions used to determine channel permeability ratios for various ions. Compositions of pipette and perfusion solutions are described in Materials and methods. Selected data points for Iopen and Iclosed are plotted in green and pink, respectively. Red lines are linear fits to Iclosed-Vapp data points, and green ones are polynomial fits to Iopen-Vapp data points (linear for B; quadratic for A, E, and F; cubic for C and D). Vrev at the intersection of the Iopen-Vapp and Iclosed-Vapp fits are marked by dashed lines and tabulated. (A) Eight Vapp ramps in one of four experiments to determine PK:PCl. Junction potential corrected (Vjunction) = −9.3 ± 0.4 mV. (B) 15 Vapp ramps in one of three experiments to determine PNa:PK. Vjunction = −1.5 ± 0.2 mV. (C) 10 Vapp ramps in one of three experiments to determine PMg:PK. Vjunction = −0.7 ± 0.4 mV. (D) 43 Vapp ramps in one of three experiments to determine PCa:PK. Vjunction = −1.4 ± 0.2 mV. (E) 35 Vapp ramps in one of three experiments to determine PBa:PK. Vjunction = −2.6 ± 0.3 mV. (F) 16 Vapp ramps in one of three experiments to determine PSr:PK. Vjunction = −2.2 ± 0.8 mV.
iCa through InsP3R-3 under physiological conditions
Although the InsP3R channel has a large gch in solutions with physiological KCl concentrations, the current measured under those conditions is mostly carried by K+ ions driven through the channel by Vapp. Because of the absence of a significant voltage across the ER membrane (Beeler et al., 1981; Marhl et al., 1997), Ca2+ ions are driven through open InsP3R channels under physiological conditions by the difference between [Ca2+]f in the ER lumen ([Ca2+]ER) and that in the cytoplasm ([Ca2+]i). [Ca2+]ER observed in various cell types is approximately hundreds of micromolars (Bygrave and Benedetti, 1996; Yu and Hinkle, 2000; Palmer et al., 2004), so there are significantly fewer Ca2+ ions than K+ ions to carry the current. A simple calculation based on the Goldman-Hodgkin-Katz current equation suggests that, in the absence of transmembrane voltage, with [Ca2+]ER = 1 mM and [Ca2+]i = 70 nM, iCa is ∼3 pA for a channel with gch = 545 pS in 140 mM KCl and PCa:PK:PCl = 15:1:0.27. However, the actual iCa is expected to be substantially smaller because divalent cations act as permeant blockers that reduce gch (Mak and Foskett, 1998; Mak et al., 2000). Despite their higher permeabilities, divalent cations bind strongly to site(s) in the channel pore so that they pass through the channel significantly more slowly than monovalent cations. In addition, the high [K+] in the cytoplasm and ER lumen (∼140 mM) and the relatively weak selectivity of the InsP3R channel for Ca2+ over K+ (PCa:PK = 15) make K+ a potentially effective competing ion to also reduce iCa. iCa might be further reduced as a result of competition from free Mg2+, which exists at significant levels (∼200 µM–1.1 mM) in both the cytoplasm and ER lumen (Halvorson et al., 1992; Morelle et al., 1994a,b; Silverman et al., 1994; Golding and Golding, 1995; Singh and Wisdom, 1995; Tashiro and Konishi, 1997), and has a permeability similar to that of Ca2+ (PCa:PMg = 1.5).
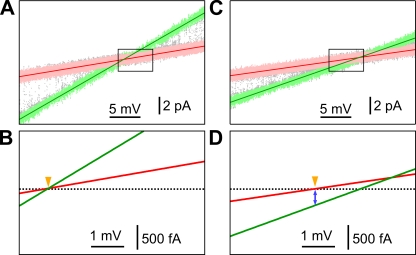
To measure iCa, we monitored the transmembrane current I through excised lum-out nuclear membrane patches containing a single active InsP3R channel. The capability to switch the perfusion solutions around the excised patch was used to minimize systematic measurement errors by calibrating the patch clamp setup for each individual experiment. The lum-out membrane patch was first exposed to a perfusion solution with the same ionic composition as the pipette solution (symmetric ionic conditions). I-Vapp data were recorded with Vapp ramped from −20 to 10 mV, and data points for Iopen-Vapp and Iclosed-Vapp were fitted linearly (Fig. 3 A). With no permeant ion concentration gradient across the excised nuclear membrane patch, the current passing through the InsP3R channel (Iopen–Iclosed) and the leak current across the nuclear membrane patch (Iclosed) must both be zero when Vapp = 0. Thus, the intersection of the Iopen-Vapp and Iclosed-Vapp lines established the zero-current level for the patch clamp current recording system during each experiment (Fig. 3 B).
Figure 3.
Measuring iCa in physiological ionic conditions. (A) Linear fits (green and red lines) to selected Iopen and Iclosed data (plotted in green and pink, respectively) from 25 Vapp ramps recorded from an excised lum-out nuclear membrane patch containing one active InsP3R channel under symmetric ionic conditions, with pipette and perfusion solutions containing 140 mM KCl, 0.5 mM MgCl2, and 3 µM [Ca2+]f. Pipette solution contained 2 µM InsP3. (B) Graph of the fitted Iopen-Vapp and Iclosed-Vapp lines in the I-Vapp region marked by the black rectangle in A. Zero-current level (black dotted line) established at the intersection of the I-Vapp fits at Vapp = 0 (marked by orange arrowhead). (C) Linear fits to Iopen and Iclosed data (same convention as in A) from 25 Vapp ramps recorded for the same membrane patch under asymmetric ionic conditions, with perfusion solution containing 2 mM CaCl2. I and Vapp ranges in A and C are the same. (D) Graph of the fitted I-Vapp lines in the same I-Vapp region with the same zero-current level as in B. Vapp = 0 (marked by orange arrowhead) at the intersection of the Iclosed-Vapp line and zero-current level. iCa is Iopen at Vapp = 0 (marked by blue arrow).
The perfusion solution was then switched to one with higher [Ca2+]f (asymmetric ionic conditions). Higher [Ca2+]ER reduced both the InsP3R gch, as a result of an increase in permeant divalent cation block, and the leak conductance (Fig. 3 C). However, the zero-current level of the recording system was not affected by the perfusion solution switch. For the leak current (Iclosed) through the excised membrane patch, Vrev = 0 because there are no significant concentration differences for the major ionic components in the pipette and perfusion solutions, K+ and Cl−, which have significantly higher mobility across the membrane than divalent cations (Beeler et al., 1981; Marhl et al., 1997). Therefore, Vapp = 0 at the point where Iclosed = 0 (Fig. 3 D). The open-channel current (Iopen) at Vapp = 0 is driven only by the [Ca2+]f gradient across the channel (Δ[Ca2+] = [Ca2+]ER−[Ca2+i]) and therefore is iCa (Fig. 3 D).
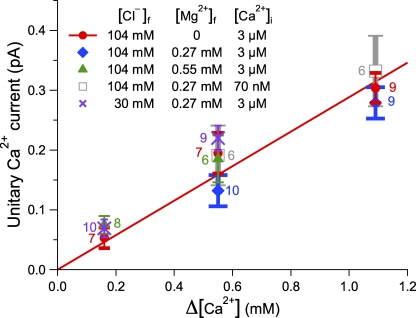
We first measured iCa in symmetric 140 mM KCl with no Mg2+. To cover the range of reported [Ca2+]ER (Bygrave and Benedetti, 1996; Yu and Hinkle, 2000; Palmer et al., 2004), the luminal side of the excised membrane patch was exposed to perfusion solutions with [Ca2+]f = 160 µM, 550 µM, and 1.1 mM. [Ca2+]i in the pipette solution was fixed at 3 µM to keep channel Po high. The observed magnitude of iCa was well described by a linear relation with Δ[Ca2+] (Fig. 4), as predicted by the Goldman-Hodgkin-Katz current equation. However, the value for PCa (1.5 × 10−18 m3s−1) derived from the slope of the iCa versus Δ[Ca2+] line is an order of magnitude smaller than that (1.5 × 10−17 m3s−1) estimated using gch in 140 mM KCl and the measured permeability ratios of PCa:PK:PCl. This discrepancy results from interactions between the channel and the permeant ions (K+ and Ca2+) and among permeant ions in the channel that are not considered in the Goldman-Hodgkin-Katz equation.
