Abstract
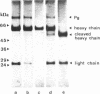
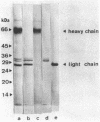
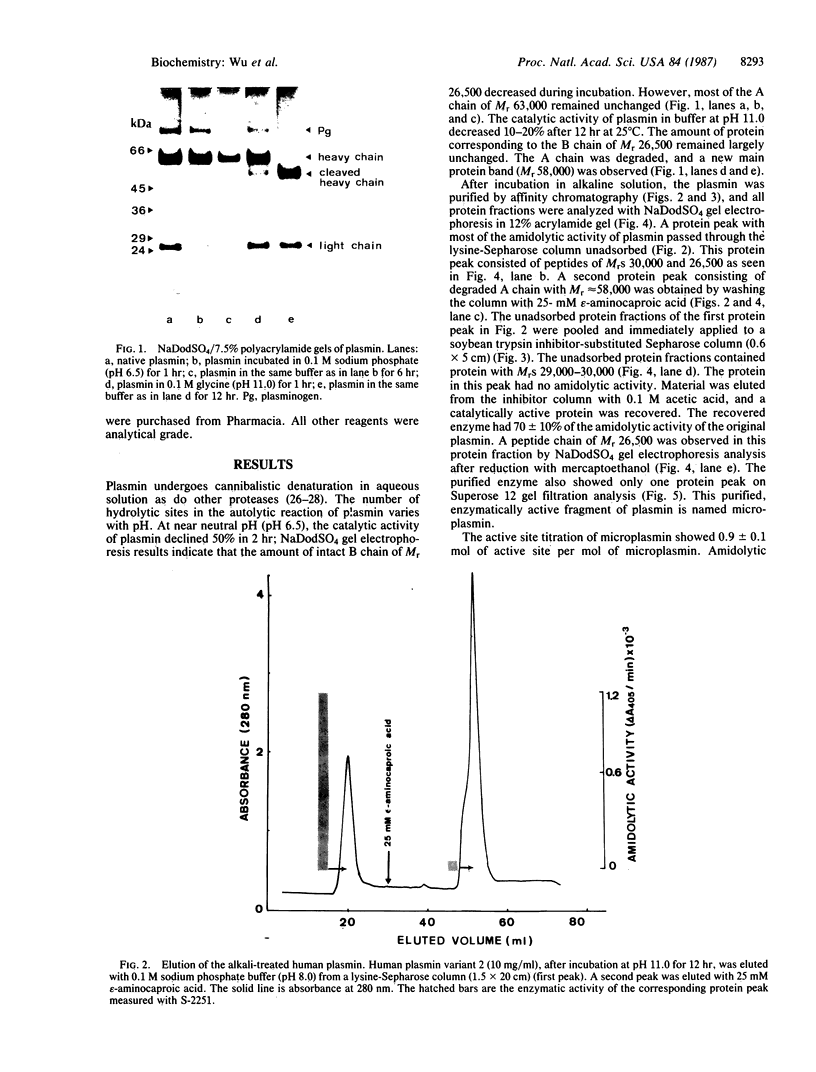
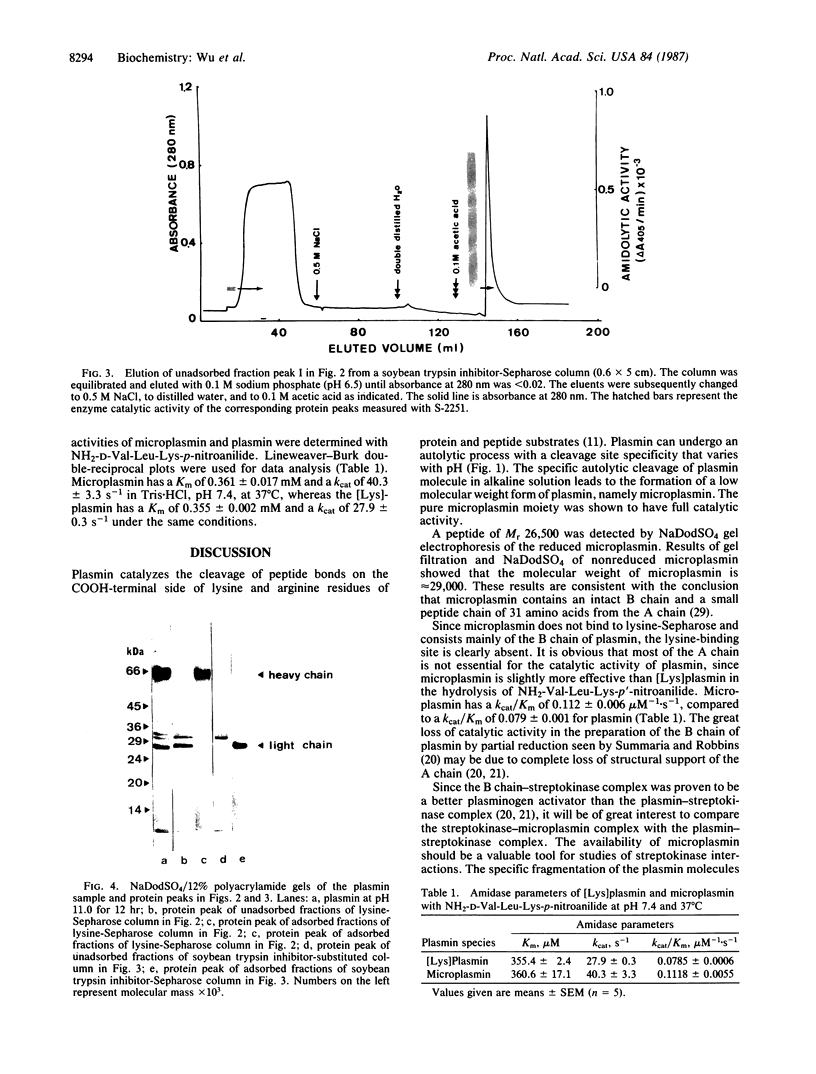
A catalytically active, human microplasmin was produced by incubation of [Lys]plasmin in buffer at pH 11.0 for up to 12 hr. The microplasmin was purified by affinity chromatography that used lysine-Sepharose and soybean trypsin inhibitor-Sepharose columns. It is homogeneous and pure by electrophoretic analysis in NaDodSO4/polyacrylamide gels and by gel filtration on a Superose 12 column. The molecular weight of the microplasmin determined by NaDodSO4 gel electrophoresis is 29,000 and 26,500 under reducing condition, whereas the molecular weight of native plasmin is 76,500. Microplasmin consists mainly of the ligh (B) chain of native human plasmin and possesses one active site per protein molecule when titrated with p-nitrophenyl p'-guanidinobenzoate. Microplasmin hydrolyzes the peptide substrate NH2-D-Val-Leu-Lys-p-nitroanilide (S-2251) with a Km of 0.361 +/- 0.017 mM and a kcat of 40.3 +/- 3.3 s-1 at pH 7.4 and 37 degrees C, whereas native plasmin has a Km of 0.355 +/- 0.002 mM and a kcat of 27.9 +/- 0.3 s-1 under the same conditions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow G. H., Summaria L., Robbins K. C. Molecular weight studies on human plasminogen and plasmin at the microgram level. J Biol Chem. 1969 Mar 10;244(5):1138–1141. [PubMed] [Google Scholar]
- Brockway W. J., Castellino F. J. Measurement of the binding of antifibrinolytic amino acids to various plasminogens. Arch Biochem Biophys. 1972 Jul;151(1):194–199. doi: 10.1016/0003-9861(72)90488-2. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. Comparison of the esterase activities of trypsin, plasmin, and thrombin on guanidinobenzoate esters. Titration of the enzymes. Biochemistry. 1969 May;8(5):2212–2224. doi: 10.1021/bi00833a063. [DOI] [PubMed] [Google Scholar]
