Abstract
The resinous portions of Aquilaria plants, called agarwood, have been used as medicines and incenses. Agarwood contains a great variety of sesquiterpenes, and a study using cultured cells of Aquilaria showed the production of sesquiterpenes (α-guaiene, α-humulene, and δ-guaiene) to be induced by treatment with methyl jasmonate (MJ). In this study, the accumulation and production of sesquiterpenes were quantified. The amounts accumulated and produced reached a maximum at 12 h, and the most abundant product was α-humulene at 6 h and δ-guaiene after 12 h. However, a headspace analysis of the cells revealed that α-humulene is likely to be volatilized; so overall, the most abundant sesquiterpene in the cells was δ-guaiene. A cDNA library from RNA isolated from MJ-treated cells was screened using PCR methodologies to isolate five clones with very similar amino acid sequences. These clones were expressed in Escherichia coli, and enzymatic reactions using farnesyl pyrophosphate revealed that three of the clones yielded the same compounds as extracted from MJ-treated cells, the major product being δ-guaiene. These genes and their encoded enzymes are the first sesquiterpene synthases yielding guaiane-type sesquiterpenes as their major products to be reported. Expression of a fourth terpene synthase gene in bacteria resulted in the accumulation of the protein in insoluble forms. Site-directed mutagenesis of the inactive clone and three-dimensional homology modeling suggested that the structure of the N-terminal domain was important in facilitating proper folding of the protein to form a catalytically active structure.
The genera Aquilaria and Gyrinops of the Thymelaeaceae are large evergreen trees found mainly in Southeast Asia. The resinous portions of their branches and trunks, known as agarwood, have been used in natural medicine as a digestive, sedative, and antiemetic, and also as incense. However, the source of agarwood is facing serious depletion because of uncontrolled collection in forests and the rapid loss of tropical rain forests. Consequently, Aquilaria and Gyrinops have been listed in Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora since 2005 (Ito and Honda, 2008), and the international import and export of their products are under strict control.
The main fragrant compounds of agarwood are sesquiterpenes and phenylethyl chromone derivatives, and a great variety of sesquiterpenes are contained in high-quality agarwood (Varma et al., 1963; Nakanishi et al., 1981; Hashimoto et al., 1985; Ishihara et al., 1993; Yagura et al., 2003). Terpenoids form one of the largest and most diverse groups of natural products, mainly composed of monoterpene (C10), sesquiterpene (C15), diterpene (C20), and triterpene (C30) compounds, and these compounds and their derivatives include valuable natural products used for pharmaceuticals or perfumes. Their diverse cyclic and acyclic structures are known to be synthesized from prenyl diphosphates or squalene by various terpene synthases (TPSs); a few species of substrates are transformed into a number of terpenoids with various structural types. Mechanisms of reaction control and structures of enzymes are studied by many groups trying to clarify the structure-function relationship in TPSs (Steele et al., 1998; Felicetti and Cane, 2004; Deguerry et al., 2006; Landmann et al., 2007; Lee and Chappell, 2008). The resinous portion of agarwood is rich in sesquiterpenes such as guaiane, eudesmane, and their oxidized forms, jinkoh-eremol and agarospirol, both of which are peculiar to agarwood and are known to have sedative and analgesic effects (Okugawa et al., 1996, 2000). Our recent studies on the effects of the fragrance of agarwood on mice by inhalation suggest that these compounds might induce sedative effect (Takemoto et al., 2008) and thus have promise for therapeutic applications.
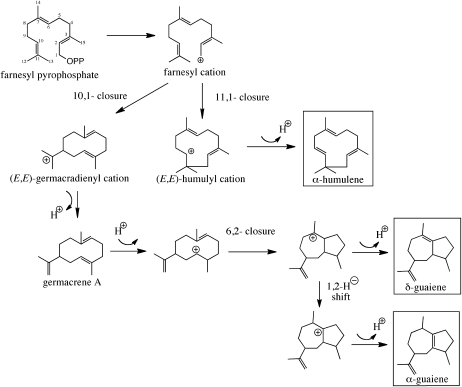
However, the biosynthetic pathways responsible for these constituents are currently unknown. As a model of plants producing oleoresins, conifers have been used to study the mechanisms by which the resin forms, including the biosynthesis of terpenoids. It has been reported that both the resin and terpenoids are produced in response to biotic and abiotic stress (Lewinsohn et al., 1991; Bohlmann et al., 1998a; Martin et al., 2002; Fäldt et al., 2003). The sesquiterpenes found in agarwood are also considered to be produced as phytoalexins under stress, but there are no reports about the biosynthesis of fragrant compounds in agarwood. This is in part because all agarwood-producing plants are timber species that require considerable time to grow, the resinous portions are formed inside of the wood, and the cells thought to contain the relevant enzymes are difficult to obtain. Because studies with fresh plants are difficult, we used cell cultures derived from the leaves of Aquilaria sp. Treatment with methyl jasmonate (MJ), an elicitor of plant defensive responses, was performed to determine if fragrant-like compounds accumulated, and three species of sesquiterpene (α-guaiene, α-humulene, and δ-guaiene) were found to be induced in the cultured cells (Ito et al., 2005; Okudera and Ito, 2009). Guaiane-type sesquiterpenes are thought to be synthesized via two cyclization reactions, the first constituting a C1-to-C10 cyclization, yielding a macrocyclic germacrene-like intermediate, and the second cyclization event occurring between C2 and C6 to generate the guaiene product (Fig. 1). Although guaiane-type sesquiterpenes are common in nature and a few enzymes described as producing guaianes as secondary reaction products have been described (Steele et al., 1998; Deguerry et al., 2006), an enzyme catalyzing the formation of this class of sesquiterpenes as its dominant reaction product has yet to be described. Guaiane-type structures are unique, being composed of five- and seven-membered rings. Given the unique five- and seven-membered ring systems in guaiane sesquiterpenes, the identification of the corresponding TPSs responsible for their biosynthesis should provide yet another tool for the molecular dissection of TPSs in general and possibly provide another perspective on the evolution and diversity of TPSs found in nature.
Figure 1.