Figure 4.
iCa’s through the InsP3R channel under various asymmetric ionic conditions. Error bars show SEM. The number of measurements performed for each ionic condition is tabulated next to the corresponding data point. The line is the linear fit to the data points in 140 mM KCl, 0 mM [Mg2+]f, and 3 µM [Ca2+]i, with slope of 0.30 ± 0.02 pA/mM.
To assess the effect on iCa of physiological concentrations of free Mg2+, ranging from 200 µM to 1.1 mM in both the cytoplasm and ER lumen (Halvorson et al., 1992; Morelle et al., 1994a,b; Silverman et al., 1994; Golding and Golding, 1995; Singh and Wisdom, 1995; Tashiro and Konishi, 1997), we measured iCa in symmetric 270 or 550 µM [Mg2+]f with [Ca2+]i = 3 µM and [Ca2+]ER = 550 µM or 1.1 mM. Interestingly, iCa observed in the presence of physiological [Mg2+]f was not significantly different from iCa measured in 0 [Mg2+]f (Fig. 4).
Under normal physiological conditions, [Ca2+]ER is significantly higher than [Ca2+]i, so iCa should have little dependence on [Ca2+]i. Indeed, we observed no statistical difference between iCa for [Ca2+]i at resting (70 nM) and activating (3 µM) levels (Fig. 4).
Free chloride ion concentration [Cl−]f is significantly lower than [K+]f in the cytoplasm as a result of the Gibbs-Donnan effect of negative charges in cytoplasmic proteins (Foskett, 1990). To verify if physiological cytoplasmic [Cl−]f significantly affects iCa, we measured iCa with pipette and perfusion solutions containing symmetric 100 mM potassium methanesulfonate (KCH3SO3), 40 mM KCl ([K+]f ≈ 104 mM, [Cl−]f ≈ 30 mM), and 270 µM [Mg2+]f, with [Ca2+]i = 3 µM and [Ca2+]ER = 550 µM or 1.1 mM. Again, iCa observed was not significantly different from that observed in 140 mM KCl (Fig. 4), indicating that iCa is to a large extent independent of [Cl−]f under physiological ionic conditions. Interestingly, gch = 293 ± 4 pS in symmetric 270 µM [Mg2+]f and 30 mM [Cl−]f, the same as that observed in symmetric 270 µM [Mg2+]f and 104 mM [Cl−]f (292 ± 5 pS). This correspondence probably arises because decreasing [Cl−]f reduces Cl− current through the channel and also reduces Cl− competition with K+ to move through the channel, with the two opposing effects on InsP3R gch cancelling each other out.
These results indicate that, in physiological ionic conditions, with symmetric 104 mM [K+]f, 30–104 mM [Cl−]f, 0–550 µM [Mg2+]f, 70 nM to 3 µM [Ca2+]i, the iCa for rat homotetrameric type 3 InsP3R channel in native ER membrane is (0.30 ± 0.02 pA mM−1) × [Ca2+]ER. This is equivalent to having an asymptotic Ca2+ slope conductance (gch as Vapp in the ER relative to the cytoplasm→∞) of (23.4 ± 1.6 pS mM−1) × [Ca2+]ER. For a filled store with 500 µM [Ca2+]ER, iCa = 0.15 ± 0.01 pA and the asymptotic Ca2+ slope conductance is 11.7 ± 0.8 pS.
Block of InsP3R channel conduction by permeant divalent cations
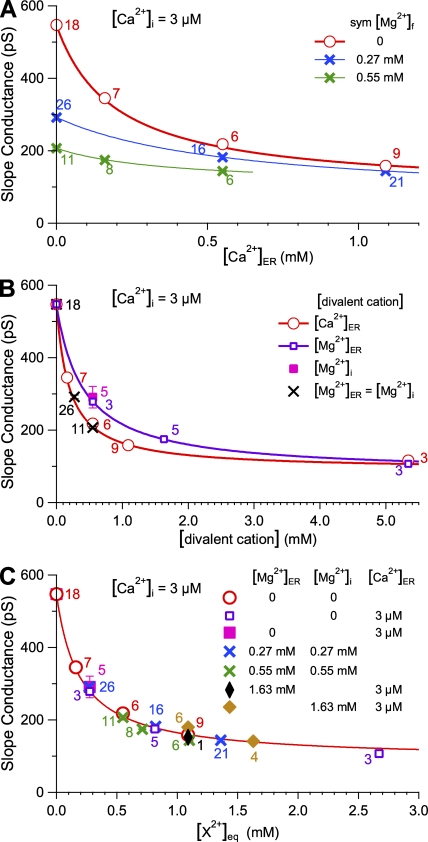
Another indication of the inadequacy of the Goldman-Hodgkin-Katz equation to describe ionic flow through the InsP3R channel is the substantial reduction of gch by Ca2+ or Mg2+ (in millimolar concentrations) on the luminal or cytoplasmic side of the channel, despite their high permeability ratios (Fig. 5A). Similar partial reduction of gch by permeant divalent cations (Mg2+, Ca2+, and Ba2+) was observed in endogenous type 1 InsP3R channels in Xenopus laevis oocyte nuclear membrane (Mak and Foskett, 1998) and recombinant type 1 InsP3R channels in the plasma membrane of DT40-KO-r-InsP3R-1 cells (Dellis et al., 2006). This reduction suggests that movement of divalent cations through the InsP3R channel is substantially slowed down by strong interactions between the ions and the pore, causing temporary block of ion flow through the channel (Hille, 2001). The channel conductance gch at the reversal potential Vrev was systematically evaluated in experiments performed to measure iCa in the presence of 3 µM, 160 µM, 550 µM, and 1.1 mM [Ca2+]ER, and symmetric 0, 270 µM and 550 µM [Mg2+]f. Channel blocking effects of Ca2+ and Mg2+ were not mutually exclusive, as the addition of Mg2+ further reduced gch already suppressed by Ca2+, and vice versa (Fig. 5 A). The observed reduction of gch by Ca2+ could be described by an empirical saturating partial inhibition equation:
| (2) |
where g0 is the channel slope conductance at 0 [Ca2+]ER, g∞CaER is the channel slope conductance at saturating [Ca2+]ER, and KCaER is the half-maximal blocking [Ca2+]ER. g0, g∞CaER, and KCaER have different values in different [Mg2+]f (corresponding to the different curves in Fig. 5 A). In the absence of Mg2+ (Fig. 5 A, red curve), KCaER = 210 ± 20 µM, g∞CaER = 82 ± 8 pS, and g0 is 545 pS, the channel slope conductance with no divalent cations.
Figure 5.
Slope conductance of an InsP3R-3 channel at Vrev in symmetric 140-mM KCl solutions with various divalent cation concentrations. SEM for all data points are shown as error bars, some of which are smaller than the size of the symbols. The number of measurements performed for each ionic condition is tabulated next to the corresponding data point in the same color. The same symbols in the same color are used for the same set of data points plotted in the different graphs. (A) gch versus [Ca2+]ER in the presence of various symmetric [Mg2+]f as tabulated. Curves are fits to gch data by the empirical inhibition equation (Eq. 2), with different KCaER for various [Mg2+]f. The red curve is for gch in 0 [Mg2+]f. (B) gch versus concentrations of divalent cations in the cytoplasmic or luminal side of the channel as tabulated. The purple curve is described by Eq. 3, and the red one is described by Eq. 2. (C) gch versus equivalent divalent cation concentrations [X2+]eq = [Ca2+]ER + 0.5 × ([Mg2+]i + [Mg2+]ER) for various combinations of [Ca2+]ER, [Mg2+]ER, and [Mg2+]i as tabulated. The red curve is described by Eq. 5.
