Abstract
Scrape loading and sonication loading are two recently described methods of introducing macromolecules into living cells. We have tested the efficacy of these methods for transfection of mammalian cells with exogenous DNA, using selection systems based either on resistance to the drug G418 (Geneticin) or on acquisition of the ability to utilize the salvage pathway of pyrimidine biosynthesis. These loading methods can be employed to generate cell lines that express the gene product of the transfected DNA molecules both transiently and stably. Optimal transfection is observed when the DNA is added to cells in physiological saline lacking divalent cations and containing K+ in place of Na+. DNA molecules 7.1 to 30 kilobases long have been introduced by the scrape loading procedure. In addition, the scrape loading procedure has been employed for cotransfection and subsequent expression of nonselectable genes encoded on DNA molecules added in a mixture with DNA molecules whose expression is selected. Cell lines expressing oncogenes or proteins that are important for regulation of cell growth and division have been obtained by this procedure. The scrape loading procedure is also useful for studies of the cellular changes that occur upon expression of an exogenous gene. As many as 80% of cells scrape loaded with the plasmid pC6, which encodes the simian virus 40 large tumor antigen, contained this protein in the nucleus between 1 and 5 days after transfection. Thus, scrape loading and sonication loading are simple, economical, and reproducible methods for introduction of DNA molecules into adherent and nonadherent cells, and these methods may be useful in the future for experimentation at both fundamental and applied levels.
Full text
PDF

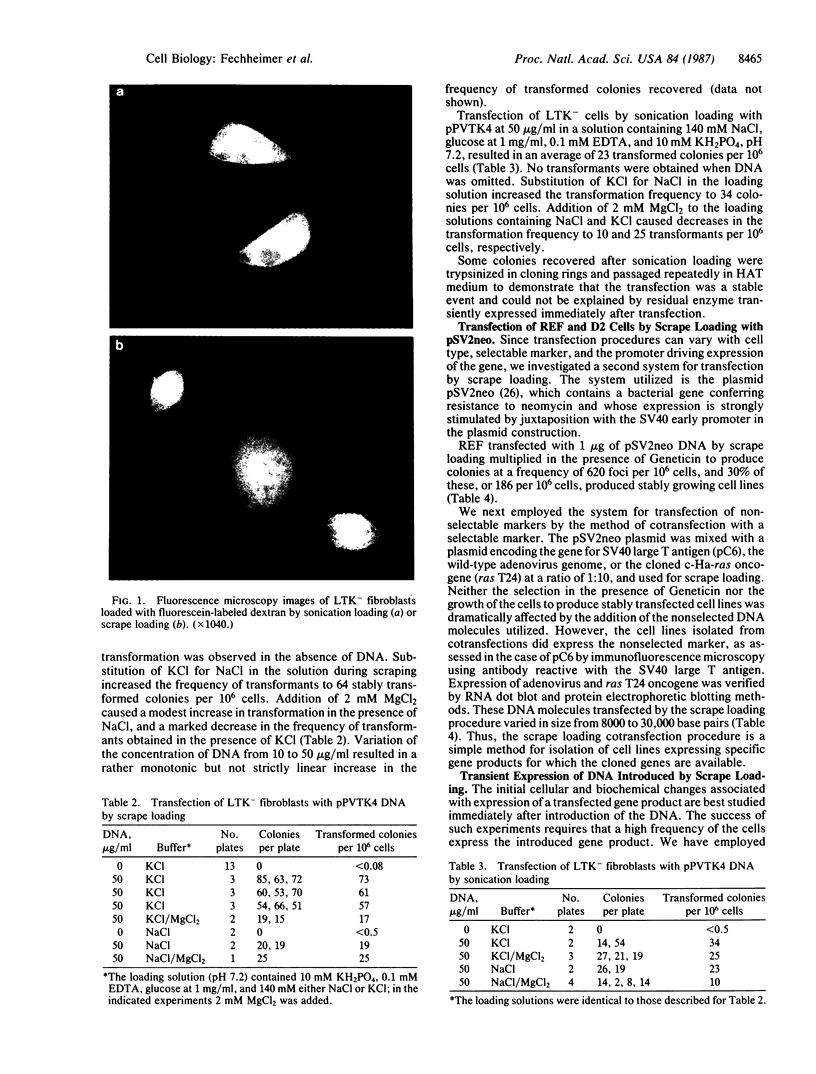


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Barton K. A., Brill W. J. Prospects in plant genetic engineering. Science. 1983 Feb 11;219(4585):671–682. doi: 10.1126/science.6297007. [DOI] [PubMed] [Google Scholar]
- Borle A. B., Freudenrich C. C., Snowdowne K. W. A simple method for incorporating aequorin into mammalian cells. Am J Physiol. 1986 Aug;251(2 Pt 1):C323–C326. doi: 10.1152/ajpcell.1986.251.2.C323. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Celis J. E. Microinjection of somatic cells with micropipettes: comparison with other transfer techniques. Biochem J. 1984 Oct 15;223(2):281–291. doi: 10.1042/bj2230281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshayes A., Herrera-Estrella L., Caboche M. Liposome-mediated transformation of tobacco mesophyll protoplasts by an Escherichia coli plasmid. EMBO J. 1985 Nov;4(11):2731–2737. doi: 10.1002/j.1460-2075.1985.tb03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacumakos E. G. Methods for micromanipulation of human somatic cells in culture. Methods Cell Biol. 1973;7:287–311. doi: 10.1016/s0091-679x(08)61783-5. [DOI] [PubMed] [Google Scholar]