Putative biosynthetic pathways for sesquiterpenes found in agarwood and cell suspension cultures.
RESULTS
Quantification of the Sesquiterpenes Produced in the MJ-Treated Cells and Enzymatic Activities of Crude Cell Extracts from MJ-Treated Cells
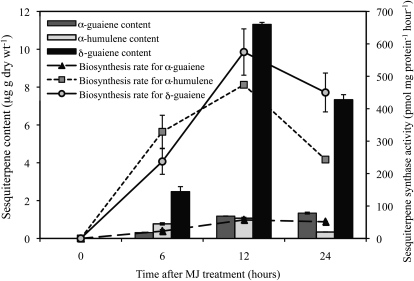
Our previous study showed that MJ induced the production of three species of sesquiterpenes (α-guaiene, α-humulene, and δ-guaiene; Fig. 1) in cultured cells of Aquilaria crassna (Okudera and Ito, 2009). Here, the amount of sesquiterpene produced in response to MJ was examined over a 24-h period. There was a temporary increase in the amount of sesquiterpene in the cells, though little accumulated in untreated control cells (Fig. 2). The total amount of sesquiterpenes accumulating reached a maximum at 12 h then decreased, of which δ-guaiene was the most abundant. Oxidized sesquiterpenes were not detected.
Figure 2.
Time course of sesquiterpene content and sesquiterpene synthase activities in cultured cells after treatment with MJ. Columns are for sesquiterpene content, and line graphs show sesquiterpene synthase activities. Data are the means ± se of duplicate or triplicate analyses.
The activities of TPSs in the crude extracts of control and MJ-treated cells were observed over 24 h as for the quantification of sesquiterpenes. The activity levels increased, reached a maximum at 12 h, and then gradually decreased in the extracts of MJ-treated cells, while no activity was detected in the control extracts, similar to the profile of sesquiterpene accumulating (Fig. 2). The most abundant of the products was α-humulene in the first 6 h, while δ-guaiene biosynthesis dominated thereafter. The biosynthesis rate for α-humulene was greater than α-guaiene during the entire period. Together, these results suggest that the different sesquiterpenes are synthesized and accumulate at different rates after the MJ treatment.
Analysis of Volatile Compounds in the Headspace of the Culture
The most abundant sesquiterpene accumulated in the MJ-treated cells was δ-guaiene, whereas the major product of the crude extracts was α-humulene at 6 h after MJ treatment. To clarify the reason of this difference, the volatile compounds emitted from MJ-treated cells were analyzed by solid-phase microextraction (SPME)-gas chromatography (GC)-mass spectrometry (MS) using an airtight container, and the major product was revealed to be α-humulene (α-guaiene, 13%; α-humulene, 61%; δ-guaiene, 26%). These results confirmed that α-humulene was the major compound produced by MJ-treated cells, at least during the first 6 h after treatment, but was quickly emitted; therefore, the most abundant compound in the cells was δ-guaiene.
Construction of cDNA Library and Sequencing
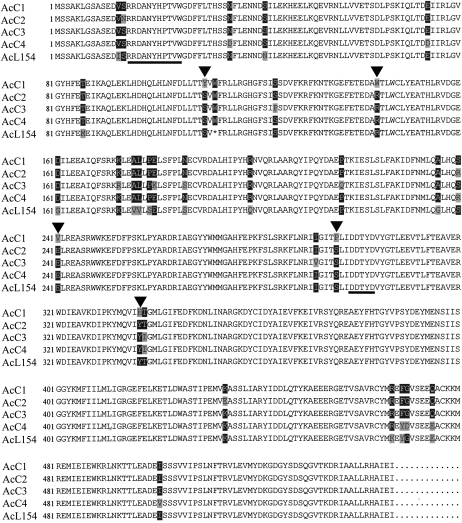
Because our findings suggested that unique TPSs might be selectively activated in the MJ-treated cells, a cDNA library was constructed from the cells to isolate the genes responsible for the sesquiterpene biosynthesis. Two hundred seventy-four clones randomly selected from the cDNA library were sequenced and their function deduced using the BLASTP algorithm. One clone, AcL154, shared 40% identity in amino acid sequence with the germacrene D synthase from Vitis vinifera (GenBank accession no. AY561842; Lücker et al., 2004). AcL154 contains an open reading frame of 1641 bp encoding a protein of 547 amino acids harboring motifs highly conserved among TPSs, such as the RPx8W motif (Bohlmann et al., 1998b) at the N terminus and the DDxxD motif (Starks et al., 1997; Whittington et al., 2002) known to be a divalent metal-ion substrate-binding site (Fig. 3). However, AcL154 does not have the transit peptide usually found in monoterpene synthases at the N terminus nor sequences specific to diterpene synthases (Bohlmann et al., 1998b), consistent with it encoding for a sesquiterpene synthase.
Figure 3.
Alignment of deduced amino acid sequences of sesquiterpene synthases from cultured cells of A. crassna. Shading indicates levels of sequence conservation (100%, black on white; over 50%, white on black; under 50%, black on gray). Triangles indicate residues mutated in this study. The conserved RPx8W and DDxxD motifs are underlined.
Isolation and Functional Expression of Sesquiterpene Synthase from Cultured Cells of Aquilaria
Although AcL154 harbors start and stop codons at appropriate locations, it has an extra stop codon (bp 334 approximately 336 from the 5′ end). Therefore, a pair of primers was designed according to the sequence of AcL154 and used for PCR with cDNA derived from the MJ-treated cells of Aquilaria as a template. Four clones (AcC1–AcC4) of the same length as AcL154 were obtained, and their sequences turned out to be almost the same as the AcL154 sequence, with a difference of 17 to 19 amino acids (Fig. 3).
In an attempt to express and functionally characterize AcL154 as well as AcC1 to AcC4, the stop codon of AcL154 was substituted with that for Trp, which is the conserved residue at this position among AcC1 to AcC4. Then, AcC1 to AcC4 and AcL154’ were expressed in Escherichia coli [BL21 (DE3) Codon Plus-RIL] for functional characterization. Heterologously expressed proteins were visualized by SDS-PAGE (data not shown), but the AcC1 protein was detected only in the insoluble fraction, and even attempts to be in the soluble fraction with various species of E. coli were unsuccessful. All clones except AcC1 were functionally characterized.