To compare the capacities of Mg2+ and Ca2+ to block the channel, we measured gch with various [Mg2+]ER, in 0 [Mg2+]i and symmetric 3 µM [Ca2+]f (Fig. 5 B, purple open squares). gch data could be fitted using an equation similar to Eq. 2:
| (3) |
Eq. 3 describes the purple curve in Fig. 5, with g0 again being the channel conductance in the absence of divalent cations (545 pS). Interestingly, g∞MgER = 81 ± 5 pS is very similar to g∞CaER, suggesting that Ca2+ and Mg2+ are equally efficacious in blocking the InsP3R channel. Furthermore, KMgER = 410 ± 20 µM, which is ≈ 2 × KCaER, suggesting that the affinity for Ca2+ of the site in the channel responsible for blockage by luminal divalent cations is twice that for Mg2+; i.e., Mg2+ is half as potent in blocking the InsP3R channel as Ca2+.
Although inhibition of InsP3R gating by high [Ca2+]i (>20 µM) (Mak et al., 2001b) prevents comparison of the effectiveness of Ca2+ from the cytoplasmic and luminal sides to block permeation, the effectiveness of Mg2+ to block permeation from either side of the channel can be compared because physiological [Mg2+]i has no significant effect on channel gating (Mak et al., 1999). gch observed in 0.55 mM [Mg2+]i and 0 [Mg2+]ER (Fig. 5 B, magenta filled square) was the same as that in 0 [Mg2+]i and 0.55 mM [Mg2+]ER. This indicates that Mg2+ blocks the InsP3R channel with equal potency from either side. Thus, the site responsible for permeant divalent cation block is probably located inside the pore along the ion permeation pathway, with cations from either side of the channel having similar access to the site.
The data for gch in symmetric [Mg2+]f (Fig. 5 B, black crosses) fall on the curve described by Eq. 2 (Fig. 5 B, red curve), so that
Using g∞CaER = g∞MgER = g∞, and KCaER = 0.5 KMgER,
| (4) |
suggesting that contributions to channel block by Mg2+ on either side can be combined by simply summing the [Mg2+]f on the two sides as if all the Mg2+ was on one side. This relation can even be extended to include [Ca2+]ER. gch data observed in various combinations of [Ca2+]ER, [Mg2+]i, and [Mg2+]ER (whether [Mg2+]i = [Mg2+]ER or not), when plotted against the equivalent divalent ion concentrations [X2+]eq defined as {[Ca2+]ER +0.5×([Mg2+]ER + [Mg2+]i)}, are all well fitted by a curve similar to that described by Eq. 2 (Fig. 5 C, red curve); i.e.,
| (5) |
This result strongly indicates that channel block by different permeant divalent cations is caused by binding to a unique, saturable site in the ion permeation pathway that is equally accessible from either side of the channel, with an affinity for Ca2+ twice that for Mg2+. This site is probably located at or near the selectivity filter of the channel where the channel pore size is most restricted.
DISCUSSION
Magnitude of iCa through the InsP3R channel in physiological ionic conditions
In this study, the conduction properties of a homotetrameric recombinant rat InsP3R-3 channel were examined under various ionic conditions, especially under physiological monovalent and divalent ion concentrations. Under physiological ranges of [K+]f, [Cl−]f, [Mg2+]f, [Ca2+]i, and [Ca2+]ER, iCa through an open InsP3R channel depends only on [Ca2+]ER with a linear relation. iCa = 0.15 ± 0.01 pA for a filled ER store with 500 µM [Ca2+]ER. This value is compatible with the magnitude of Ca2+ flux estimated by the imaging of Ca2+ release events (puffs) of 0.4–2.5 pA (Sun et al., 1998) and 0.12–0.95 pA (Bruno et al., 2010) in Xenopus oocytes, where multiple active InsP3R channels were involved in generating a puff. The value is also in reasonable agreement with the values for iCa estimated from Ca2+ release events (blips and puffs): ∼0.4 pA (Shuai et al., 2006) and ∼0.1 pA (Bruno et al., 2010) for endogenous InsP3R-1 in Xenopus oocytes, and ∼0.05 pA iCa estimated for endogenous InsP3R-1 channels in human neuroblastoma SH-SY5Y cells (Smith and Parker, 2009). Accordingly, our experimental approach, with the advantages of observing Ca2+ currents with single-channel resolution and rigorous control of ionic conditions on both sides of the channel, appears to accurately reflect the physiological behavior of InsP3R channels in intact cells. Furthermore, our measured value of iCa is comparable to the iCa of 0.1 pA (Swillens et al., 1999), 0.07 pA (Thul and Falcke, 2004), and 0.2 pA (Shuai et al., 2008), assumed in various efforts to numerically simulate Ca2+ release through InsP3R channels, thus providing experimental support for the validity of those modeling efforts.
iCa for InsP3R measured here is comparable to but smaller than those determined for RYR channels, the other major family of intracellular Ca2+ release channels. iCa driven by 500 µM [Ca2+]ER through purified amphibian type 1 RYR (RYR1) and mammalian type 2 RYR (RYR2) channels reconstituted in artificial lipid bilayers was estimated to be 0.26 and 0.27 pA, respectively, in the presence of symmetric 150 mM KCl and 1 mM MgCl2 (Kettlun et al., 2003). Under similar ionic conditions (140 mM KCl and 1 mM MgCl2), iCa through an open rat InsP3R-3 channel is 0.15 pA. However, as discussed in more detail below, if the lipid environment impinges on the conductance properties of the release channels, a comparison of the iCa may not yet be possible because iCa measurements for the two channel types were obtained in different membrane environments.
InsP3R channel conductance
gch of various InsP3R channel isoforms in symmetric 140 mM KCl has been observed, mainly by nuclear patch clamp experiments (Mak and Foskett, 1998; Mak et al., 2000; Boehning et al., 2001a; Ionescu et al., 2006; Betzenhauser et al., 2009), to be comparable to but smaller than those for RYR channels reconstituted into lipid bilayers under the same KCl concentration: ≈750 pS for RYR1 (Wang et al., 2005) and 740 pS for RYR2 (Lindsay et al., 1991). Comparisons of primary sequences of different InsP3R isoforms from various species with those of RYR isoforms reveal that a conserved sequence GGGXGDX (amino acid residues 2545–2551 in rat InsP3R-1 SI+ SII+ isoform [Mignery et al., 1990] and 2472–2478 in rat InsP3R-3 [Blondel et al., 1993]; X stands for I or V) between putative transmembrane helices 5 and 6 in the InsP3R pore-forming domain is highly homologous to the sequence GGGIGDE (amino acid residues 4895–4901 in human RYR1 [Fujii et al., 1991] and 4824–4830 in human RYR2 [Zorzato et al., 1990]), which is conserved in all three RYR isoforms. Site-directed mutagenesis in the homologous RYR sequence alters the conductance properties of RYR channels (Zhao et al., 1999; Gao et al., 2000; Du et al., 2001; Chen et al., 2002; Wang et al., 2005), suggesting that those amino acids lie in or near the selectivity filter that determines, at least in part, the conductance of the RYR channel. Point mutations in the corresponding sequence in the rat InsP3R-1 also altered its conductance properties (Boehning et al., 2001b). A mutation changing the GGGVGDV sequence to GGGIGDV made it more similar to that of the RYR and increased InsP3R channel conductance (Boehning et al., 2001b). Conversely, GGGIGDE to GGGVGDE (Gao et al., 2000) and GGGIGDE to GGGIGDQ (Wang et al., 2005) substitutions in the RYR sequence generated channels with reduced conductance. Furthermore, invertebrate InsP3R isoforms have a GGGIGDI sequence (Yoshikawa et al., 1992; Baylis et al., 1999; Iwasaki et al., 2002) that resembles the RYR sequence and have a higher single-channel conductance (477 ± 3 pS; Ionescu et al., 2006) than the vertebrate isoforms (360 – 390 pS; Mak and Foskett, 1998; Mak et al., 2000; Boehning et al., 2001a; Betzenhauser et al., 2009), which have a GGGVGDX sequence. Collectively, these observations strongly suggest that the GGGXGDX sequence in InsP3R is close to or forms part of the selectivity filter in the tetrameric channel, and is therefore a major factor that determines the conductance properties of InsP3R channels. However, mutagenesis suggests that amino acids outside the GGGXGDX sequence also play a role in determining the conductance of the InsP3R (unpublished data) and RYR (Gao et al., 2000; Du et al., 2001; Xu et al., 2006) channels, possibly through electrostatic effects to concentrate ions in the channel.
