Abstract
Disruption of the Esr1 gene encoding estrogen receptor alpha (ERα) by insertion of a neomycin resistance gene (neo) into exon 2 (αERKO mice) was shown previously to cause infertility in male mice. While full-length ERα protein was not expressed in αERKO mice, alternative splicing resulted in the low level expression of a truncated form lacking the N-terminus A/B domain and containing the DNA- and ligand-binding domains. Thus, it was unclear whether the reproductive phenotype in αERKO males was due only to the lack of full-length ERα or was affected by the presence of the variant ERα isoform. The present study examined male mice with exon 3 of Esr1 deleted, lacking the DNA-binding domain, and null for ERα (Ex3αERKO). Dilation of some seminiferous tubules was apparent in male Ex3αERKO mice as early as postnatal day 10 and was pronounced in all tubules from day 20 onward. At 6 weeks of age, sperm numbers and sperm motility were lower in Ex3αERKO than in wild type mice and the rete testis and efferent ductules were dilated. Mating studies determined that adult Ex3αERKO males were infertile and failed to produce copulatory plugs. Serum testosterone levels and Hsd17b3 and Cyp17a1 transcript levels were significantly higher, but serum estradiol, progesterone, LH and FSH levels and Cyp19a1 transcript levels were not significantly different from those in WT mice. These results confirm and extend those seen in other studies on male mice with exon 3 of Esr1 deleted. In addition, the reproductive phenotype of male Ex3αERKO mice recapitulated the phenotype of αERKO mice, strongly suggesting that the αERKO male infertility was not due to the presence of the DNA-binding domain in the truncated form of ERα and that full-length ERα is essential for maintenance of male fertility.
Keywords: testis, epididymis, spermatogenesis, sperm, male fertility, estrogen receptor alpha
Introduction
Previous studies showed that male αERKO mice homozygous for a targeted disruption of the Esr1 gene encoding estrogen receptor alpha (ERα) were infertile due to compromised fluid reabsorption by the efferent ductules and the initial segment of the epididymis (Eddy et al. 1996, Hess et al. 1997). This resulted in progressive dilation of the seminiferous tubule lumen, reduction in motility and loss of fertilizing ability by sperm, and disruption of the seminiferous epithelium leading to collapse of spermatogenesis. In addition, αERKO males produced significantly fewer copulatory plugs when mated with wild-type (WT) female mice, contributing to the infertility phenotype (Eddy et al. 1996, Ogawa et al. 1997). When germ cells from αERKO males were transplanted into the testes of WT mice depleted of germ cells, the recipients became fertile and sired offspring with the same genotype as the transplanted cells. This demonstrated that ERα is not required by male germ cells, but is required by somatic cells of the testis and/or male reproductive tract that provide the environment in which male gametes develop and mature (Mahato et al. 2000, Mahato et al. 2001). However, it is of interest that estrogen was reported to act through a G-protein-coupled receptor (GPR-30) on GC-1 spermatogonial cells to activate the epidermal growth factor receptor transduction pathway and stimulate their proliferation (Sirianni et al. 2008).
Several studies have provided evidence that estrogen is involved in regulating steroidogenesis in Leydig cells. The cytochrome P450, family 17, subfamily a, polypeptide 1 (CYP17A1) enzyme has an essential role in the steroidogenesis pathway in the synthesis of androstenedione and the hydroxysteroid (17-beta) dehydrogenase 3 (HSD17B3) enzyme is responsible for reducing androstenedione to testosterone. Diethylstilbestrol (DES) treatment inhibited CYP17A1 activity in testes of adult mice (Samuels et al. 1964) and in Leydig cells from fetal rats (Majdic et al. 1996). The Cyp17a1 mRNA and CYP17A1 protein levels also were reduced in mice lacking estrogen sulfotransferase (SULT1E1), the enzyme responsible for sulfo-conjugation and inactivation of estrogens (Tong et al. 2004). Transgenic mice expressing human aromatase (AROM+ mice; CYP19A1) had reduced testicular levels of Cyp17a1 and Hsd17b3 mRNA and low testosterone and high estrogen levels (Strauss et al. 2009). In contrast, the levels of Cyp17a1 and Hsd17b3 mRNA and CYP17A1 and HSD17B3 enzymatic activity were elevated significantly in the testis of αERKO mice (Akingbemi et al. 2003). Other studies with the AROM+ mice indicated that estrogen regulates Leydig cell steroidogenesis through ERα and not estrogen receptor beta (ERβ) (Strauss et al. 2009).
The original αERKO mice were generated using the technology available at the time, which involved insertion of a neomycin resistance gene (neo) into exon 2 of the Esr1 gene (Lubahn et al. 1993). Although this eliminated expression of full length ERα, an alternative splicing event resulted in low level expression of a truncated form of ERα lacking the N-terminal A/B domain, but containing the DNA- and ligand-binding domains (Couse et al. 1995, Pendaries et al. 2002). The present study examines mice generated with exon 3 of the Esr1 gene flanked by loxP sites and crossed with Sox2-cre transgenic mice to produce mice with a deletion in the Esr1 gene of the region encoding the DNA-binding domain (Ex3αERKO) and lacking a functional ERα (Hewitt et al. in press). Other investigators have generated mice with exon 3 of the Esr1 gene deleted, referred to as ERαKO (Dupont et al. 2000) and ACTB-Cre/ERα−/− mice (Chen et al. 2009a), or with a knock-in of mutant forms of the Esr1 gene, referred to as NERKI (McDevitt et al. 2007, Weiss et al. 2008) and ENERKI mice (Sinkevicius et al. 2009). The Esr1 allele in NERKI mice have point mutations in the first zinc finger of the DNA-binding domain of ERα that replace the glutamic acid at position 207 and the glycine at position 208 with alanines and disrupts the ability of ERα to bind DNA (Jakacka et al. 2002). The Esr1 allele in ENERKI mice has a mutation in the ligand binding pocket of ERα that replaces the glycine at position 525 with lysine and disrupts the ability of ERα to bind estradiol (Sinkevicius et al. 2008). Male αERKO (Eddy et al. 1996), ERαKO (Dupont et al. 2000) and ACTB-Cre/ERα−/− mice (Chen et al. 2009a) were found to be infertile in continuous mating studies. While ENERKI males did not undergo the disruption of the testis and excurrent ducts seen in αERKO, ERαKO and ACTB-Cre/ERα−/− males, they were subfertile (Sinkevicius et al. 2009). Disruption of the morphology of the testis and excurrent ducts in NERKI males was delayed compared to αERKO males, but they were infertile primarily due to defects in male mating behavior (McDevitt et al. 2007). The present study analyzes the reproductive phenotype of male Ex3αERKO mice and relates the findings to the reproductive phenotypes of αERKO, ERαKO, ACTB-Cre/ERα−/−, NERKI, and ENERKI males.