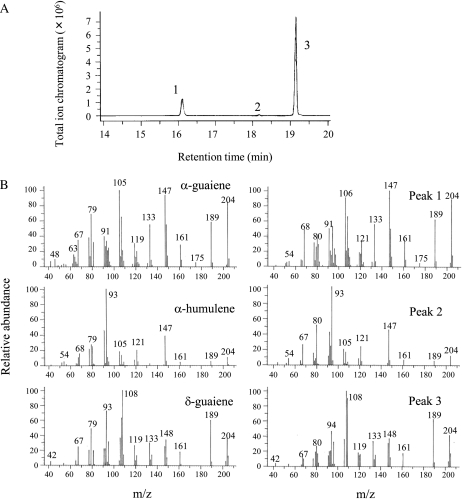
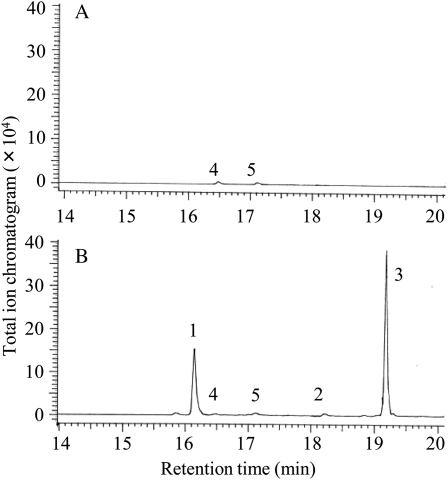
The enzyme assays were performed by using farnesyl pyrophosphate (FPP) as a substrate, and the reaction products were analyzed by GC-MS. All clones except AcC1 yielded the same compounds (α-guaiene, α-humulene, and δ-guaiene), which are those accumulated in the MJ-treated cells, and the major product was δ-guaiene (Fig. 4; Table I). The assays that did not include silica in the workup confirmed that these cyclases did not generate sesquiterpene alcohols. These results suggest that these TPSs are expressed in the MJ-treated cells and contribute to the biosynthesis of particular sesquiterpenes that accumulate.
Figure 4.
GC-MS profiles. A, Total ion chromatogram of the products formed by sesquiterpene synthases (AcC2) with FPP as a substrate. B, Mass spectra of the sesquiterpenes and their authentic standards. GC-MS profiles of control assay are provided in Supplemental Figure S1.
Table I. Products of sesquiterpene synthases expressed in E. coli.
| Clone Name | Total Products | Kinetic Parameters | ||||
| α-Guaiene | α-Humulene | δ-Guaiene | kcat | Km | kcat/Km | |
| % | s−1 | μm | s−1 mm−1 | |||
| AcC2 | 18.1 | 0.7 | 81.2 | 4.99 × 10−3 | 2.71 | 1.836 |
| AcC3 | 44.6 | 1.7 | 53.7 | 9.72 × 10−5 | 0.51 | 0.189 |
| AcC4 | 20.9 | 0.9 | 78.2 | 7.34 × 10−3 | 3.05 | 2.404 |
| AcL154’ | 20.8 | 0.7 | 78.5 | 1.58 × 10−5 | 0.45 | 0.035 |
Kinetic characterization of these clones revealed that AcC2 and AcC4 showed similar kinetic profile, but Km values of AcC3 and AcL154 were one-sixth and one-seventh, and the catalytic efficiencies (kcat/Km) were one-thirteenth and one-seventieth of those of AcC4, respectively (Table I). However, Km values of these clones were within the range of those previously reported for sesquiterpene synthases (0.1–10 μm; Cane, 1999).
Confirmation of Induction of TPS Gene Expression by MJ Treatment in Cultured Cells
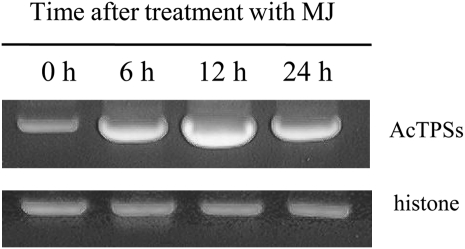
A quantitative reverse transcription (RT)-PCR method was employed to measure the transcript levels for the TPS genes in MJ-treated cells (Fig. 5). Primer pairs were designed specifically for these TPSs and histone as an internal control. Similar to the results above, the transcript levels for TPS genes were elevated by MJ treatment, reached a maximum at 12 h, and then decreased.
Figure 5.
RT-PCR analysis of A. crassna TPS genes 0, 6, 12, and 24 h after the MJ treatment. Amplification of the histone fragment was used as the PCR control.
Mutagenesis of AcC1
AcC1 expression, unsuccessful in E. coli, was carried out in an in vitro system to investigate whether the clone had TPS activities. The protein of AcC1 was visualized on a membrane by western blotting to confirm successful expression in vitro system (data not shown) and was subjected to an enzymatic reaction to reveal that it did not have any TPS activity with FPP or geranyl pyrophosphate as a substrate (Fig. 6).
Figure 6.
GC-MS profiles. A, Total ion chromatogram of products formed by AcC1 with FPP as a substrate. B, Total ion chromatogram of products formed by M42 with FPP as a substrate. GC-MS profiles of control assay are provided in Supplemental Figure S2.
AcC1, which alone was inactive among the four clones, was mutated differently from active clones at five amino acids, 110Y, 144W, 241V, 296P, and 337H (Fig. 3). These five were replaced with the corresponding amino acids in the active clones so as to investigate their individual contributions to the enzymatic activity. Possible single, double, triple, quadruple, and quintuple mutants of AcC1 were constructed, and Table II shows the quadruple and quintuple mutants among them. Expression of all mutants and enzymatic reactions revealed that only M42 and M50 had activity for sesquiterpene synthesis to almost the same product ratio as AcC2 to AcC4 and almost the same catalytic efficiencies (kcat/Km) as AcC3 (Fig. 6; Table II). These results suggest that the mutations Y110S, V241E, P296S, and H337Y are important to the activity for sesquiterpene synthesis.