Other factors also appear to contribute to the channel conductance properties. Different single-channel conductances have been observed for the same recombinant InsP3R-1 isoform expressed in DT40-InsP3R-KO cells, depending on whether it was localized in the outer nuclear membrane (373 ± 2 pS; Betzenhauser et al., 2009) or the plasma membrane (214 ± 17 pS; Dellis et al., 2006). Moreover, the conductance of the homotetrameric recombinant InsP3R-3 channel expressed in the outer nuclear envelope of DT40-KO-r-InsP3R-3 cells measured here (545 ± 7 pS) is nearly 50% larger than that observed for the same recombinant InsP3R-3 channel expressed in the outer nuclear envelope of Xenopus oocytes (370 ± 8 pS; Mak et al., 2000). Conductance values (∼125 and 200 pS) smaller than the one we observed here were reported for the same channel in the same location from the same cell type (Taufiq-Ur-Rahman et al., 2009), but those smaller and variable conductances were likely a result of heavy contamination by Mg2+ of the Na2ATP used (Rahman and Taylor, 2009). The conductance of the r-InsP3R-3 channels in the outer nuclear membrane of DT40-KO-r-InsP3R-3 cells is even larger than that for the invertebrate InsP3R (477 ± 3 pS; Ionescu et al., 2006), despite the more RYR-like putative selectivity filter sequence of the latter. Because all of these patch clamp studies of various InsP3R channels were all performed in symmetric 140 mM KCl ([K+]f = 104 mM), these observations suggest that besides the primary sequences of the InsP3R, other factor(s)—lipid environment, interacting proteins or peptides, etc., that are different in various expression systems (outer nuclear membrane vs. plasma membrane; DT40-KO-r-InsP3R-3 nucleus vs. Xenopus oocyte nucleus)—can affect the conductance of InsP3R channels substantially.
The factors that cause the differences of the InsP3R-3 gch observed in the same rigorously controlled ionic conditions (symmetric 140 mM KCl) in different cellular locations and different cells may also affect the size of iCa through InsP3R-3 channels in those contexts. Because ours is the first measurement of iCa through an InsP3R channel under physiological ionic conditions, how iCa correlates with gch in various cell systems cannot be clearly gauged until similar measurements are made in different systems. It should be pointed out that although the ionic conditions ([Ca2+]ER, [Mg2+]f, [K+], and [Cl−]) under which iCa was measured in this study were physiological or near-physiological, the ionic conditions under which gch in symmetric 140 mM KCl were measured (in this study and all published reports) were nonphysiological because of the absence of divalent cations, especially Mg2+, on either side of the channels. It is possible that the observed variability of gch is a result of the use of nonphysiological ionic conditions, as a study on the effects of divalent cations on InsP3R gch suggested (Mak and Foskett, 1998). This issue can be clarified with more direct measurements of iCa for other InsP3R channels in different cell systems. At this point, the applicability of the iCa of homotetrameric type 3 InsP3R channels in DT40-KO-r-InsP3R-3 cells to other InsP3R channels and cell types should be considered judiciously with recognition of its potential limits. However, this first direct measurement is of significant value for improving our understanding of the mechanisms of InsP3R-mediated Ca2+ release and for modeling efforts to simulate Ca2+ signaling.
Selective permeant ion block of the InsP3R channel by Mg2+
Physiological [Mg2+]f (200 µM–1.1 mM in both the cytoplasm and ER lumen) substantially reduced gch of the InsP3R channel in symmetric 140-mM KCl solutions (Fig. 5) by acting as a permeant blocking cation. This indicates that under physiological conditions, Mg2+ significantly affects the passage of K+ through the InsP3R channel pore. Accordingly, it is not immediately obvious why iCa was not measurably different in the presence or absence of physiological [Mg2+]f (Fig. 4). Similarly, iCa’s passing through RYR channels under physiological (∼500 µM) [Ca2+]ER (Chen et al., 2003; Kettlun et al., 2003; Gillespie and Fill, 2008) were not significantly affected by the presence or absence of 1 mM MgCl2.
Because of the lack, to date, of a quantitative model to describe the conductance properties of the InsP3R channel pore, we attempt to explain qualitatively the apparently contradicting observations by using models developed to account for conductance properties of RYR channels, which have a putative selectivity filter sequence, and therefore structure, highly homologous to those of InsP3R channels.
In a barrier model that describes the RYR channel as a single-ion occupancy channel with four energy barriers in the selectivity filter (Tinker et al., 1992), the channel is blocked when any cation enters the vacant selectivity filter because the channel cannot accommodate two cations simultaneously. Despite higher permeability of Mg2+ than K+, because [K+]f in the cytoplasm and ER lumen is about two orders of magnitude higher than [Mg2+]f, K+ is likely to be the major permeant blocker of iCa through the InsP3R channel. Furthermore, because of the higher affinity of Ca2+ for the selectivity filter relative to that of Mg2+, as revealed by its more potent reduction of InsP3R gch (Fig. 5), the energy released as Ca2+ enters the channel selectivity filter from the luminal side can compensate for the energy required to push an occupying Mg2+ out the cytoplasmic side, especially if the Mg2+ is bound to the side energy well close to the cytoplasmic end of the filter. Thus, it is possible that the iCa through the InsP3R channel is already significantly suppressed by physiological [K+]f, such that Mg2+, with lower affinity for the selectivity filter than Ca2+, cannot reduce the magnitude of iCa further by a detectable amount.
In Poisson-Nernst Planck models with (Gillespie et al., 2005) and without (Chen et al., 1997, 2003) density function theory, a cation-selective channel is described as one with a constricted selectivity filter region containing negative charges from acidic amino side chains or carbonyl backbones. Cations move through the pore by electrodiffusion under electrical and concentration gradients, interacting with the channel both electrostatically and chemically. According to these models, concentrations of cations in the selectivity filter of the RYR/InsP3R channel are higher than those in the bulk solutions because of the permanent negative charges from the Asp (in GGGXGDX in InsP3R) or Asp and Glu (GGGIGDE in RYR) located in or near the selectivity filter of the channel. This concentrating effect is stronger for Ca2+ and Mg2+ than for K+ because of the more negative chemical potentials of Ca2+ and Mg2+ in the selectivity filter as a result of either chemical interactions between the divalent cations and the channel (Chen et al., 2003), or because of the smaller ionic radii and therefore smaller excluded volumes and higher charge densities of Ca2+ and Mg2+ (Gillespie, 2008). The high divalent cation concentration in or near the selectivity filter in turn lowers [K+] there as a result of electrostatic repulsion between the cations (Chen et al., 2003; Gillespie et al., 2005; Gillespie, 2008). This can cause the observed reduction of gch by physiological concentrations of Mg2+ or Ca2+. In InsP3R channels, the conserved sequence GGGXGDX in or near the selectivity filter has fewer acidic residues than the corresponding sequence GGGIGDE for the RYR channels. This may reduce the permanent negative charge density in the InsP3R channel selectivity filter relative to that for the RYR channel and consequently weaken the electrostatic interaction between the selectivity filter and the cations (Gillespie et al., 2005), making the effect of the more negative chemical potential of Ca2+ than Mg2+ in the filter even more prominent in the InsP3R than RYR, as indicated by the substantial higher potency of Ca2+ than Mg2+ to reduce InsP3R gch (Fig. 5). High [Ca2+]f in the InsP3R selectivity filter will suppress entry of Mg2+ through electrostatic repulsion. With only physiological [Mg2+]f in the bulk solution around the channel, there may not be enough Mg2+ in the filter to significantly impede the flux of Ca2+.