Materials and Methods
Animals
All experiments involving animals were carried out according to U.S. Public Health Service (USPHS) guidelines and the studies were approved by the National Institute of Environmental Health Sciences (NIEHS) Institutional Animal Care and Use Committee. The generation of mice lacking exon 3 of Esr1 was described previously (Hewitt et al. 2010).
Fertility studies
The fertility and fecundity of 7 to 14 week-old C57BL/6 male Ex3αERKO (n=9) and their wild type (WT) litter-mates (n=6) was determined in a continuous mating study in which each male was mated with two C57BL/6 females (6 weeks of age) for one month and with two different females the following month. The females were monitored for pregnancy during and after the mating periods and the number of litters and offspring were recorded. At the end of the second month, the males were euthanized and organs weighed, sperm numbers and sperm motility determined and serum hormone levels assayed. A separate study was carried out with Ex3αERKO males (n=4) to determine if they exhibited typical mating characteristics. Each male was mated with two C57BL/6 females (6 weeks of age) for 5 days and the females were checked daily for copulatory plugs and this was repeated 2 weeks later.
Sample collection
Ex3αERKO and WT males 6–7 and 18–33 weeks of age were anesthetized with carbon dioxide and euthanized by cervical dislocation. Blood for measuring serum hormone levels was collected by heart puncture or orbital bleeding on anesthetized mice prior to euthanasia. Body weights were determined and the reproductive organs were excised and cleaned of fat and blotted before the testis, epididymal and combined seminal vesicle and coagulating gland weights were determined. Testes and epididymides were fixed in Bouin’s solution for 12–16 h, washed in PBS, dehydrated in increasing concentrations of ethanol, embedded in paraffin, sectioned and stained with hematoxylin and eosin for histological examination. In addition, 10 and 20-day-old and 6 week-old Ex3αERKO and WT mice were euthanized, weights (body, testis and epididymis) recorded, and testes and epididymides prepared for histological examination as described. Images were recorded using an Axioplan microscope (Carl Zeiss, Thornewood, NJ, USA), and QImaging camera and software (QImaging, Tucson, AZ, USA).
Sperm motility assays and counts
The caudae epididymides of 6–7 week-old and 14–35 week-old Ex3αERKO and WT mice were collected in PBS (Ca2+/Mg2+-free) at room temperature, cleaned, and transferred to 500µl of M2 medium(Chemicon, Phillipsburg, NJ, USA). Cuts were made with iridectomy scissors, sperm were allowed to swim out into the medium for 10 min at room temperature, and motility was assayed using computer-assisted sperm analysis (CASA). Sperm tracks (1.5 sec, 30 frames) were captured at 60 Hz and analyzed using HTM-IVOS Sperm Analyzer software (Hamilton Thorne Biosciences, version 12.2L, Beverly, MA, USA). Sperm collected in the same manner from 6-week-old and adult Ex3αERKO and WT mice were diluted 1:10 or 1:2 in water and sperm counts determined on duplicate samples using a hemocytometer.
Serum hormone levels
The serum hormone levels were assayed for individual 6–7-week-old and adult male mice in the NIEHS Clinical Pathology Support Laboratory using an APEX Automatic Gamma Counter (ICN Micromedic Systems, Inc., Huntsville, AL, USA). Radioimmunoassay kits were used to measure testosterone, estradiol, progesterone (Coat-A-Count, Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA), FSH and LH serum levels (ALPCO Diagnostics, Salem, NH, USA).
Quantitative real-time RT-PCR (qPCR) assay
Male mice (3 WT and 6 Ex3αERKO) were euthanized at 2–3 months of age and testes snap frozen in liquid nitrogen. Ovaries were collected from adult female WT mice. Testes and ovaries were pulverized individually, homogenized in Trizol, and RNA was prepared as recommended by the manufacturer (Invitrogen, Carlsbad, CA, USA). Analysis was performed in duplicate for each testis (6 WT and 12 Ex3αERKO) and ovary (2 WT) by qPCR with an ABI PRISM 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) as described previously (Hewitt et al. in press). Primer sequences included: Cyp17a1: F - GATCGGTTTATGCCTGAGCG, R - TCCGAAGGGCAAATAACTGG; Hsd17b3: F - ATGGAGTCAAGGAGGAAAGGC, R - GGCTGTAAAGAGGCCAGGG; Cyp19a1: F - TGATCATGGGCCTCCTTCTC, R – CCCAGACAGTAGCCAGGACCT; Rpl7: F - AGCTGGCCTTTGTCATCAGAA, R - GACGAAGGAGCTGCAGAACCT. Expression ratios were calculated according to the method of Pfaffl (2001).
Statistical analysis
Statistical analyses were performed using Student’s t-test (two sample, assuming unequal variances) to calculate the mean and the standard error of the mean (SEM).
Results
Fertility studies
Two-month continuous breeding studies were performed to compare the fertility and fecundity of WT and Ex3αERKO male mice. The 6 WT males sired 29 litters and 175 offspring during this period, while the 8 Ex3αERKO males sired no offspring (Table 1). In addition, 4 Ex3αERKO males were housed twice for 5 days each with 2 WT females and the females were examined each morning for copulatory plugs. No copulatory plugs were detected, suggesting that the males failed to breed.
Table 1.
