Abstract
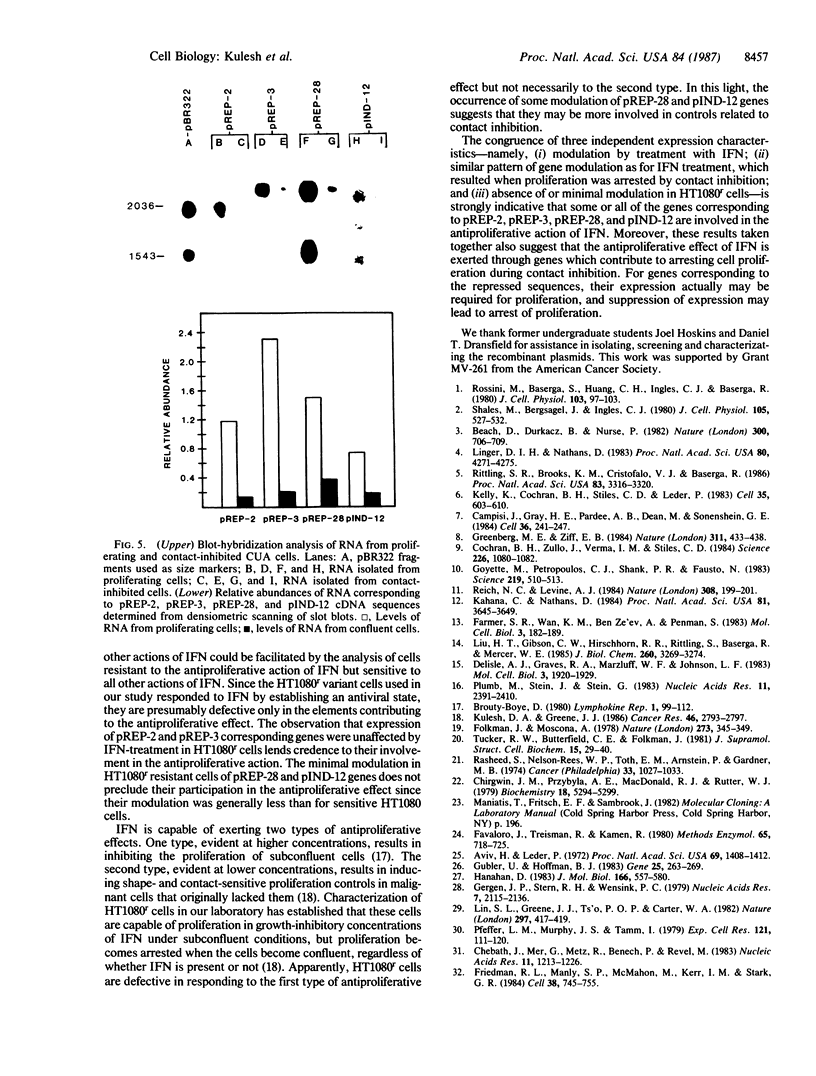
To identify genes mediating the antiproliferative action of interferon (IFN), two cDNA libraries were constructed with mRNA from IFN-treated and untreated human fibrosarcoma (HT1080) cells previously shown to be highly sensitive to the antiproliferative effects of IFN. Differential screening of these two libraries identified cloned sequences whose expression was either induced or repressed with IFN treatment. Rescreening of these sequences with cDNA probes constructed from proliferating or quiescent cells led to the identification of one IFN-induced and three IFN-repressed sequences whose expressions also appeared to be modulated by cell proliferation. Blot-hybridization analysis revealed that RNA levels corresponding to the three repressed genes decreased when HT1080 cells were treated with IFN or when proliferation of normal CUA foreskin fibroblast cells became naturally arrested by contact inhibition. Levels of RNA corresponding to the induced gene increased in HT1080 cells within 24 hr after IFN-treatment but declined below basal levels by 48 hr. Expression of these genes was unaffected or only slightly affected by IFN treatment in variant cells resistant to the antiproliferative effects of IFN. Collectively, these results suggest that the identified cDNAs correspond to genes that are involved in the antiproliferative action of IFN. Moreover, these results also suggest that IFN's antiproliferative action may be exerted through genes that contribute to arresting cell proliferation during contact inhibition.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach D., Durkacz B., Nurse P. Functionally homologous cell cycle control genes in budding and fission yeast. Nature. 1982 Dec 23;300(5894):706–709. doi: 10.1038/300706a0. [DOI] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Chebath J., Merlin G., Metz R., Benech P., Revel M. Interferon-induced 56,000 Mr protein and its mRNA in human cells: molecular cloning and partial sequence of the cDNA. Nucleic Acids Res. 1983 Mar 11;11(5):1213–1226. doi: 10.1093/nar/11.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Gergen J. P., Stern R. H., Wensink P. C. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 1979 Dec 20;7(8):2115–2136. doi: 10.1093/nar/7.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Expression of a cellular oncogene during liver regeneration. Science. 1983 Feb 4;219(4584):510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Isolation of cloned cDNA encoding mammalian ornithine decarboxylase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3645–3649. doi: 10.1073/pnas.81.12.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kulesh D. A., Greene J. J. Shape-dependent regulation of proliferation in normal and malignant human cells and its alteration by interferon. Cancer Res. 1986 Jun;46(6):2793–2797. [PubMed] [Google Scholar]
- Lin S. L., Greene J. J., Ts'o P. O., Carter W. A. Sensitivity and resistance of human tumor cells to interferon and rIn . rCn. Nature. 1982 Jun 3;297(5865):417–419. doi: 10.1038/297417a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. T., Gibson C. W., Hirschhorn R. R., Rittling S., Baserga R., Mercer W. E. Expression of thymidine kinase and dihydrofolate reductase genes in mammalian ts mutants of the cell cycle. J Biol Chem. 1985 Mar 25;260(6):3269–3274. [PubMed] [Google Scholar]
- Pfeffer L. M., Murphy J. S., Tamm I. Interferon effects on the growth and division of human fibroblasts. Exp Cell Res. 1979 Jun;121(1):111–120. doi: 10.1016/0014-4827(79)90450-6. [DOI] [PubMed] [Google Scholar]
- Plumb M., Stein J., Stein G. Coordinate regulation of multiple histone mRNAs during the cell cycle in HeLa cells. Nucleic Acids Res. 1983 Apr 25;11(8):2391–2410. doi: 10.1093/nar/11.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Rittling S. R., Brooks K. M., Cristofalo V. J., Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(10):3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini M., Baserga S., Huang C. H., Ingles C. J., Baserga R. Changes in RNA polymerase II in a cell cycle-specific temperature-sensitive mutant of hamster cells. J Cell Physiol. 1980 Apr;103(1):97–103. doi: 10.1002/jcp.1041030114. [DOI] [PubMed] [Google Scholar]
- Shales M., Bergsagel J., Ingles C. J. Defective RNA polymerase II in the G1 specific temperature sensitive hamster cell mutant TsAF8. J Cell Physiol. 1980 Dec;105(3):527–532. doi: 10.1002/jcp.1041050317. [DOI] [PubMed] [Google Scholar]
- Tucker R. W., Butterfield C. E., Folkman J. Interaction of serum and cell spreading affects the growth of neoplastic and non-neoplastic fibroblasts. J Supramol Struct Cell Biochem. 1981;15(1):29–40. doi: 10.1002/jsscb.1981.380150104. [DOI] [PubMed] [Google Scholar]