[Ca2+]i at various distances from an open InsP3R channel as a result of iCa
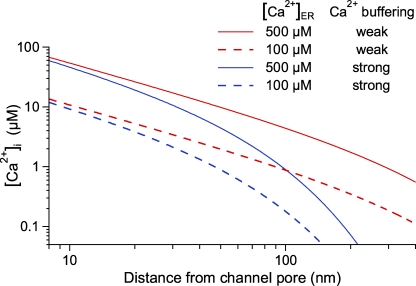
The size of iCa through an open InsP3R channel in the ER membrane obviously directly affects the [Ca2+]i immediately surrounding it. Measurement of iCa in physiological ionic conditions now enables us to estimate the [Ca2+]i in the vicinity of an open InsP3R. For the estimation, the channel pore can be treated as a point source in a semi-infinite region (Smith, 1996). Within short distances from the open-channel pore (<15 nm), the concentrations and Ca2+-binding rates of endogenous cytoplasmic Ca2+ buffers are too low to significantly affect the local [Ca2+]i. Thus, local [Ca2+]i in the immediate vicinity of the open channel is mainly determined by the equilibrium between Ca2+ flux through the channel and diffusion of unbound Ca2+ through the cytosol, and can be estimated with reasonable accuracy as
| (6) |
where r is the distance from the channel pore, D is the diffusion coefficient for Ca2+ in the cytoplasm with no buffering, and zCa and F are as defined in Eq. 1 (Smith, 1996; Neher, 1998), using the value of iCa measured here and D ≈ 225 µm2s−1 (Allbritton et al., 1992). According to cryo-electron microscopy (Jiang et al., 2002; da Fonseca et al., 2003; Hamada et al., 2003; Serysheva et al., 2003; Sato et al., 2004; Wolfram et al., 2010) and electron microscopy (Cárdenas et al., 2010a) measurements, the radius of a tetrameric InsP3R channel in a plane perpendicular to the axis of the channel pore is ∼10–12 nm. There is no structural information concerning the locations of various Ca2+-binding sites in the channel relative to the pore, but it is reasonable to assume that the distance between the sites and the pore is <10–12 nm. Using 8 nm as an estimate of the distance between the channel pore and the activating and inhibitory Ca2+-binding sites of the channel, within the physiological range of [Ca2+]ER (100–500 µM), [Ca2+]i is sensed by the channel; i.e., [Ca2+]i (8 nm) ≈ 14 – 69 µM (Fig. 6). This [Ca2+]i level is reached within a short time (microsecond) after the opening of the channel (Neher, 1998). With a filled ER store, [Ca2+]i (8 nm) is high enough that even at saturating [InsP3], Po of the open channel itself is significantly inhibited for most InsP3R isoforms that have been studied in single-channel experiments (Foskett et al., 2007). This can provide negative feedback to terminate the Ca2+ release. On the other hand, when the ER store is partially depleted, Ca2+ released by the InsP3R channel may not be sufficient to raise [Ca2+]i high enough to suppress activity of the releasing channel to terminate the Ca2+ release.
Figure 6.
Spatial profiles of [Ca2+]i at various distances from an open InsP3R channel for different [Ca2+]ER with different cytoplasmic Ca2+-buffering capacities. [Ca2+]i was calculated using Eq. 7 with characteristic length λ = 55 and 440 nm for strong and weak cytoplasmic Ca2+ buffering, respectively.
Farther away from the channel pore, [Ca2+]i is strongly affected by cytoplasmic Ca2+ buffers. Estimates of concentrations and Ca2+-binding rates of endogenous Ca2+ buffer(s) (BT and kon, respectively) vary widely (Wagner and Keizer, 1994; Smith et al., 1996; Falcke, 2003; Shuai et al., 2008), so only a first-order estimate of [Ca2+]i(r) is feasible for a general case. For simplicity, so that [Ca2+]i(r) can be evaluated analytically, the excess buffer approximation is assumed (Smith, 1996) for our rough estimation, so
| (7) |
where λ, the characteristic length, is (D/konBT)1/2. From parameters used in Wagner and Keizer (1994), Smith et al. (1996), and Falcke (2003), we determined a high and low estimate for λ as 440 and 55 nm, respectively, and estimated the range of [Ca2+]i(r) using Eq. 7 (Fig. 6).
For r > 200 nm, [Ca2+]i(r) calculated from the two estimates for λ differs by over two orders of magnitude (Fig. 6), and it is no longer meaningful to consider [Ca2+]i(r) for a general case. More specific values for kon and BT are needed to give a better evaluation of [Ca2+]i.
From our estimation, it is clear that as [Ca2+]i(r) decreases for larger r, there is a range of r within which the Ca2+ released by an open InsP3R channel can activate a neighboring channel at that distance away, as long as [InsP3] is sufficiently elevated, for all InsP3Rs studied (Foskett et al., 2007). Thus, the magnitude of iCa observed confirms that CICR can be a mechanism to couple neighboring InsP3R channels to coordinate concerted Ca2+ release by multiple channels to generate various intracellular Ca2+ signals.
In this simple consideration, the [Ca2+]i profile was estimated around an open channel with Po = 1. In reality, the amount of Ca2+ moving through the releasing channel depends not only on iCa, but also on the stochastic gating of the releasing channel, which is dynamically regulated by the local [InsP3] and [Ca2+]i (Foskett et al., 2007), which in turn are affected by Ca2+ released by the releasing channel and any neighboring active channels. Furthermore, the activation of an InsP3R channel by CICR is a complex dynamic process regulated by stochastic binding and unbinding of InsP3 and Ca2+ to activating and inhibitory sites, which are affected not only by local [Ca2+]i, but also by the on- and off-rates of the sites that can be allosterically coupled (Atri et al., 1993; Tang et al., 1996; Kaftan et al., 1997; Moraru et al., 1999; Dawson et al., 2003; Mak et al., 2003). To properly take into consideration all of these complicated factors affecting the regulation of Ca2+ signaling, quantitative kinetic modeling (De Young and Keizer, 1992; Swillens et al., 1994, 1998, 1999; Dupont and Swillens, 1996; Tang et al., 1996; Falcke et al., 2000; Sneyd and Falcke, 2005; Swaminathan et al., 2009) using the right parameters, of which iCa is a critical one, is necessary.
In summary, we have described the first direct electrophysiological measurements of the iCa’s driven by physiological [Ca2+] gradients across single InsP3R channels in a native ER membrane environment under physiological ionic conditions. These measurements will enable more accurate evaluations of the amount of Ca2+ released in a fundamental Ca2+ release event mediated by a single InsP3R channel, contribute to a better estimation of the coupling between the activities of neighboring InsP3R channels through CICR, and provide insights to improve future understanding and modeling of intracellular Ca2+ signals.
Acknowledgments
We thank John E. Pearson for helpful discussions.
This work was supported by National Institutes of Health grants R01 GM074999 (to D.-O.D. Mak), R01 GM065830 (to D.-O.D. Mak and J.K. Foskett), and R01 MH059937 to (J.K. Foskett).
Richard L. Moss served as editor.