- Doxsey S. J., Sambrook J., Helenius A., White J. An efficient method for introducing macromolecules into living cells. J Cell Biol. 1985 Jul;101(1):19–27. doi: 10.1083/jcb.101.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechheimer M., Denny C., Murphy R. F., Taylor D. L. Measurement of cytoplasmic pH in Dictyostelium discoideum by using a new method for introducing macromolecules into living cells. Eur J Cell Biol. 1986 Apr;40(2):242–247. [PubMed] [Google Scholar]
- Fechheimer M., Taylor D. L. Introduction of exogenous molecules into the cytoplasm of Dictyostelium discoideum amoebae by controlled sonication. Methods Cell Biol. 1987;28:179–190. doi: 10.1016/s0091-679x(08)61644-1. [DOI] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Pursel V. G., Rexroad C. E., Jr, Wall R. J., Bolt D. J., Ebert K. M., Palmiter R. D., Brinster R. L. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985 Jun 20;315(6021):680–683. doi: 10.1038/315680a0. [DOI] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature. 1986 Mar 20;320(6059):275–277. doi: 10.1038/320275a0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Krippl B., Ferguson B., Rosenberg M., Westphal H. Functions of purified E1A protein microinjected into mammalian cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6988–6992. doi: 10.1073/pnas.81.22.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., Murphy R. F., Lanni F., Taylor D. L. A method for incorporating macromolecules into adherent cells. J Cell Biol. 1984 Apr;98(4):1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada C. Y., Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982 May;29(1):33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Huang L. Interaction of phospholipid vesicles with cultured mammalian cells. II. Studies of mechanism. J Cell Biol. 1975 Oct;67(1):49–60. doi: 10.1083/jcb.67.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R. A., Rechsteiner M. C. Microinjection of thymidine kinase and bovine serum albumin into mammalian cells by fusion with red blood cells. Cell. 1975 Aug;5(4):371–379. doi: 10.1016/0092-8674(75)90056-2. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Weintraub H., Cheng P. F., Conrad K. Expression of transfected DNA depends on DNA topology. Cell. 1986 Jul 4;46(1):115–122. doi: 10.1016/0092-8674(86)90865-2. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]