The total number of litters and offspring produced by adult Ex3αERKO and WT male mice during a two month mating tudy.
| Litters | Offspring | |
|---|---|---|
| Ex3αERKO (n=8) | 0 | 0 |
| WT (n=6) | 29 | 175 |
Reproductive organ weights
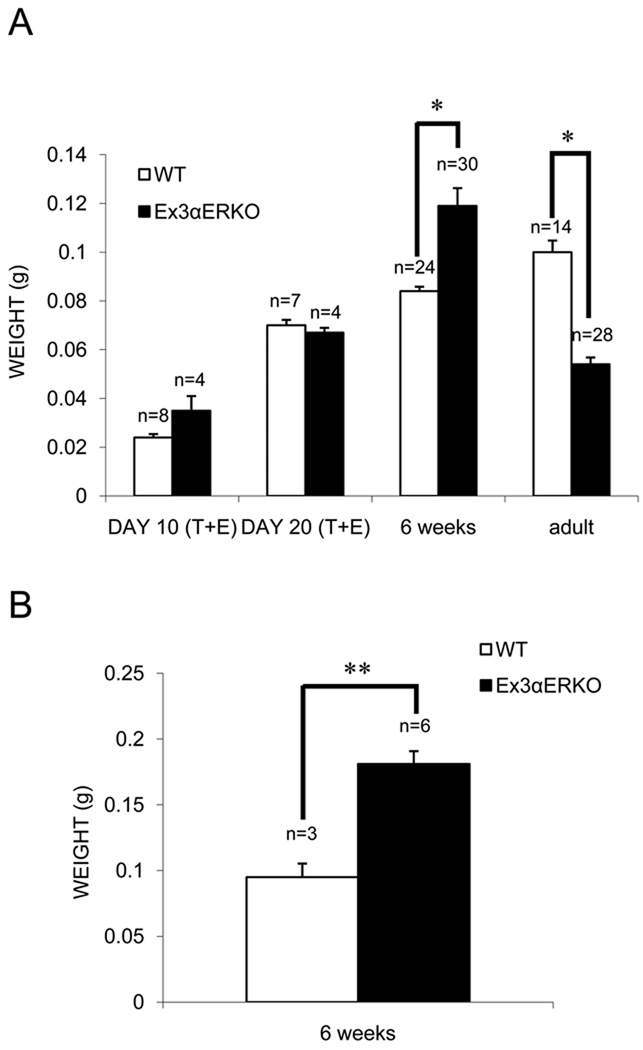
The combined testis and epididymis weights of 10 and 20-day-old WT and Ex3αERKO mice were not different. However, the testis weights of 6-week-old Ex3αERKO males were significantly greater than those of the WT males, while the testis weights of 18 to 33-week-old Ex3αERKO animals were significantly lower than those of WT males (Figure 1A). In addition, the seminal vesicle weights of 6-week-old Ex3αERKO animals were significantly higher than those of WT mice (Figure 1B). Other studies determined there were no differences in body weights between WT and Ex3αERKO males (Hewitt et al. in press).
Figure 1.
Organ weights of wild-type (WT) and Ex3αERKO mice. (A) Combined testis and epididymis (T+E) weights were determined for 10- and 20-day-old WT and Ex3αERKO mice. Testis weights were determined for 6-week-old and adult WT and Ex3αERKO mice. Data are expressed as mean ± SEM; (*p<0.001). (B) Combined seminal vesicle and coagulating gland weights were determined for 6-week-old WT and Ex3αERKO mice. Data are expressed as the mean ± SEM; (**p<0.005).
Sperm function
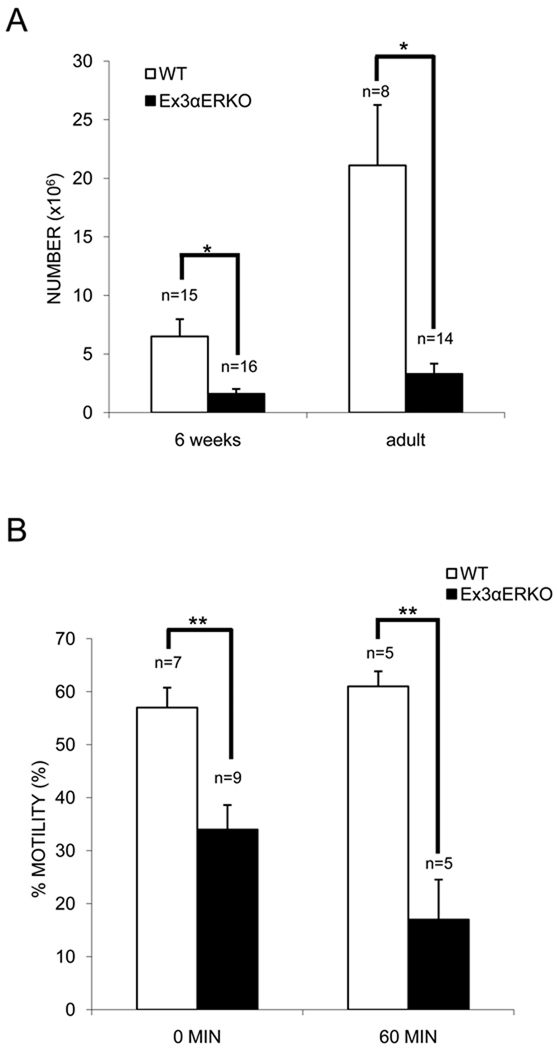
Sperm from the cauda epididymis of Ex3αERKO males did not appear different morphologically than sperm from WT males at the light microscope level, but the numbers were significantly lower for Ex3αERKO than WT males for both 6-week-old [1.6 vs. 6.5 × 106/ml] and adult [3.3 vs. 21.1 × 106/ml] mice (Figure 2A). The use of CASA revealed that the percent of sperm from the cauda epididymis of 6-week-old Ex3αERKO males that were motile was significantly less than of sperm from WT mice at time 0 [34% (n=9) vs. 57% (n=7)] and after a 60 minute incubation in M2 medium at RT [17% (n=5) vs. 61% (n=5)] (Figure 2B). However, sperm from 18–36-week-old Ex3αERKO mice were not motile (data not shown).