Footnotes
Abbreviations used in this paper:
- iCa
- unitary Ca2+ current
- InsP3
- inositol 1,4,5-trisphosphate
- InsP3R
- InsP3 receptor
References
- Allbritton N.L., Meyer T., Stryer L. 1992. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 258:1812–1815 10.1126/science.1465619 [DOI] [PubMed] [Google Scholar]
- Atri A., Amundson J., Clapham D., Sneyd J. 1993. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys. J. 65:1727–1739 10.1016/S0006-3495(93)81191-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis H.A., Furuichi T., Yoshikawa F., Mikoshiba K., Sattelle D.B. 1999. Inositol 1,4,5-trisphosphate receptors are strongly expressed in the nervous system, pharynx, intestine, gonad and excretory cell of Caenorhabditis elegans and are encoded by a single gene (itr-1). J. Mol. Biol. 294:467–476 10.1006/jmbi.1999.3229 [DOI] [PubMed] [Google Scholar]
- Beeler T.J., Farmen R.H., Martonosi A.N. 1981. The mechanism of voltage-sensitive dye responses on sarcoplasmic reticulum. J. Membr. Biol. 62:113–137 10.1007/BF01870205 [DOI] [PubMed] [Google Scholar]
- Berridge M.J. 1993. Inositol trisphosphate and calcium signalling. Nature. 361:315–325 10.1038/361315a0 [DOI] [PubMed] [Google Scholar]
- Berridge M.J. 1997. Elementary and global aspects of calcium signalling. J. Physiol. 499:291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J., Lipp P., Bootman M.D. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Betzenhauser M.J., Wagner L.E., II, Park H.S., Yule D.I. 2009. ATP regulation of type-1 inositol 1,4,5-trisphosphate receptor activity does not require Walker A-type ATP-binding motifs. J. Biol. Chem. 284:16156–16163 10.1074/jbc.M109.006452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. 1995. The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 145:205–216 [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda J., Janssen H., Seino S., Bell G.I. 1993. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J. Biol. Chem. 268:11356–11363 [PubMed] [Google Scholar]
- Boehning D., Joseph S.K., Mak D.-O.D., Foskett J.K. 2001a. Single-channel recordings of recombinant inositol trisphosphate receptors in mammalian nuclear envelope. Biophys. J. 81:117–124 10.1016/S0006-3495(01)75685-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning D., Mak D.-O.D., Foskett J.K., Joseph S.K. 2001b. Molecular determinants of ion permeation and selectivity in inositol 1,4,5-trisphosphate receptor Ca2+ channels. J. Biol. Chem. 276:13509–13512 [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Berridge M.J., Lipp P. 1997. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 91:367–373 10.1016/S0092-8674(00)80420-1 [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Collins T.J., Peppiatt C.M., Prothero L.S., MacKenzie L., De Smet P., Travers M., Tovey S.C., Seo J.T., Berridge M.J., et al. 2001. Calcium signalling—an overview. Semin. Cell Dev. Biol. 12:3–10 10.1006/scdb.2000.0211 [DOI] [PubMed] [Google Scholar]
- Braet K., Cabooter L., Paemeleire K., Leybaert L. 2004. Calcium signal communication in the central nervous system. Biol. Cell. 96:79–91 10.1016/j.biolcel.2003.10.007 [DOI] [PubMed] [Google Scholar]
- Bruno L., Solovey G., Ventura A.C., Dargan S., Dawson S.P. 2010. Quantifying calcium fluxes underlying calcium puffs in Xenopus laevis oocytes. Cell Calcium. 47:273–286 10.1016/j.ceca.2009.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J.N. 1968. The thermodynamic activity of calcium ion in sodium chloride-calcium chloride electrolytes. Biophys. J. 8:1426–1433 10.1016/S0006-3495(68)86564-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F.L., Benedetti A. 1996. What is the concentration of calcium ions in the endoplasmic reticulum? Cell Calcium. 19:547–551 10.1016/S0143-4160(96)90064-0 [DOI] [PubMed] [Google Scholar]
- Cárdenas C., Escobar M., García A., Osorio-Reich M., Härtel S., Foskett J.K., Franzini-Armstrong C. 2010a. Visualization of inositol 1,4,5-trisphosphate receptors on the nuclear envelope outer membrane by freeze-drying and rotary shadowing for electron microscopy. J. Struct. Biol. 171:372–381 10.1016/j.jsb.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas C., Miller R.A., Smith I., Bui T., Molgó J., Müller M., Vais H., Cheung K.H., Yang J., Parker I., et al. 2010b. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 142:270–283 10.1016/j.cell.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.P., Xu L., Tripathy A., Meissner G., Eisenberg B. 1997. Permeation through the calcium release channel of cardiac muscle. Biophys. J. 73:1337–1354 10.1016/S0006-3495(97)78167-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.P., Xu L., Eisenberg B., Meissner G. 2003. Calcium ion permeation through the calcium release channel (ryanodine receptor) of cardiac muscle. J. Phys. Chem. B. 107:9139–9145 10.1021/jp0354191 [DOI] [Google Scholar]
- Chen S.R.W., Li P., Zhao M., Li X., Zhang L. 2002. Role of the proposed pore-forming segment of the Ca2+ release channel (ryanodine receptor) in ryanodine interaction. Biophys. J. 82:2436–2447 10.1016/S0006-3495(02)75587-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. 1995. Calcium signaling. Cell. 80:259–268 10.1016/0092-8674(95)90408-5 [DOI] [PubMed] [Google Scholar]
- da Fonseca P.C., Morris S.A., Nerou E.P., Taylor C.W., Morris E.P. 2003. Domain organization of the type 1 inositol 1,4,5-trisphosphate receptor as revealed by single-particle analysis. Proc. Natl. Acad. Sci. USA. 100:3936–3941 10.1073/pnas.0536251100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A.P., Lea E.J., Irvine R.F. 2003. Kinetic model of the inositol trisphosphate receptor that shows both steady-state and quantal patterns of Ca2+ release from intracellular stores. Biochem. J. 370:621–629 10.1042/BJ20021289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Young G.W., Keizer J. 1992. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc. Natl. Acad. Sci. USA. 89:9895–9899 10.1073/pnas.89.20.9895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellis O., Dedos S.G., Tovey S.C., Taufiq-Ur-Rahman S.J., Dubel S.J., Taylor C.W. 2006. Ca2+ entry through plasma membrane IP3 receptors. Science. 313:229–233 10.1126/science.1125203 [DOI] [PubMed] [Google Scholar]
- Du G.G., Guo X., Khanna V.K., MacLennan D.H. 2001. Functional characterization of mutants in the predicted pore region of the rabbit cardiac muscle Ca2+ release channel (ryanodine receptor isoform 2). J. Biol. Chem. 276:31760–31771 10.1074/jbc.M102751200 [DOI] [PubMed] [Google Scholar]
- Dupont G., Swillens S. 1996. Quantal release, incremental detection, and long-period Ca2+ oscillations in a model based on regulatory Ca2+-binding sites along the permeation pathway. Biophys. J. 71:1714–1722 10.1016/S0006-3495(96)79373-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcke M. 2003. Buffers and oscillations in intracellular Ca2+ dynamics. Biophys. J. 84:28–41 10.1016/S0006-3495(03)74830-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcke M., Tsimring L., Levine H. 2000. Stochastic spreading of intracellular Ca2+ release. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Topics. 62:2636–2643 [DOI] [PubMed] [Google Scholar]
- Foskett J.K. 1990. Optical studies of ion and water transport. Noninvasive Techniques in Cell Biology. Foskett J.K., Grinstein S., Wiley-Liss, New York: 237–272 [Google Scholar]
- Foskett J.K., Mak D.-O.D. 2010. Regulation of IP3R channel gating by Ca2+ and Ca2+ binding proteins. In Current Topics in Membranes Vol. 66: Structure-Function of Ca2+ Release Channels. Serysheva I.I., editor Elsevier, Amsterdam: 235–272 [Google Scholar]
- Foskett J.K., White C., Cheung K.H., Mak D.-O.D. 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87:593–658 10.1152/physrev.00035.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii J., Otsu K., Zorzato F., de Leon S., Khanna V.K., Weiler J.E., O’Brien P.J., MacLennan D.H. 1991. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 253:448–451 10.1126/science.1862346 [DOI] [PubMed] [Google Scholar]
- Furuichi T., Mikoshiba K. 1995. Inositol 1, 4, 5-trisphosphate receptor-mediated Ca2+ signaling in the brain. J. Neurochem. 64:953–960 10.1046/j.1471-4159.1995.64030953.x [DOI] [PubMed] [Google Scholar]