- Christensen U., Sottrup-Jensen L., Magnusson S., Petersen T. E., Clemmensen I. Enzymic properties of the neo-plasmin-Val-422 (miniplasmin). Biochim Biophys Acta. 1979 Apr 12;567(2):472–481. doi: 10.1016/0005-2744(79)90133-5. [DOI] [PubMed] [Google Scholar]
- Claeys H., Sottrup-Jensen L., Zajdel M., Petersen T. E., Magnusson S. Multiple gene duplication in the evolution of plasminogen. Five regions of sequence homology with the two internally homologous structures in prothrombin. FEBS Lett. 1976 Jan 1;61(1):20–24. doi: 10.1016/0014-5793(76)80161-5. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Groskopf W. R., Summaria L., Robbins K. C. Studies on the active center of human plasmin. Partial amino acid sequence of a peptide containing the active center serine residue. J Biol Chem. 1969 Jul 10;244(13):3590–3597. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Paoni N. F., Castellino F. J. Isolation of a low molecular weight form of plasminogen. Biochem Biophys Res Commun. 1975 Jul 22;65(2):757–764. doi: 10.1016/s0006-291x(75)80210-5. [DOI] [PubMed] [Google Scholar]
- Paoni N. F., Violand B. N., Castellino F. J. Isolation and characterization of native and lower molecular weight forms of sheep plasminogen. J Biol Chem. 1977 Nov 10;252(21):7725–7732. [PubMed] [Google Scholar]
- Powell J. R., Castellino F. J. Activation of human neo-plasminogen-Val442 by urokinase and streptokinase and a kinetic characterization of neoplasmin-Val442. J Biol Chem. 1980 Jun 10;255(11):5329–5335. [PubMed] [Google Scholar]
- Rickli E. E., Cuendet P. A. Isolation of plasmin-free human plasminogen with N-terminal glutamic acid. Biochim Biophys Acta. 1971 Nov 13;250(2):447–451. doi: 10.1016/0005-2744(71)90202-6. [DOI] [PubMed] [Google Scholar]
- Rickli E. E., Otavsky W. I. Release of an N-terminal peptide from human plasminogen during activation with urokinase. Biochim Biophys Acta. 1973 Jan 25;295(1):381–384. doi: 10.1016/0005-2795(73)90106-2. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Bernabe P., Arzadon L., Summaria L. The primary structure of human plasminogen. II. The histidine loop of human plasmin: light (B) chain active center histidine sequence. J Biol Chem. 1973 Mar 10;248(5):1631–1633. [PubMed] [Google Scholar]