Figure 2.
Sperm counts and motility for WT and Ex3αERKO mice. (A) Sperm from the cauda epididymis of 6-week-old and adult WT and Ex3αERKO mice were counted. Data are expressed as the mean ± SEM; (*p<0.05). (B) The motility of sperm from the cauda epididymis of 6-week-old WT and Ex3αERKO mice was determined using CASA after 0 min and 60 min incubation in M2 medium. Data are expressed as the mean ± SEM; (**p<0.01).
Serum hormone levels
Serum testosterone levels were significantly higher (P<0.001) in 6-week-old and 10 to 36-week-old male Ex3αERKO mice than in WT mice of the same ages (Table 2), while estrogen, progesterone, FSH and LH levels were not significantly different (Table 2).
Table 2.
Serum hormone levels (ng/dl) for 6-week-old and adult Ex3αERKO and WT male mice.
| Testosterone | Progesterone | Estradiol | FSH | LH | |
|---|---|---|---|---|---|
| 6-week-old | |||||
| Ex3αERKO | 661 ± 134.8a, b (n=12) |
NDc | ND | 17 ± 1.8 (n=14) |
ND |
| WT | 64 ± 39.3 (n=7) |
ND | ND | 22 ± 2.4 (n=10) |
ND |
| Adult | |||||
| Ex3αERKO | 641 ± 108.6d (n=8) |
0.81 ± 0.07 (n=8) |
11.8 ± 2.0 (n=8) |
31 ± 1.9 (n=11) |
0.72 ± 0.21 (n=14) |
| WT | 93 ± 45.3 (n=6) |
0.55 ± 0.12 (n=6) |
14.3 ± 2.2 (n=6) |
32 ± 1.1 (n=11) |
1.08 ± 0.17 (n=11) |
Values are the mean ± SEM
Serum testosterone levels in 6-week-old Ex3αERKO males were significantly higher than in 6-week-old WT males (p<0.001)
ND = not determined
Serum testosterone levels in adult Ex3αERKO males were significantly higher than in adult WT males (p>0.001)
Histology
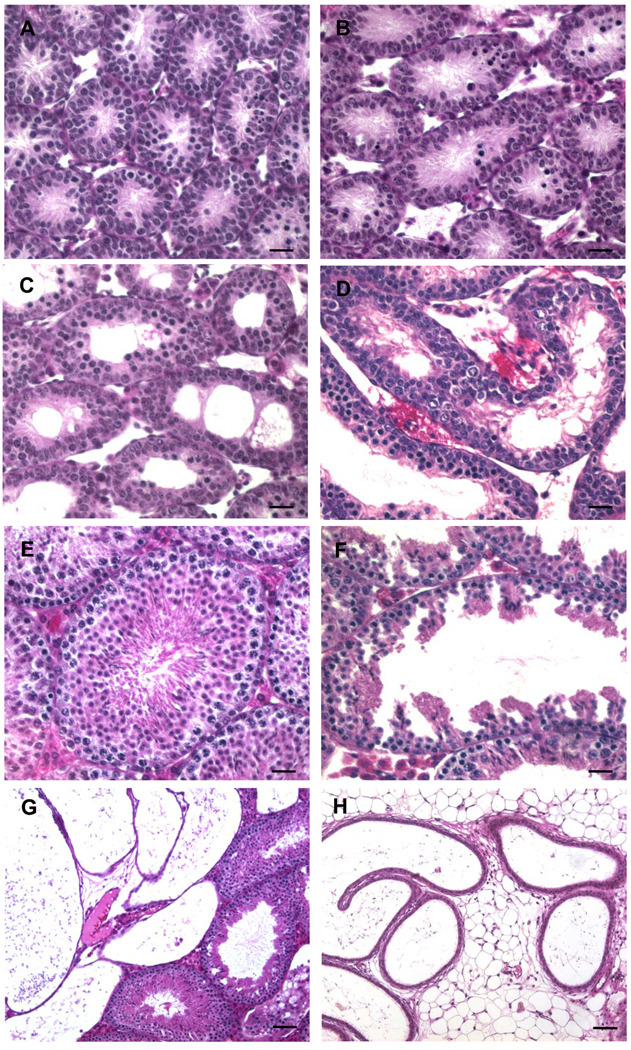
The lumen of seminiferous tubules of 10-day-old Ex3αERKO mice varied from mildly (Figure 3B) to severely (Figures 3C, 3D) dilated compared to WT mice (Figure 3A), while all tubules showed marked dilation in 20-day-old Ex3αERKO mice (data not shown). At 6 weeks of age, the seminiferous tubules (Figures 3F), rete testis (Figure 3G) and efferent ductules (Figure 3H) in Ex3αERKO mice were considerably dilated, compared to the seminiferous tubules (Figure 3E), rete testis and efferent ductules (data not shown) of WT mice. In most adult Ex3αERKO males (18 to 36 weeks of age), the testicular interstitium was expanded and the seminiferous tubules were reduced in diameter and had a low seminiferous epithelium and dilated lumen or lacked a lumen and contained a vacuolated epithelium (data not shown). These features were identical to what was observed in αERKO males (Eddy et al. 1996).
Figure 3.