- Gao L., Balshaw D., Xu L., Tripathy A., Xin C., Meissner G. 2000. Evidence for a role of the lumenal M3-M4 loop in skeletal muscle Ca2+ release channel (ryanodine receptor) activity and conductance. Biophys. J. 79:828–840 10.1016/S0006-3495(00)76339-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D. 2008. Energetics of divalent selectivity in a calcium channel: the ryanodine receptor case study. Biophys. J. 94:1169–1184 10.1529/biophysj.107.116798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Fill M. 2008. Intracellular calcium release channels mediate their own countercurrent: the ryanodine receptor case study. Biophys. J. 95:3706–3714 10.1529/biophysj.108.131987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Xu L., Wang Y., Meissner G. 2005. (De)constructing the ryanodine receptor: modeling ion permeation and selectivity of the calcium release channel. J. Phys. Chem. B. 109:15598–15610 10.1021/jp052471j [DOI] [PubMed] [Google Scholar]
- Goldberg R.N., Nuttall R.L. 1978. Evaluated activity and osmotic coefficients for aqueous solutions: the alkaline earth metal halides. J. Phys. Chem. Ref. Data. 7:263–310 10.1063/1.555569 [DOI] [Google Scholar]
- Golding E.M., Golding R.M. 1995. Interpretation of 31P MRS spectra in determining intracellular free magnesium and potassium ion concentrations. Magn. Reson. Med. 33:467–474 10.1002/mrm.1910330403 [DOI] [PubMed] [Google Scholar]
- Halvorson H.R., Vande Linde A.M., Helpern J.A., Welch K.M. 1992. Assessment of magnesium concentrations by 31P NMR in vivo. NMR Biomed. 5:53–58 10.1002/nbm.1940050202 [DOI] [PubMed] [Google Scholar]
- Hamada K., Terauchi A., Mikoshiba K. 2003. Three-dimensional rearrangements within inositol 1,4,5-trisphosphate receptor by calcium. J. Biol. Chem. 278:52881–52889 10.1074/jbc.M309743200 [DOI] [PubMed] [Google Scholar]
- Hamer W.J., Wu Y.-C. 1972. Osmotic coefficients and mean activity coefficients of uni-univalent electrolytes in water at 25°C. J. Phys. Chem. Ref. Data. 1:1047–1099 10.1063/1.3253108 [DOI] [Google Scholar]
- Hille B. 2001. Ionic Channels of Excitable Membranes. 3rd edition Sinauer Associates, Inc, Sunderland, MA: 814 pp [Google Scholar]
- Ionescu L., Cheung K.H., Vais H., Mak D.-O.D., White C., Foskett J.K. 2006. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal Ca2+ release. J. Physiol. 573:645–662 10.1113/jphysiol.2006.109504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H., Chiba K., Uchiyama T., Yoshikawa F., Suzuki F., Ikeda M., Furuichi T., Mikoshiba K. 2002. Molecular characterization of the starfish inositol 1,4,5-trisphosphate receptor and its role during oocyte maturation and fertilization. J. Biol. Chem. 277:2763–2772 10.1074/jbc.M108839200 [DOI] [PubMed] [Google Scholar]
- Jiang Q.X., Thrower E.C., Chester D.W., Ehrlich B.E., Sigworth F.J. 2002. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 Å resolution. EMBO J. 21:3575–3581 10.1093/emboj/cdf380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S.K., Hajnóczky G. 2007. IP3 receptors in cell survival and apoptosis: Ca2+ release and beyond. Apoptosis. 12:951–968 10.1007/s10495-007-0719-7 [DOI] [PubMed] [Google Scholar]
- Joseph S.K., Lin C., Pierson S., Thomas A.P., Maranto A.R. 1995. Heteroligomers of type-I and type-III inositol trisphosphate receptors in WB rat liver epithelial cells. J. Biol. Chem. 270:23310–23316 10.1074/jbc.270.40.23310 [DOI] [PubMed] [Google Scholar]
- Kaftan E.J., Ehrlich B.E., Watras J. 1997. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor–dependent calcium signaling. J. Gen. Physiol. 110:529–538 10.1085/jgp.110.5.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettlun C., González A., Ríos E., Fill M. 2003. Unitary Ca2+ current through mammalian cardiac and amphibian skeletal muscle ryanodine receptor channels under near-physiological ionic conditions. J. Gen. Physiol. 122:407–417 10.1085/jgp.200308843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C.A. 1979. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J. Physiol. 286:417–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wang X., Vais H., Thompson C.B., Foskett J.K., White C. 2007. Apoptosis regulation by Bcl-xL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc. Natl. Acad. Sci. USA. 104:12565–12570 10.1073/pnas.0702489104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay A.R., Manning S.D., Williams A.J. 1991. Monovalent cation conductance in the ryanodine receptor-channel of sheep cardiac muscle sarcoplasmic reticulum. J. Physiol. 439:463–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. 1994. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J. Biol. Chem. 269:29375–29378 [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. 1997. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J. Gen. Physiol. 109:571–587 10.1085/jgp.109.5.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. 1998. Effects of divalent cations on single-channel conduction properties of Xenopus IP3 receptor. Am. J. Physiol. 275:C179–C188 [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. 1998. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. USA. 95:15821–15825 10.1073/pnas.95.26.15821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. 1999. ATP regulation of type 1 inositol 1,4,5-trisphosphate receptor channel gating by allosteric tuning of Ca2+ activation. J. Biol. Chem. 274:22231–22237 10.1074/jbc.274.32.22231 [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Raghuram V., Yue Y., Joseph S.K., Foskett J.K. 2000. Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J. Gen. Physiol. 115:241–256 10.1085/jgp.115.3.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. 2001a. ATP regulation of recombinant type 3 inositol 1,4,5-trisphosphate receptor gating. J. Gen. Physiol. 117:447–456 10.1085/jgp.117.5.447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. 2001b. Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels. Ca2+ activation uniquely distinguishes types 1 and 3 InsP3 receptors. J. Gen. Physiol. 117:435–446 10.1085/jgp.117.5.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S.M., Foskett J.K. 2003. Spontaneous channel activity of the inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R). Application of allosteric modeling to calcium and InsP3 regulation of InsP3R single-channel gating. J. Gen. Physiol. 122:583–603 10.1085/jgp.200308809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., White C., Ionescu L., Foskett J.K. 2005. Nuclear patch clamp electrophysiology of inositol trisphosphate receptor Ca2+ release channels. Calcium Signaling. 2nd ed Putney J.W., Jr, editor CRC Press, Boca Raton, FL: 203–229 [Google Scholar]
- Mak D.-O.D., Pearson J.E., Loong K.P.C., Datta S., Fernández-Mongil M., Foskett J.K. 2007. Rapid ligand-regulated gating kinetics of single inositol 1,4,5-trisphosphate receptor Ca2+ release channels. EMBO Rep. 8:1044–1051 10.1038/sj.embor.7401087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko S.M., Yarotskyy V.V., Kovalenko T.N., Kostyuk P.G., Thomas R.C. 2005. Spontaneously active and InsP3-activated ion channels in cell nuclei from rat cerebellar Purkinje and granule neurones. J. Physiol. 565:897–910 10.1113/jphysiol.2004.081299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhl M., Schuster S., Brumen M., Heinrich R. 1997. Modeling the interrelations between the calcium oscillations and ER membrane potential oscillations. Biophys. Chem. 63:221–239 10.1016/S0301-4622(96)02248-X [DOI] [PubMed] [Google Scholar]
- Mignery G.A., Newton C.L., Archer B.T., III, Südhof T.C. 1990. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 265:12679–12685 [PubMed] [Google Scholar]
- Moraru I.I., Kaftan E.J., Ehrlich B.E., Watras J. 1999. Regulation of type 1 inositol 1,4,5-trisphosphate–gated calcium channels by InsP3 and calcium: simulation of single channel kinetics based on ligand binding and electrophysiological analysis. J. Gen. Physiol. 113:837–849 10.1085/jgp.113.6.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelle B., Salmon J.M., Vigo J., Viallet P. 1994a. Are intracellular ionic concentrations accessible using fluorescent probes? The example of Mag-indo-1. Cell Biol. Toxicol. 10:339–344 10.1007/BF00755780 [DOI] [PubMed] [Google Scholar]
- Morelle B., Salmon J.M., Vigo J., Viallet P. 1994b. Measurement of intracellular magnesium concentration in 3T3 fibroblasts with the fluorescent indicator Mag-indo-1. Anal. Biochem. 218:170–176 10.1006/abio.1994.1156 [DOI] [PubMed] [Google Scholar]
- Neher E. 1995. Voltage offsets in patch-clamp experiments. Single-channel recording. 2nd edition Sakmann B., Neher E., Plenum Press, New York: 147–153 [Google Scholar]