Table II. Scheme of the mutants of AcC1 and their TPS activities.
Kinetic parameters are measured by using proteins expressed in E. coli. X indicates mutation sites included in each clone.
| Clone Name | Mutation Sites | Total Products | Kinetic Parameters | ||||||||
| Y110S | W144R | V241E | P296S | H337Y | α-Guaiene | α-Humulene | δ-Guaiene | kcat | Km | kcat/Km | |
| % | s−1 | μm | s−1 mm−1 | ||||||||
| M41 | X | X | X | X | – | – | – | – | – | – | |
| M42 | X | X | X | X | 34.1 | 1.3 | 64.6 | 1.25 × 10−3 | 6.85 | 0.182 | |
| M43 | X | X | X | X | – | – | – | – | – | – | |
| M44 | X | X | X | X | – | – | – | – | – | – | |
| M45 | X | X | X | X | –a | – | –a | – | – | – | |
| M50 | X | X | X | X | X | 31.1 | 1.5 | 67.4 | 5.86 × 10−4 | 4.29 | 0.137 |
The amount of the products is under the detection limit.
Comparison of AcC1 and AcC2 in 3D Homology Models
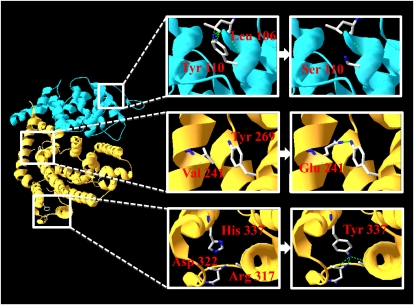
X-ray crystallography revealed the structure of 5-epi-aristolochene synthase from Nicotiana tabacum (Starks et al., 1997), trichodiene synthase from Fusarium sporotrichoides (Rynkiewicz et al., 2001), pentalenene synthase from Streptomyces UC5319 (Lesburg et al., 1997), and aristolochene synthase from Penicillium roqueforti (Caruthers et al., 2000). These vegetative sesquiterpene synthases share almost the same three-dimensional structure despite the absence of any significant sequence similarity (Greenhagen and Chappell, 2001). The locations of four amino acids necessary for restoration of the enzymatic activities were examined in models of AcC1 and AcC2 created using Deep View/Swiss-PDBViewer and SWISS-MODEL based on the structure of 5-epi-aristolochene synthase from N. tabacum (Protein Data Bank ID code 5EAT; Fig. 7). The 5-epi-aristolochene synthase is made up of α-helices and short connecting loops and turns and is separated into two structural domains, an NH2 terminus domain (helices 1–8) and a COOH terminus domain (helices A–K; Starks et al., 1997). The putative three-dimensional structures of AcC1 and AcC2 showed that all of the four amino acids existed on the α-helices. These models suggested that the hydrogen bonds of 241E (in helix A) and 269Y (in helix C), and 337Y (in helix E), 322D (in loop D1 to D2), and 317R (in helix D1) were formed by the mutations V241E and H337Y, respectively. On the other hand, the CH-πbond, the weak hydrogen bond of 110Y and 106L in the same α-helix, disappeared when the mutation Y110S was present. The distortion of the α-helix caused by the disappearance of the hydrogen bond, or the change in space between α-helices caused by the replacement of a bulky Tyr with a nonbulky Ser would explain the structural changes at the N terminus of AcC1. Furthermore, it has been reported that Pro is rarely found in α-helices because it distorts their structure (Madison, 1977). It was considered that the mutation P296S (in helix D) resolved the distortion of the α-helix and led to a recovery of the enzymatic activity. The DDxxD motif was found in Helix D, whose structure was suggested to play a major role in the enzymatic activity.
Figure 7.
Three-dimensional homology model of AcC1 based on the structure of 5-epi-aristolochene synthase from N. tabacum. The N-terminal domain is shown in blue, and the C-terminal domain is shown in orange. The insets show the mutated residues. [See online article for color version of this figure.]
DISCUSSION
In this article, we reported the first cloning and functional characterization of δ-guaiene synthases from cell cultures of Aquilaria. The enzymes that catalyze the formation of sesquiterpenes via germacrene A, such as valencene synthase (Sharon-Asa et al., 2003), vetispiradiene synthase (Back and Chappell, 1995), and β-selinene synthase (Iijima et al., 2004), have been cloned, and their reactions are proposed to involve eudesmane cations formed by closure of C2 and C7 of germacrene A. However, guaiane-type sesquiterpenes have a unique adjoining five- and seven-membered ring, which is thought to be formed by closure of C2 and C6 of germacrene A (Fig. 1). The TPSs from Aquilaria cells produced α-guaiene, α-humulene, and δ-guaiene simultaneously, which indicates that the cyclization mechanisms for these sesquiterpenes are closely related to each other. Most of the genes encoding sesquiterpene synthases have been isolated by homology-based PCR approaches based on their similarity to known gene sequences; however, the TPSs cloned in this study were derived from the screening of a cDNA library and shared only 40% identity in amino acid sequence with other sesquiterpene synthases. Moreover, although these TPSs had almost the same amino acid sequences, they had various ranges of Km and kcat values. These results suggest that these TPSs may provide some information for the molecular dissection of TPSs in general.
Of the three species of sesquiterpenes produced by the enzymatic reaction of cloned enzymes, α-humulene is known as a feeding deterrent to insects and pathogens (direct defense; Suga et al., 1993) and as a volatile attracting natural enemies of herbivores (indirect defense; Mattiacci et al., 2001; Rodriguez-Soana et al., 2003). However, no reports have referred to the role of guaiane-type sesquiterpenes in plant metabolism. Analysis of volatile compounds in the headspace of the culture and the data in Figure 2 revealed that α-humulene was emitted more quickly than δ-guaiene from the cells, which suggests that δ-guaiene has some different biological roles from those of α-humulene.