Histology of testes from 10-day-old and 6-week-old WT and Ex3αERKO mice on sections stained with hematoxylin and eosin. Seminiferous tubules in testis of (A) 10-day-old WT and (B, C, D) Ex3αERKO mice. Seminiferous tubules in testis of 6-week-old (E) WT and (F) Ex3αERKO mice. (G) Rete testis in 6-week-old Ex3αERKO mouse. (H) Efferent ductules in 6-week-old Ex3αERKO mouse. Bars: 25µm.
qPCR assay
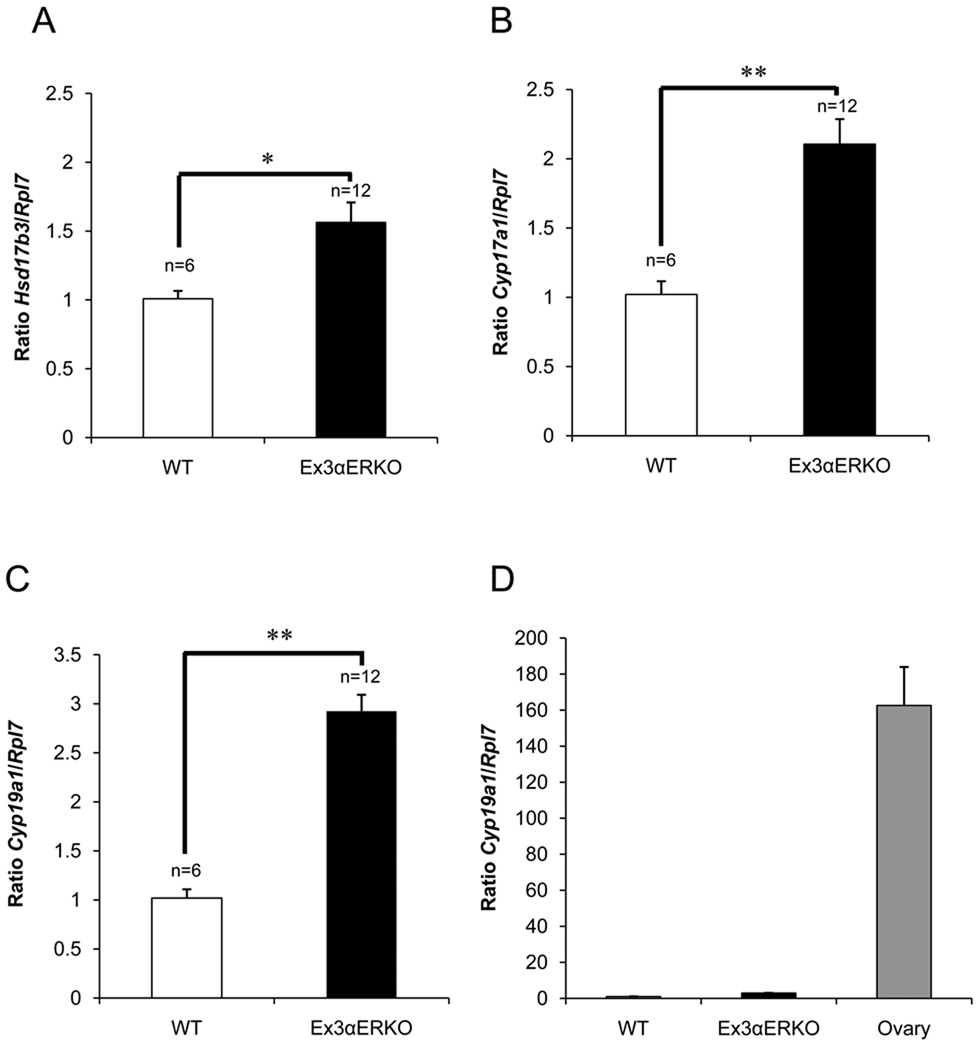
Previous studies demonstrated that Cyp17a1 and Hsd17b3 transcript levels and CYP17A1 and HSD17B3 enzyme activities were higher in the testes of αERKO than in WT mice (Akingbemi et al. 2003). Using qPCR, we found similarly that Hsd17b3 and Cyp17a1 transcript levels also were elevated significantly in the testes of adult Ex3αERKO mice (Figure 4A, 4B). While Cyp19a1 levels were elevated slightly in the testis of Ex3αERKO males compared to WT males, they were far lower than the levels in ovaries of WT females (Figure 4C, 4D).
Figure 4.
Real-time RT-PCR with RNA from testes and ovaries of WT and Ex3αERKO mice. The values shown are the average of two replicates of RNA from testes of WT and Ex3αERKO mice that were 2–3 months of age and calculated as the ratio of Hsd17b3 (A), Cyp17a1(B) and Cyp19a1 in testes (C) to Rpl7 expression levels. The value shown for ovary is the average of two replicates of RNA from ovaries of WT female mice and calculated as the ratio of Cyp19a1 to Rpl7 expression levels. Data are express as the mean ± SEM (Hsd17b3, *p<0.01; Cyp17a1 and Cyp19a1, **p <0.001). N= number of animals (N = 2 for ovary).
Discussion
The original αERKO mice were generated by insertion of a Pgk1-neo targeting and drug selection cassette into the Not1 site of exon 2 of the Esr1 gene (Lubahn et al. 1993). Homozygous αERKO mice did not express full-length ERα protein, but a truncated form of ERα containing the DNA-binding and ligand-binding domains was expressed at low levels due to alternative splicing (Couse et al. 1995, Pendaries et al. 2002). Generating mice with a global Esr1 gene exon 3 deletion (Ex3αERKO) and lacking ERα protein (Hewitt et al. in press) allowed us to determine if the truncated form of ERα contributed to the αERKO male infertility phenotype. Male Ex3αERKO mice mated with WT female mice failed to sire offspring during a two-month continuous mating trail, the same result seen with αERKO male mice (Eddy et al. 1996). This strongly suggests that the overriding cause of male infertility in αERKO mice was the absence of full-length ERα.
There were multiple underlying causes for the infertility in αERKO male mice, including lack of normal mating behavior, dilation of the rete testis and seminiferous tubules, and reduced sperm counts and sperm motility (Eddy et al. 1996; Hess et al. 1997; Lee et al. 2009). The Ex3αERKO male mice failed to produce copulatory plugs, consistent with observations that disruption of ERα caused altered mating behavior in adult αERKO male mice (Eddy et al. 1996, Ogawa et al. 1997, Couse & Korach 1999, Rissman et al. 1999). Young ENERKI males were subfertile (Sinkevicius et al. 2009), but lacked the dysmorphology seen in the testis and excurrent ducts of EX3αERKO, αERKO (Eddy et al. 1996, Hess et al. 1997; Lee et al. 2009), ERαKO (Dupont et al. 2000), NERKI (Weiss et al. 2008) and ACTB-Cre/ER−/− mice (Chen et al. 2009a). This suggests that although the ERα in ENERKI mice is unable to bind estradiol, it can partially rescue the male reproductive phenotype seen in mice with other mutant forms of ERα.