- Neher E. 1998. Usefulness and limitations of linear approximations to the understanding of Ca++ signals. Cell Calcium. 24:345–357 10.1016/S0143-4160(98)90058-6 [DOI] [PubMed] [Google Scholar]
- Palmer A.E., Jin C., Reed J.C., Tsien R.Y. 2004. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. USA. 101:17404–17409 10.1073/pnas.0408030101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R.L., Boehning D., Snyder S.H. 2004. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 73:437–465 10.1146/annurev.biochem.73.071403.161303 [DOI] [PubMed] [Google Scholar]
- Rahman T., Taylor C.W. 2009. Dynamic regulation of IP3 receptor clustering and activity by IP3. Channels (Austin). 3:226–232 [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Trautmann A. 2004. Ca2+ signals and T lymphocytes; “New mechanisms and functions in Ca2+ signalling”. Biol. Cell. 96:69–78 10.1016/j.biolcel.2003.10.008 [DOI] [PubMed] [Google Scholar]
- Sato C., Hamada K., Ogura T., Miyazawa A., Iwasaki K., Hiroaki Y., Tani K., Terauchi A., Fujiyoshi Y., Mikoshiba K. 2004. Inositol 1,4,5-trisphosphate receptor contains multiple cavities and L-shaped ligand-binding domains. J. Mol. Biol. 336:155–164 10.1016/j.jmb.2003.11.024 [DOI] [PubMed] [Google Scholar]
- Serysheva I.I., Bare D.J., Ludtke S.J., Kettlun C.S., Chiu W., Mignery G.A. 2003. Structure of the type 1 inositol 1,4,5-trisphosphate receptor revealed by electron cryomicroscopy. J. Biol. Chem. 278:21319–21322 10.1074/jbc.C300148200 [DOI] [PubMed] [Google Scholar]
- Shuai J., Rose H.J., Parker I. 2006. The number and spatial distribution of IP3 receptors underlying calcium puffs in Xenopus oocytes. Biophys. J. 91:4033–4044 10.1529/biophysj.106.088880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai J., Pearson J.E., Parker I. 2008. Modeling Ca2+ feedback on a single inositol 1,4,5-trisphosphate receptor and its modulation by Ca2+ buffers. Biophys. J. 95:3738–3752 10.1529/biophysj.108.137182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman H.S., Di Lisa F., Hui R.C., Miyata H., Sollott S.J., Hanford R.G., Lakatta E.G., Stern M.D. 1994. Regulation of intracellular free Mg2+ and contraction in single adult mammalian cardiac myocytes. Am. J. Physiol. 266:C222–C233 [DOI] [PubMed] [Google Scholar]
- Singh J., Wisdom D.M. 1995. Second messenger role of magnesium in pancreatic acinar cells of the rat. Mol. Cell. Biochem. 149:175–182 10.1007/BF01076575 [DOI] [PubMed] [Google Scholar]
- Smith G.D. 1996. Analytical steady-state solution to the rapid buffering approximation near an open Ca2+ channel. Biophys. J. 71:3064–3072 10.1016/S0006-3495(96)79500-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.D., Wagner J., Keizer J. 1996. Validity of the rapid buffering approximation near a point source of calcium ions. Biophys. J. 70:2527–2539 10.1016/S0006-3495(96)79824-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I.F., Parker I. 2009. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc. Natl. Acad. Sci. USA. 106:6404–6409 10.1073/pnas.0810799106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneyd J., Falcke M. 2005. Models of the inositol trisphosphate receptor. Prog. Biophys. Mol. Biol. 89:207–245 10.1016/j.pbiomolbio.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Sugawara H., Kurosaki M., Takata M., Kurosaki T. 1997. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 16:3078–3088 10.1093/emboj/16.11.3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.P., Callamaras N., Marchant J.S., Parker I. 1998. A continuum of InsP3-mediated elementary Ca2+ signalling events in Xenopus oocytes. J. Physiol. 509:67–80 10.1111/j.1469-7793.1998.067bo.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan D., Ullah G., Jung P. 2009. A simple sequential-binding model for calcium puffs. Chaos. 19:037109 10.1063/1.3152227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillens S., Combettes L., Champeil P. 1994. Transient inositol 1,4,5-trisphosphate-induced Ca2+ release: a model based on regulatory Ca2+-binding sites along the permeation pathway. Proc. Natl. Acad. Sci. USA. 91:10074–10078 10.1073/pnas.91.21.10074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillens S., Champeil P., Combettes L., Dupont G. 1998. Stochastic simulation of a single inositol 1,4,5-trisphosphate-sensitive Ca2+ channel reveals repetitive openings during ‘blip-like’ Ca2+ transients. Cell Calcium. 23:291–302 10.1016/S0143-4160(98)90025-2 [DOI] [PubMed] [Google Scholar]
- Swillens S., Dupont G., Combettes L., Champeil P. 1999. From calcium blips to calcium puffs: theoretical analysis of the requirements for interchannel communication. Proc. Natl. Acad. Sci. USA. 96:13750–13755 10.1073/pnas.96.24.13750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Stephenson J.L., Othmer H.G. 1996. Simplification and analysis of models of calcium dynamics based on IP3-sensitive calcium channel kinetics. Biophys. J. 70:246–263 10.1016/S0006-3495(96)79567-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Konishi M. 1997. Basal intracellular free Mg2+ concentration in smooth muscle cells of guinea pig tenia cecum: intracellular calibration of the fluorescent indicator furaptra. Biophys. J. 73:3358–3370 10.1016/S0006-3495(97)78360-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taufiq-Ur-Rahman A., Skupin M., Falcke, Taylor C.W. 2009. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+. Nature. 458:655–659 10.1038/nature07763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.W., Richardson A. 1991. Structure and function of inositol trisphosphate receptors. Pharmacol. Ther. 51:97–137 10.1016/0163-7258(91)90043-L [DOI] [PubMed] [Google Scholar]
- Thul R., Falcke M. 2004. Release currents of IP(3) receptor channel clusters and concentration profiles. Biophys. J. 86:2660–2673 10.1016/S0006-3495(04)74322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A., Lindsay A.R., Williams A.J. 1992. A model for ionic conduction in the ryanodine receptor channel of sheep cardiac muscle sarcoplasmic reticulum. J. Gen. Physiol. 100:495–517 10.1085/jgp.100.3.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vais H., Siebert A.P., Ma Z., Fernández-Mongil M., Foskett J.K., Mak D.-O.D. 2010. Redox-regulated heterogeneous thresholds for ligand recruitment among InsP3R Ca2+-release channels. Biophys. J. 99:407–416 10.1016/j.bpj.2010.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J., Keizer J. 1994. Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations. Biophys. J. 67:447–456 10.1016/S0006-3495(94)80500-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Xu L., Pasek D.A., Gillespie D., Meissner G. 2005. Probing the role of negatively charged amino acid residues in ion permeation of skeletal muscle ryanodine receptor. Biophys. J. 89:256–265 10.1529/biophysj.104.056002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras J., Bezprozvanny I., Ehrlich B.E. 1991. Inositol 1,4,5-trisphosphate-gated channels in cerebellum: presence of multiple conductance states. J. Neurosci. 11:3239–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfram F., Morris E., Taylor C.W. 2010. Three-dimensional structure of recombinant type 1 inositol 1,4,5-trisphosphate receptor. Biochem. J. 428:483–489 10.1042/BJ20100143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Wang Y., Gillespie D., Meissner G. 2006. Two rings of negative charges in the cytosolic vestibule of type-1 ryanodine receptor modulate ion fluxes. Biophys. J. 90:443–453 10.1529/biophysj.105.072538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S., Tanimura T., Miyawaki A., Nakamura M., Yuzaki M., Furuichi T., Mikoshiba K. 1992. Molecular cloning and characterization of the inositol 1,4,5-trisphosphate receptor in Drosophila melanogaster. J. Biol. Chem. 267:16613–16619 [PubMed] [Google Scholar]
- Yu R., Hinkle P.M. 2000. Rapid turnover of calcium in the endoplasmic reticulum during signaling. Studies with cameleon calcium indicators. J. Biol. Chem. 275:23648–23653 10.1074/jbc.M002684200 [DOI] [PubMed] [Google Scholar]
- Zhao M., Li P., Li X., Zhang L., Winkfein R.J., Chen S.R. 1999. Molecular identification of the ryanodine receptor pore-forming segment. J. Biol. Chem. 274:25971–25974 10.1074/jbc.274.37.25971 [DOI] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N.M., Lai F.A., Meissner G., MacLennan D.H. 1990. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 265:2244–2256 [PubMed] [Google Scholar]