The major product was δ-guaiene in the enzymatic reactions using active enzymes (AcC2, AcC3, and AcC4) expressed in E. coli but α-humulene in the assays using crude enzymes extracted from the cells cultured for 6 h after MJ treatment. These results suggested that in the MJ-treated cells, there existed other enzymes that produced α-humulene as a main product in addition to the cloned enzymes that produced δ-guaiene as a main product.
Among the five clones isolated in this study, one clone (AcC1) had no TPS activity, and another (AcL154) harbored an extra stop codon, which suggested that the two might be pseudogenes. In fact, the mutant AcL154’, which was made by replacing the stop codon in AcL154 with Trp, had little catalytic efficiencies (kcat/Km = 0.035). However, the five clones had very similar amino acid sequences and produced the same products at very similar rates in enzymatic reactions performed using heterologously expressed proteins. Furthermore, phylogenetic analysis showed that the evolutional distances among the five were quite small compared to those of TPSs from other plants cloned with multiple copies (data not shown). The cells used in this study were cultured in media containing 2,4-dichlorophenoxyacetic acid, which is suggested to maintain plant cells in an undifferentiated state and promote cell division (Dixon, 1985). Mutations are known to occur during DNA replication or as a result of damage caused by radiation and mutagenic chemicals (Bertram, 2000); thus, the differences in amino acid sequence among the five clones are likely to be derived from the accumulation of mutations that occurred during DNA replication. This is one possibility for the explanation of existing similar multiple genes, and this can be evaluated by looking at the corresponding genes isolated from intact plants.
The heterologously expressed AcC1 in E. coli could not be found in the soluble fraction, and the clone was expressed by the in vitro system but was lacking in TPS activity. These results show that there are differences in protein structures between AcC1 and the other clones with TPS activity. This idea is supported by the finding that the mutants M42 and M50, both of which were active mutants of AcC1, were found their proteins in the soluble fraction in the E. coli expression system (data not shown). The DDxxD motif located at the C terminus has already been reported to play a major role in the enzymatic activity; however, mutagenesis and homology modeling of AcC1 and AcC2 showed that the space between and structures within α-helices was also important to the enzymatic activities. In recent years, evidence has emerged that this domain acts as a scaffold facilitating proper folding of the catalytically active C-terminal domain (Köllner et al., 2004), which may be supported by the results of this study. Our findings suggested that the structure and orientation between and within α-helices at the N terminus, especially around helix 4 where 110S is located, are important in facilitating proper folding of the protein to form a catalytically active structure.
MATERIALS AND METHODS
Cell Cultures and MJ Treatment
The methods used to prepare cultures of cells from Aquilaria crassna leaves were as described previously (Okudera and Ito, 2009). Cell suspension cultures were incubated with reciprocal shaking at 25°C in Murashige and Skoog medium containing 3% (w/v) Suc, 10−6 m 2,4-dichlorophenoxyacetic acid, and 10−6 m 6-benzyladenine and subcultured in fresh medium every 2 weeks. Cells cultured for 5 d after the inoculation were used. MJ (Sigma-Aldrich) was dissolved in DMSO to a concentration of 300 mm, and added to the culture at a final concentration of 0.1 mm.
Extraction of Terpenes from MJ-Treated Cells
The cell cultures were harvested by filtration 0 (control), 6, 12, and 24 h after MJ treatment. The cells and filtrate were extracted with 30 and 20 mL of pentane containing 48 ng mL−1 of limonene as an internal standard, respectively. The cells and medium were extracted over 14 h with constant shaking at room temperature, and the extracts were filtered through a Pasteur pipette filled with 0.4 g of silica gel overlaid with 0.6 g of anhydrous MgSO4. Each column was washed with 1 mL of pentane three times, and the two eluates were combined. The samples were concentrated to approximately 10 μL under nitrogen before analysis by GC-flame ionization detection (FID). For the identification of compounds, 3 μL of these samples was transferred into a 4-mL vial (Supelco) with a polypropylene cap, extracted by SPME fiber (100 μm bonded polydimethylsiloxane coating; Supelco), and analyzed by GC-MS. The assays without silica preparation were also performed, and sesquiterpene alcohols were not detected. The dry weights of extracted cells were determined after 48 h at 65°C. Means and standard errors were calculated from three replicates per time point per treatment.
Analysis of Sesquiterpenes
For quantification, sesquiterpenes were analyzed with a G-5000 GC system (Hitachi) equipped with a FID fitted with a TC-WAX column (0.25 mm × 0.25 μm × 60 m; GL Science). The flow rate was 40 mL H2 min−1 and 0.8 mL He min−1, and the FID was operated at 250°C. Split injections (1 μL) were made at a ratio of 5:1 with an injector temperature of 250°C. The GC was programmed with an initial oven temperature of 50°C, increasing at a rate of 3°C min−1 until 110°C, and then a ramp of 5°C min−1 until 240°C (10-min hold). The identification of terpenes was based on retention times with authentic standards, and concentrations of sesquiterpenes were calculated by comparing the integrated peak area with that of the internal standard limonene.
For the SPME analysis, a fiber was inserted directly into the injection port (250°C) of a GC-MS system (G-7000-M9000/3DQMS, 15 eV; Hitachi), the program was immediately started, and the fiber was removed after a period of 10 min. The GC was fitted with a TC-WAX column, and the flow rate of He was 1 mL min−1. The program had an initial oven temperature of 80°C, a ramp of 5°C min−1 until 220°C (10-min hold), and then a ramp of 10°C min−1 until 240°C (3-min hold). The identification of terpenes was based on a comparison of retention times and mass spectra with authentic standards.
Extraction of Enzymes from MJ-Treated Cells and Enzyme Assays
Using a mortar and pestle, 2.5 g of the MJ-treated cells was ground to a fine powder in liquid nitrogen and combined with 5 mL of extraction buffer containing 50 mm Tris-HCl, pH 7.5, 10% (v/v) glycerol, 100 mm MgCl2, 5 mm dithiothreitol (DTT), 1 mm EDTA, 1% (w/v) polyvinylpyrrolidone, and 1% (w/v) polyvinylpolypyrrolidone. The extract was vortexed and centrifuged at 10,000g for 30 min at 4°C. The supernatant was filtered through filter paper, divided into 1-mL aliquots, and kept at −80°C. Extracts were thawed only once before the enzyme assay. The total protein concentration of each extract was determined by the Bradford assay (Bio-Rad) using bovine serum albumin as the standard.