Dilation of the seminiferous tubules was apparent in 10-day-old Ex3αERKO mice and the rete testes and efferent ductules were dilated by 6 weeks of age. The seminiferous tubule dilation in αERKO males was suggested to be due either to increased fluid production in the seminiferous tubules or by reduced fluid reabsorption in the efferent ductules (Eddy et al. 1996). The latter possibility seemed more likely because the efferent ductules reabsorb the majority of the fluid coming from the testis (Jones & Jurd 1987, Veeramachaneni et al. 1990) and the efferent ductules have higher ER levels than other regions of the male reproductive tract (Schleicher et al. 1984, West & Brenner 1990, Cooke et al. 1991). This was confirmed by showing that less fluid is secreted from the testis in αERKO than WT mice, and that isolated segments of efferent ductules from αERKO mice are less effective at reabsorbing luminal fluid than segments from WT mice (Hess et al. 1997). In addition, the testis weights in Ex3αERKO mice were significantly greater than in WT mice at 6 weeks of age, but significantly less at 18 weeks and later. Similar changes observed in αERKO mice were suggested to be due to the initial accumulation of fluid and subsequent testicular atrophy (Hess et al. 1997). A higher pH was observed in the epididymal lumen in ERαKO mice and addition of cAMP rescued the defective motility of sperm from the epididymis of these mice (Joseph et al. 2010). A principal difference between Ex3αERKO and αERKO males was that seminiferous tubule dilation occurred as early as 10 days of age in Ex3αERKO mice, but not until later in αERKO mice (Eddy et al. 1996), suggesting that tubule dilation due to fluid accumulation begins even earlier in Ex3αERKO mice that are null for truncated ERα variants.
Sperm counts and sperm motility declined with age and were significantly lower in Ex3αERKO mice than in WT mice, comparable to what was observed in αERKO mice (Eddy et al. 1996). An age-dependent decline in sperm counts also was seen in ACTB-Cre/ERα−/− (Chen et al. 2009a), NERKI (Weiss et al. 2008), and ENERKI mice (Sinkevicius et al. 2009). Sperm motility was relatively unchanged with age in NERKI mice, suggesting that the hormone responsiveness is regulated by a gene tethering mechanism (Jakacka et al. 2002). The age-dependent infertility in ENERKI males was reminiscent of results in ArKO mice with a targeted disruption in the aromatase (Cyp19a1) gene. This might be expected because even though a full-length ERα is present in both ENERKI and ArKO mice, ArKO mice do not produce estrogen and the ERα in ENERKI mice cannot bind estrogen. Sperm motility was not evaluated in ACTB-Cre/ERα−/− mice, but sperm counts, sperm motility and fertility were normal in most ArKO males at 4.5 months (Robertson et al. 1999, Robertson et al. 2001), and fertility was reduced considerably by 3 months in ENERKI mice (Sinkevicius et al. 2009).
There was a significant increase in serum testosterone levels in Ex3αERKO males compared to WT males. This also was observed in αERKO (Eddy et al. 1996, Lindzey et al. 1998, Akingbemi et al. 2003), ACTB-Cre/ERα−/− (Chen et al. 2009a), NERKI (McDevitt et al. 2007, Weiss et al. 2008) and ENERKI mice (Sinkevicius et al. 2009). Although there was a slight increase in the level of Cyp19a1 transcripts in the testis of Ex3αERKO mice compared to WT mice, it was well below the level found in the ovary of WT mice. In addition, there was not a significant difference in serum estradiol levels in Ex3αERKO male mice compared to WT mice. This suggests the increased levels are due to the increased CYP17A1 and 17βHSD3 levels that result in a feed-forward effect on steroidogenesis. We observed in αERKO females similar elevated levels of serum testosterone and expression of these enzymes in thecal cells and found that estrogen and ERα participate in a short-loop-feedback gonadal regulation (Taniguchi et al. 2007). The elevated testosterone levels presumably were responsible for the increased seminal vesicle weights seen in Ex3αERKO, αERKO (Eddy et al. 1996, Lindzey et al. 1998), and ACTB-Cre/ERα−/− mice (Chen et al. 2009a).
The CYP17A1 enzyme is essential for the synthesis of androstenedione and the HSD17B3 enzyme converts androstenedione to testosterone. We found that the Cyp17a1 and Hsd17b3 mRNA levels were increased significantly in testes of Ex3αERKO mice. This also was seen in αERKO mice (Akingbemi et al. 2003). This increase in Hsd17b3 was consistent with the increased serum testosterone levels in Ex3αERKO and αERKO males, the reduced Leydig cell steroidogenic activity caused by DES treatment (Samuels et al. 1964, Majdic et al. 1996), the reduced CYP17A1 and estrogen levels in Leydig cells lacking estrogen sulfotransferase (Tong et al. 2004) and the elevated estrogen levels in transgenic AROM+ mice over-expressing human aromatase (Strauss et al. 2009). Crossing AROM+ and αERKO mice resulted in expression of normal levels of Cyp17a1 and Hsd17b3 mRNA expression in testes of offspring lacking full-length ERα, while expression of these transcripts remained low in offspring lacking estrogen receptor beta (ERβ) from crosses of AROM+ and βERKO mice. These results strongly suggest that estrogen regulates Leydig cell steroidogenesis through ERα (Strauss et al. 2009). In addition, the ovaries of αERKO females contained cells similar to Leydig cells and elevated levels of Cyp17a1 and Hsd17b3 mRNA, and the serum testosterone levels of these mice were comparable to the levels in wild type males (Couse et al. 2003, Couse et al. 2006, Taniguchi et al. 2007). Taken together, these results provide strong evidence that estrogen regulates Leydig cell steroidogenesis intragonadally through ERα (Akingbemi et al. 2003; Strauss et al. 2009).