- Robbins K. C., Summaria L., Hsieh B., Shah R. J. The peptide chains of human plasmin. Mechanism of activation of human plasminogen to plasmin. J Biol Chem. 1967 May 25;242(10):2333–2342. [PubMed] [Google Scholar]
- Summaria L., Boreisha I., Wohl R. C., Robbins K. C. Recombinant human Lys-plasmin and the Lys-plasmin . streptokinase complex. J Biol Chem. 1979 Jul 25;254(14):6811–6814. [PubMed] [Google Scholar]
- Summaria L., Hsieh B., Groskopf W. R., Robbins K. C. The isolation and characterization of the S-carboxymethyl beta (light) chain derivative of human plasmin. The localization of the active site on the beta (light) chain. J Biol Chem. 1967 Nov 10;242(21):5046–5052. [PubMed] [Google Scholar]
- Summaria L., Hsieh B., Robbins K. C. The specific mechanism of activation of human plasminogen to plasmin. J Biol Chem. 1967 Oct 10;242(19):4279–4283. [PubMed] [Google Scholar]
- Summaria L., Robbins K. C. Isolation of a human plasmin-derived, functionally active, light (B) chain capable of forming with streptokinase an equimolar light (B) chain-streptokinase complex with plasminogen activator activity. J Biol Chem. 1976 Sep 25;251(18):5810–5813. [PubMed] [Google Scholar]
- Violand B. N., Castellino F. J. Mechanism of the urokinase-catalyzed activation of human plasminogen. J Biol Chem. 1976 Jul 10;251(13):3906–3912. [PubMed] [Google Scholar]
- Wu H. L., Kundrot C., Bender M. L. The denaturation of trypsin. Biochem Biophys Res Commun. 1982 Jul 30;107(2):742–745. doi: 10.1016/0006-291x(82)91553-4. [DOI] [PubMed] [Google Scholar]
- Wu H. L., Wastell A., Bender M. L. Ageing of alpha-chymotrypsin: Cannibalistic and hydroxide ion reactions. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4116–4117. doi: 10.1073/pnas.78.7.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies L. D., Kaplan A. P. Partial characterization of a low molecular weight fragment derived from human plasminogen. Thromb Res. 1979;14(4-5):729–738. doi: 10.1016/0049-3848(79)90128-2. [DOI] [PubMed] [Google Scholar]