Enzymatic activity was assessed with 1 mL of extract with the addition of 87 μm FPP (Sigma-Aldrich). All assays were done in duplicate, with 1 mL of pentane overlaid to collect volatiles and incubation at 30°C for 4 h. After the addition of 1.6 μg mL−1 of limonene as an internal standard, the pentane overlay was removed and filtered through a Pasteur pipette filled with 0.2 g of silica gel overlaid with MgSO4. Each assay mixture was extracted with an additional 1 mL of pentane twice, and these fractions were also passed through the same column and pooled with the initial eluent. Subsequently, the column was washed with 1 mL of pentane twice, and the total eluent was concentrated to approximately 10 μL under nitrogen and then analyzed immediately by GC-FID and SPME-GC-MS. The assays without silica preparation were also performed, and sesquiterpene alcohols were not detected.
Collection of Volatiles
After the administration of MJ, the cells were transferred to flasks with a silicon cap and incubated for 6 h with reciprocal shaking. The SPME fiber was inserted into each flask and left for 1 min, and immediately GC-MS was conducted.
Isolation of RNA and Construction of a cDNA Library
Total RNA was extracted from 1 g of the cultured cells incubated for 6 or 10 h with MJ. mRNA was purified using a TaKaRa mRNA purification kit from total RNA extracted with an RNeasy plant mini kit (Qiagen). Two micrograms of the mRNA was employed to construct a cDNA library using the Super Script Plasmid System (Invitrogen) following the manufacturer’s protocol.
Sequence Analysis
Two hundred seventy-four clones of the cDNA library were randomly sequenced; plasmids extracted using the Mini plus Plasmid DNA Extraction System (Viogene) were sequenced at Biomatrix Co. Ltd. The deduced amino acid sequences of the clones were compared with GenBank data using BLASTP search algorisms of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/blast/blast.cig).
Cloning of the Putative Sesquiterpene Synthases
The sequence of the clone named AcL154 was very similar to that of the germacrene D synthase isolated from Vitis vinifera (GenBank accession no. AY561842). A pair of forward (5′-ATGTCTTCGGCAAAACTAGGTTCTGCCTCC-3′) and reverse (5′-GATTTCAATAGCAT GACGCAACAAGGCAGC-3′) primers were used for PCR to generate the fragment for expression. Each reaction mixture contained 2 μL of 10× reaction buffer (TaKaRa), 0.2 mm of each deoxyribonucleic acid triphosphate, 0.5 units of ExTaq polymerase (TaKaRa), 0.2 μm of the forward and reverse primers, 0.2 μL of DMSO, and 0.5 μL of template cDNA prepared from total RNA isolated from MJ-treated cells using ReverTra Ace (Toyobo) in a final volume of 20 μL. The thermal cycling conditions for PCR performed with a Program Temp Control System (Astec) were as follows: a denaturing step at 94°C for 30 s, followed by 30 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 1 min 40 s. Finally, the reaction mixture was heated at 72°C for 3 min. Following gel purification, the PCR products were ligated into the expression vector pCR T7/CT-TOPO (Invitrogen) and introduced into competent Escherichia coli TOP 10 F’ cells (Invitrogen).
Heterologous Expression of Sesquiterpene Synthases in E. coli and Enzyme Assays
Full-length cDNAs cloned into expression vectors were transformed into BL21 (DE) Codon plus-RIL (Stratagene). Cultures initiated from single colonies were incubated in TB medium and cultured at 37°C until the optical density at 600 nm became 0.6 to 0.7 before the administration of isopropylthio-β-galactoside at 0.5 mm, and then the cultures were incubated for 22 h at 16°C with shaking at 220 rpm. The cells were separated from the medium by centrifugation at 10,000g for 10 min at 4°C. The cells were resuspended in sonication buffer (100 mm Tris-HCl, pH 8.0, 20% glycerol, 0.5 mm EDTA, and 5 mm DTT) and sonicated five times for 30 s. Clear lysate of the sonicated sample was collected by centrifugation at 10,000g for 10 min at 4°C. The lysate was loaded on a Ni-NTA Spin column (Qiagen) to isolate the His-tagged recombinant protein. Protein concentrations were determined by the Bradford assay (Bio-Rad) using bovine serum albumin as the standard. Bacterial pellets and lysates, and purified enzyme samples were examined on SDS-PAGE gels to visualize expected proteins.
The enzyme reaction was performed in a 4-mL vial with a solid-top polypropylene cap using 50 μL of crude protein extract in a final volume of 200 μL containing Tris-HCl buffer (25 mm, pH 7.0) supplemented with 10% glycerol, 100 mm MgSO4, 5 mm DTT, and 46 μm FPP. After incubation at 30°C for 1 h, a SPME fiber was inserted into the headspace of the vial to collect volatiles for 10 s and then transferred to the injection port of a GC-MS system for desorption. The conditions for GC-MS and the identification of compounds were as described above. For kinetic analyses, purified enzymes were incubated with FPP ranging from 0.46 to 46 μm in 1-mL reaction volumes. Reactions were incubated for 1 h at 25°C before adding limonene as an internal standard and then extracting twice with 2 mL of pentane. Extracts were combined, removed the water by MgSO4, concentrated to about 10 μL under nitrogen, and then analyzed by GC-FID as described above.
RT-PCR
Total RNA was isolated from the cells incubated for 0 (control), 6, 12, and 24 h after MJ treatment using the method described above, and first-strand cDNAs were synthesized using ReverTra Ace (Toyobo) and the oligo(dT) primer (Takara) following the manufacturers’ protocols. PCR was performed with the forward primer AcTPS-f (5′-CTCACAACATCCGTTTGGTTTCGG-3′) and reverse primer AcTPS-r (5′-GTGTCATCGATCAGAGAAGTAATCCC-3′), both of which were complementary to the corresponding sequences of all TPSs obtained from Aquilaria cultured cells in this study. Histone transcripts provided the internal control for PCR using the forward primer AcHistone-f (5′-GTACCGCTACCGGAGGGAAGTTGAAGA-3′) and reverse primer AcHistone-r (5′-CTTCTTGGGCGACTTGGTAGCCTTGGT-3′).