Serum FSH and LH levels were not significantly different between Ex3αERKO and wild type males. The FSH levels were significantly higher in some studies in αERKO (Lindzey et al., 1998) and NERKI male mice (McDevitt et al. 2007, Weiss et al. 2008), but not in other studies in αERKO (Eddy et al. 1996, Akingbemi et al. 2003, Strauss et al. 2009), NERKI (Weiss et al. 2008) or ENERKI males (Sinkevicius et al. 2009). Moderate but significantly higher LH levels were seen in some studies in αERKO males (Lindzey et al. 1998, Akingbemi et al. 2003, Strauss et al. 2009) and elevated but not significantly higher levels of LH were seen in other studies in αERKO (Eddy et al. 1996), NERKI (Weiss et al. 2008), ENERKI (Sinkevicius et al. 2009) and ACTB-Cre/ERα−/− males (Chen et al. 2009a), compared to wild type males. The modest increases in LH levels in males contrasts with the high LH levels found in αERKO females (Couse et al. 2003). This is consistent with the suggestion that while ERα has a critical negative feedback role in regulating LH secretion in the female mouse, this may be mediated predominantly by the androgen receptor and largely independent of ERα in the adult male mouse (Lindzey et al. 1998).
Full length ERα was not detected in αERKO mice, but variant transcripts produced by utilization of a cryptic splice site in the neo sequence resulted in expression of a truncated form of ERα lacking the N-terminal A/B domain and containing the DNA- and ligand-binding domains (Couse et al. 1995, Dupont et al. 2000, Pendaries et al. 2002). These observations raised doubts that the αERKO mouse was a genuine null mutation and cast further doubts that the reproductive phenotype of αERKO males (Eddy et al. 1996) was due solely to the absence of full length ERα. However, Esr1 transcripts lacking the DNA-binding domain were observed in ERαKO (Dupont et al. 2000), ACTB-Cre/ERα−/− (Chen et al. 2009a) and Ex3αERKO mice (Hewitt et al. in press) and the males were infertile. In addition, ERα protein was not detected with antibodies to the N-terminal or C-terminal regions in ACTB-Cre/ERα−/− (Chen et al. 2009b), the N-terminal region in ERαKO (Dupont et al. 2000) or the N-terminal region in Ex3αERKO mice (Hewitt et al. in press). The results of the present study, along with those observed in ERαKO (Dupont et al. 2000) and ACTB-Cre/ERα−/− (Chen et al. 2009a) mice strongly suggest that the presence of a truncated ERα does not substantially affect the male reproductive phenotype.
In summary, these studies provided data not reported previously for male mice null for ERα due to a global deletion of exon 3 of Esr1. Not reported for ERαKO mice (Dupont et al. 2000; Joseph et al. 2010) were: 1) Testis and epididymis weights were significantly higher in six week-old and significantly lower in adult ERα-null mice compared to WT mice, 2) Epididymal sperm counts were significantly lower in six week-old and adult ERα-null mice compared to WT mice, 3) Motility of sperm from the cauda epididymis of ERα-null mice was significantly lower upon release and after one hour than of sperm from WT mice, 4) Testosterone levels were significantly higher in six week-old and adult ERα-null mice compared to WT mice, and 5) Expression of Hsd17b3 and Cyp17a1 were elevated significantly in the testis of ERα-null mice compared to WT mice. Not reported for ACTB-Cre/ERα−/− mice (Chen et al., 2009) were: 1) Testis and epididymis weights for 10 day-old, 20 day-old, and 6-week old ERα-null mice, 2) Combined seminal vesicle and coagulating gland weights for 6 week-old ERα-null mice, 3) Motility of sperm from the cauda epididymis of six week-old ERα-null mice, and 4) Levels of expression of Hsd17b3, Cyp17a1, and Cyp19a1 in testes of ERα-null mice.
Acknowledgements
Authors thank Mr. Linwood Koonce his excellent care of the animals, Mr. Ralph Wilson for blood collections and measuring serum hormone levels, other members of the Gamete Biology and Receptor Biology Groups for helpful discussions, Manas Ray and Masuo Goto for valuable comments on the manuscript and Manas Ray, Kelly Hartzell, and Gregory Scott from the NIEHS Knockout Mice Core for help in generating the Ex3αERKO mice.
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Z01ES70065 (KSK) and Z01ES70076 (EME).
Footnotes
Declaration of interest
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP. Estrogen receptor-α gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144:84–93. doi: 10.1210/en.2002-220292. [DOI] [PubMed] [Google Scholar]
- Chen M, Hsu I, Wolfe A, Radovick S, Huang K, Yu S, Chang C, Messing EM, Yeh S. Defects of prostate development and reproductive system in the estrogen receptor-alpha null male mice. Endocrinology. 2009a;150:251–259. doi: 10.1210/en.2008-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wolfe A, Wang X, Chang C, Yeh S, Radovick S. Generation and characterization of a complete null estrogen receptor α mouse using Cre/LoxP technology. Molecular Cell Biochemistry. 2009b;321:145–153. doi: 10.1007/s11010-008-9928-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Young P, Hess RA, Cunha GR. Estrogen receptor expression in developing epididymis, efferent ductules, and other male reproductive organs. Endocrinology. 1991;128:2874–2879. doi: 10.1210/endo-128-6-2874. [DOI] [PubMed] [Google Scholar]
- Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after target disruption of the estrogen receptor gene. Molecular Endocrinology. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocrine Reviews. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Molecular Endocrinology. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Rodriguez KR, Johnson JA, Poirier, Korach KS. The intraovarian actions of estrogen receptor-α are necessary to repress the formation of morphological and functional Leydig-like cells in the female gonad. Endocrinology. 2006;147:3666–3678. doi: 10.1210/en.2006-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptor alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Lubahn DB, Korach KS. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee K-H, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature. 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Kissling GE, Fieselman KE, Jaynes FL, Gerrish KE, Korach KS. Biological and biochemical consequences of global deletion of exon 3 from the ERα gene. FASEB Journal. 2010 doi: 10.1096/fj.10-163428. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Endocrinology. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- Jones RC, Jurd KM. Structural differentiation and fluid reabsorption in the ductule efferentes testis of the rat. Australian Journal of Biological Sciences. 1987;40:79–90. [PubMed] [Google Scholar]