Site-Directed Mutagenesis
Site-directed mutagenesis of AcL154, in which the 112th codon from the 5′-end of the sequence was replaced with Trp, yielded AcL154’. AcC1 was also mutated at 110S, 144R, 241E, 296S, and 337Y to obtain M41 to M50. The mutagenesis was performed using the QuickChange II site-directed mutagenesis kit (Stratagene) according to the manufacturer’s directions. For each mutation, the forward and reverse mutagenetic primers consisted of two complementary oligonucleotides (26–29 mer) containing the desired mutation. The incorporation of the desired mutation was verified by DNA sequencing.
Expression of the Mutants in Vitro and Enzyme Assays
Mutant plasmids were expressed in an in vitro cell-free system using the TNT Quick Coupled Transcription/Translation system (Promega). Expressed proteins were transferred to nitrocellulose (Shleicher & Schuell) after SDS-PAGE, and Mrs were confirmed by western-blot analyses using the Transcend Colorimetric Translation Detection System (Promega).
The enzyme reaction was performed in a 4-mL vial with a solid-top polypropylene cap using 50 μL of protein solution in a final volume of 200 μL containing Tris-HCl buffer (25 mm, pH 7.0) supplemented with 10% glycerol, 10 mm MgSO4, 5 mm DTT, and 43.5 μm FPP. After incubation at 30°C for 1 h, a SPME fiber was inserted into the headspace of the vial to collect volatiles for 30 min and then transferred to the injection port of a GC-MS system (M9000 GC3DQMS; Hitachi) for desorption. The conditions for GC-MS, identification of compounds, and calculation of the product ratio were as described above. The determination of the kinetic parameters of the active mutants was performed by using the proteins expressed in E. coli as described above.
Western Blotting
The Transcend Colorimetric Translation Detection System was used to visualize the proteins synthesized in vitro. The transcend biotin-Lysyl-tRNA (Promega) was added to the translation reaction, and the biotinylated Lys residues were incorporated into nascent proteins during translation. After SDS-PAGE and electroblotting to a nitrocellulose membrane, the biotinylated proteins were visualized by binding streptavidin alkaline phosphatase, followed by colorimetric detection (nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate).
Molecular Modeling
Deep View/Swiss-PDBViewer (http://spdbv.vital-it.ch/) and SWISS-MODEL were used to develop three-dimensional models of AcC1 and AcC2 based on the structure of the 5-epi-aristolochene synthase from Nicotiana tabacum containing the substrate analog farnesyl hydroxyphosphonate (Protein Data Bank ID code 5EAT).
Sequence data from this article can be found in the GenBank/EMBL libraries under accession numbers GU083696, GU083697, GU083698, GU083699, and GU083700.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. GC-MS profiles of control assay for Figure 4.
Supplemental Figure S2. GC-MS profiles of control assay for Figure 6.
Supplementary Material
Acknowledgments
We thank Professor Joe Chappell for great comments on the manuscript.
References
- Back K, Chappell J. (1995) Cloning and bacterial expression of a sesquiterpene cyclase from Hyoscyamus muticus and its molecular comparison to related terpene cyclases. J Biol Chem 270: 7375–7381 [DOI] [PubMed] [Google Scholar]
- Bertram JS. (2000) The molecular biology of cancer. Mol Aspects Med 21: 167–223 [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Crock J, Jetter R, Croteau R. (1998a) Terpenoid-based defenses in conifers: cDNA cloning, characterization, and functional expression of wound-inducible (E)-α-bisabolene synthase from grand fir (Abies grandis). Proc Natl Acad Sci USA 95: 6756–6761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann J, Meyer-Gauen G, Croteau R. (1998b) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95: 4126–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane DE. (1999) Sesquiterpene biosynthesis: cyclization mechanisms. Barton SB, Nakanishi K, Meth-Cohn O, , Comprehensive Natural Products Chemistry: Isoprenoids Including Carotenoids and Steroids, Vol 2 Pergamon, Oxford, UK, pp 155–200 [Google Scholar]
- Caruthers JM, Kang I, Rynkiewicz MJ, Cane DE, Christianson DW. (2000) Crystal structure determination of aristolochene synthase from the blue cheese mold, Penicillium roqueforti. J Biol Chem 275: 25533–25539 [DOI] [PubMed] [Google Scholar]
- Deguerry F, Pastore L, Wu S, Clark A, Chappell J, Schalk M. (2006) The diverse sesquiterpene profile of patchouli, Pogostemon cablin, is correlated with a limited number of sesquiterpene synthases. Arch Biochem Biophys 454: 123–136 [DOI] [PubMed] [Google Scholar]
- Dixon RA. (1985) Plant Cell Culture: A Practical Approach. IRL Press Ltd, Oxford [Google Scholar]
- Fäldt J, Martin D, Miller B, Rawat S, Bohlmann J. (2003) Traumatic resin defense in Norway spruce (Picea abies): methyl jasmonate-induced terpene synthase gene expression, and cDNA cloning and functional characterization of (+)-3-carene synthase. Plant Mol Biol 51: 119–133 [DOI] [PubMed] [Google Scholar]
- Felicetti B, Cane DE. (2004) Aristolochene synthase: mechanistic analysis of active site residues by site-directed mutagenesis. J Am Chem Soc 126: 7212–7221 [DOI] [PubMed] [Google Scholar]
- Greenhagen B, Chappell J. (2001) Molecular scaffolds for chemical wizardry: learning nature’s rules for terpene cyclases. Proc Natl Acad Sci USA 98: 13479–13481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Nakahara S, Inoue T, Sumida Y, Takahashi M, Masada Y. (1985) A new chromone from agarwood and pyrolysis products of chromone derivatives. Chem Pharm Bull (Tokyo) 33: 5088–5091 [Google Scholar]
- Iijima Y, Davidovich-Rikanati R, Fridman E, Gang DR, Bar E, Lewinsohn E, Pichersky E. (2004) The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil. Plant Physiol 136: 3724–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M, Tsuneya T, Uneyama K. (1993) Fragrant sesquiterpenes from agarwood. Phytochemistry 33: 1147–1155 [Google Scholar]
- Ito M, Honda G. (2008) Agarwood-its sedative effect on mice and current state in the production sites. Aroma Res 34: 122–127 [Google Scholar]
- Ito M, Okimoto K, Yagura T, Honda G. (2005) Induction of sesquiterpenoid production by methyl jasmonate in Aquilaria sinensis cell suspension culture. J Essent Oil Res 17: 175–180 [Google Scholar]
- Köllner TG, Schnee C, Gershenzon J, Degenhardt J. (2004) The variability of sesquiterpenes cultivars is controlled by allelic emitted from two Zea mays variation of two terpene synthase genes encoding stereoselective multiple product enzymes. Plant Cell 16: 1115–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann C, Fink B, Festner M, Dregus M, Engel KH, Schwab W. (2007) Cloning and functional characterization of three terpene synthases from lavender (Lavandula angustifolia). Arch Biochem Biophys 465: 417–429 [DOI] [PubMed] [Google Scholar]
- Lee S, Chappell J. (2008) Biochemical and genomic characterization of terpene synthases in Magnolia grandiflora. Plant Physiol 147: 1017–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesburg CA, Zhai G, Cane DE, Christianson DW. (1997) Crystal structure of pentalenene synthase: mechanistic insights on terpenoid cyclization reactions in biology. Science 277: 1820–1824 [DOI] [PubMed] [Google Scholar]
- Lewinsohn E, Gijzen M, Croteau R. (1991) Defense mechanisms of conifers: differences in constitutive and wound-induced monoterpene biosynthesis among species. Plant Physiol 96: 44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lücker J, Bowen P, Bohlmann J. (2004) Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (-)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 65: 2649–2659 [DOI] [PubMed] [Google Scholar]
- Madison V. (1977) Flexibility of the pyrrolidine ring in proline peptides. Biopolymers 16: 2671–2692 [Google Scholar]
- Martin D, Tholl D, Gershenzon J, Bohlmann J. (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129: 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiacci L, Rocca BA, Scascighini N, D’Alessandro M, Hern A, Dorn S. (2001) Systemically induced plant volatiles emitted at the time of “danger”. J Chem Ecol 27: 2233–2252 [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Yamagata E, Yoneda K, Miura I. (1981) Jinkohol, a prezizane sesquiterpene alcohol from agarwood. Phytochemistry 20: 1597–1599 [Google Scholar]
- Okudera Y, Ito M. (2009) Production of agarwood fragrant constituents in Aquilaria calli and cell suspension cultures. Plant Biotechnol 26: 307–315 [Google Scholar]
- Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato A. (1996) Effect of jinkoh-eremol and agarospirol from agarwood on the central nervous system in mice. Planta Med 62: 2–6 [DOI] [PubMed] [Google Scholar]
- Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato K. (2000) Effects of sesquiterpenoids from “Oriental incenses” on acetic acid-induced writhing and D2 and 5-HT2A receptors in rat brain. Phytomedicine 7: 417–422 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Soana C, Crafts-Brandner SJ, Canas LA. (2003) Volatile emissions triggered by multiple herbivore damage: beet armyworm and whitefly feeding on cotton plants. J Chem Ecol 29: 2521–2532 [DOI] [PubMed] [Google Scholar]
- Rynkiewicz MJ, Cane DE, Christianson DW. (2001) Structure of trichodiene synthase from Fusarium sporotrichioides provides mechanistic inferences on the terpene cyclization cascade. Proc Natl Acad Sci USA 98: 13543–13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon-Asa L, Shalit M, Frydman A, Bar E, Holland D, Or E, Lavi U, Lewinsohn E, Eyal Y. (2003) Citrus fruit flavor and aroma biosynthesis: isolation, functional characterization, and developmental regulation of Cstps1, a key gene in the production of the sesquiterpene aroma compound valencene. Plant J 36: 664–674 [DOI] [PubMed] [Google Scholar]
- Starks CM, Back KW, Chappell J, Noel JP. (1997) Structural basis for cyclic terpene biosynthesis by tobacco 5-epi-aristolochene synthase. Science 277: 1815–1820 [DOI] [PubMed] [Google Scholar]
- Steele CL, Crock J, Bohlmann J, Croteau R. (1998) Sesquiterpene synthases from grand fir (Abies grandis). Comparison of constitutive and wound-induced activities, and cDNA isolation, characterization, and bacterial expression of delta-selinene synthase and gamma-humulene synthase. J Biol Chem 273: 2078–2089 [DOI] [PubMed] [Google Scholar]
- Suga T, Ohta S, Munesada K, Ide N, Kurokawa M, Shimizu M, Ohta E. (1993) Endogenous pine wood nematicidal substance in pines, Pinus massioniana, P. strobus and P. palustris. Phytochemistry 33: 1395–1401 [Google Scholar]
- Takemoto H, Ito M, Shiraki T, Yagura T, Honda G. (2008) Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J Nat Med 62: 41–46 [DOI] [PubMed] [Google Scholar]
- Varma KR, Maheshwari ML, Bhattacharyya SC. (1963) The constitution of agarospirol, a sesquiterpenoid with a new skeleton. Tetrahedron 21: 1079–1090 [Google Scholar]
- Whittington DA, Wise ML, Urbansky M, Coates RM, Croteau RB, Christianson DW. (2002) Bornyl diphosphate synthase: structure and strategy for carbocation manipulation by a terpenoid cyclase. Proc Natl Acad Sci USA 99: 15375–15380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagura T, Ito M, Kiuchi F, Honda G, Shimada Y. (2003) Four new 2-(2-phenylethyl)chromone derivatives from withered wood of Aquilaria sinensis. Chem Pharm Bull (Tokyo) 51: 560–564 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.