- Joseph A, Hess RA, Schaeffer DJ, Ko CM, Hudgin-Spivey S, Chambon P, Shur BD. Absence of estrogen receptor alpha leads to physiological alterations in the mouse epididymis and consequent defects in sperm function. Biology of Reproduction. 2010;82:948–957. doi: 10.1095/biolreprod.109.079889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K-H, Park J-H, Bunick D, Lubahn DB, Bahr JM. Morphological comparison of the testis and efferent ductules between wild-type and estrogen receptor α knockout mice during postnatal development. Journal of Anatomy. 2009;214:916–925. doi: 10.1111/j.1469-7580.2009.01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, Korach KS. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotrophins in male wild-type and estrogen receptor-α knockout mice. Endocrinology. 1998;139:4092–4101. doi: 10.1210/endo.139.10.6253. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G, Sharpe RM, O’Schaughnessy PJ, Saunders PTK. Expression of cytochrome P450 17α-hydroxylase/C17–20 lyase in the fetal rat testis is reduced by maternal exposure to exogenous estrogens. Endocrinology. 1996;137:1063–1070. doi: 10.1210/endo.137.3.8603575. [DOI] [PubMed] [Google Scholar]
- Mahato D, Goulding EH, Korach KS, Eddy EM. Spermatogenic cells do not require estrogen receptor-alpha for development or function. Endocrinology. 2000;141:1273–1276. doi: 10.1210/endo.141.3.7439. [DOI] [PubMed] [Google Scholar]
- Mahato D, Goulding EH, Korach KS, Eddy EM. Estrogen receptor-alpha is required by the supporting somatic cells for spermatogenesis. Molecular and Cellular Endocrinology. 2001;178:57–63. doi: 10.1016/s0303-7207(01)00410-5. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenny C, Weiss J, Chambon P, Jameson JL, Levine JE. Estrogen response element-independent estrogen receptor (ER)-α signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERα knockout mice. Endocrinology. 2007;148:5288–5294. doi: 10.1210/en.2007-0673. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendaries C, Darblade B, Rochaix P, Krust A, Chambon P, Korach KS, Bayard F, Gaillard-Kelly M, Baron R. The AF-1 activation-function of ERα may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2205–2210. doi: 10.1073/pnas.042688499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Research. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- Robertson KM, O’Donnell L, Jones MEE, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan R, Simpson ER. Impairment of spermatogenesis in mice lacking a functional aromotase (cyp19) gene. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KM, Simpson ER, Lacham-Kaplan O, Jones MEE. Characterization of the fertility of male aromatase knockout mice. Journal of Andrology. 2001;22:825–830. [PubMed] [Google Scholar]
- Samuels L, Short JG, Huseby RA. The effects of diethylstilbesterol on testicular 17α-desmolase activities in Balb/c mice. Acta Endocrinologica. 1964;45:487–497. doi: 10.1530/acta.0.0450487. [DOI] [PubMed] [Google Scholar]
- Schleicher G, Drews U, Stumpf WE, Sar M. Differential distribution of dihydrotestosterone and estradiol binding sites in the epididymis of the mouse. Histochemistry. 1984;81:139–147. doi: 10.1007/BF00490107. [DOI] [PubMed] [Google Scholar]
- Sinkevicius KS, Burdette JE, Woloszyn, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, Greene GL. An estrogen receptor-α knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149:2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkevicius KS, Laine M, Lotan TL, Woloszyn K, Richburg JH, Greene GL. Estrogen-dependent and -independent estrogen receptor-α signaling separately regulate male fertility. Endocrinology. 2009;150:2898–2905. doi: 10.1210/en.2008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirianni R, Chimento A, Ruggiero C, De Luca A, Lappano R, Andò S, Maggioilina M, Pezzi V. The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17β-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology. 2008;149:5043–5051. doi: 10.1210/en.2007-1593. [DOI] [PubMed] [Google Scholar]
- Strauss L, Kallio J, Desai N, Pakarinen P, Miettinen T, Gylling H, Albrecht M, Mäkelä S, Mayerhofer A, Poutanen M. Increased exposure to estrogens disturbs maturation, steroidogenesis, and cholesterol homeostasis via estrogen receptor α in adult mouse Leydig cells. Endocrinology. 2009;150:2865–2872. doi: 10.1210/en.2008-1311. [DOI] [PubMed] [Google Scholar]
- Taniguchi F, Couse JF, Rodriguez KF, Emmen JMS, Poirier D, Korach KS. Estrogen receptor-α mediates an intraovarian negative feedback loop on thecal cell steroidogenesis via modulation of Cyp17a1 (cytochrome P450, steroid 17α-hydroxylase/17,20 lyase) expression. The FASEB Journal. 2007;21:586–595. doi: 10.1096/fj.06-6681com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong MH, Christenson LK, Song W-C. Aberrant cholesterol transport and impaired steroidogenesis in Leydig cells lacking estrogen sulfotransferase. Endocrinology. 2004;145:2487–2497. doi: 10.1210/en.2003-1237. [DOI] [PubMed] [Google Scholar]
- Veeramachaneni DNR, Amann RP, Palmer JS, Hinton BT. Proteins in luminal fluid of the ram excurrent ducts: changes in composition and evidence for differential endocytosis. Journal of Andrology. 1990;11:140–154. [PubMed] [Google Scholar]
- Weiss J, Bernhardt ML, Laronda MM, Hurley LA, Glidewell-Kenny, Pillai S, Tong M, Korach KS, Jameson JL. Estrogen actions in the male reproductive system involves estrogen response element-independent pathways. Endocrinology. 2008;149:6198–6206. doi: 10.1210/en.2008-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West NB, Brenner RM. Estrogen receptor in the ductuli efferentes, epididymis, and testis of rhesus and cynomolgus macaques. Biology of Reproduction. 1990;42:533–538. doi: 10.1095/biolreprod42.3.533. [DOI] [PubMed] [Google Scholar]