Abstract
Immune tolerance against enteric commensal bacteria is important for preventing intestinal inflammation. Deletion of phosphoinositide dependent protein kinase 1 (Pdk1) in T cells using Cd4-Cre induced chronic inflammation of the intestine despite the importance of PDK1 in T cell activation. Analysis of colonic intraepithelial lymphocytes of PDK1-deficient mice revealed markedly increased CD8α+ T cell receptor (TCR)γδ+ T cells, including an interleukin-17 (IL-17)-expressing population. TCRγδ+ T cells were responsible for the inflammatory colitis as deletion of Tcrd abolished spontaneous colitis in the PDK1 deficient mice. This dysregulation of intestinal TCRγδ+ T cells was attributable to a reduction in the number and functional capacity of PDK1-deficient T-regulatory (Treg) cells. Adoptive transfer of wild-type Treg cells abrogated the spontaneous activation and proliferation of intestinal TCRγδ+ T cells observed in PDK1-deficient mice and prevented the development of colitis. Therefore suppression of intestinal TCRγδ+ T cells by Treg cells maintains enteric immune tolerance.
INTRODUCTION
Colitis, including ulcerative colitis and Crohn’s disease are chronic, immunologically mediated disorders that lead to a range of symptoms including abdominal pain, severe diarrhea, rectal bleeding and wasting (Xavier and Podolsky, 2007). Studies have shown that commensal bacteria in the intestine are the main trigger of the inflammatory response and treatment with antibiotics reduces intestinal inflammation in humans and experimental animals (Elson, 2000; Videla et al., 1994). Therefore, immune tolerance towards normal commensal bacteria is critical for maintaining enteric immune homeostasis. In human diseases such as Crohn’s disease and ulcerative colitis, it has been reported that activated T cell receptor (TCR)γδ+ T cells accumulate in the inflamed region (McVay et al., 1997; Yeung et al., 2000). However, the role of TCRγδ+ T cells in inflammatory bowel disease and, in particular, whether they are involved in induction or regulation of inflammation, has remained a controversial issue (Nanno et al., 2007).
TCRγδ+ T cells were discovered nearly 25 years ago, yet even now their biological role remains to be fully understood (Hayday et al., 1985; Nanno et al., 2007). A portion of the TCRγδ+ T cell population develops in the thymus, similar to TCRαβ+ T cells (Nanno et al., 2007). However, unlike TCRαβ+ T cells, TCRγδ+ T cells can also develop outside of the thymus as evidenced by the TCRγδ+ T cell population in thymectomized mice and in athymic nude mice (Bandeira et al., 1990; Nanno et al., 2007; Saito et al., 1998). Serological studies indicate that TCRγδ+ T cells are more abundant in the intraepithelial lymphocyte (IEL) compartment (up to 30%)than peripheral blood (Nanno et al., 2007). In the IEL compartment, most of the TCRγδ+ T cells are CD8α-positive (Hayday and Tigelaar, 2003; Nanno et al., 2007). CD8α+ IEL are proposed to have an extrathymic origin, being the progeny of bone-marrow-derived stem cells that develop in novel lymphoid sites termed cryptopatches in the small and large intestinal mucosa (Saito et al., 1998).
In experimental colitis models that are induced by chemical-mediated damage such as dextran sulfate sodium (DSS) or 2,4,6-trinitrobenzene sulfonic acid (TNBS) treatment, Tcrd−/− mice display more severe colitis than wild-type control mice (Chen et al., 2002; Inagaki-Ohara et al., 2004). Transferring of self-I-Ab-reactive T-cell clones into mice induces transient graft-versus-host disease (GVHD) and these mice subsequently recover and become resistant to the GVHD. However, this resistance does not occur in Tcrd−/− mice (Shiohara et al., 1996). Based on these data, it has been suggested that TCRγδ+ T cells are important for immunoregulation. However, interestingly, in the spontaneous colitis that develops in Tcra−/− mice, TCRγδ+ T cells are involved in the induction of colitis (Nanno et al., 2008). In addition, it was recently shown that TCRγδ+ T cells contribute to the induction of experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (Ito et al., 2009; Sutton et al., 2009). In the EAE model, TCRγδ+ T cells work as a fast acting T cell population that produces the inflammatory cytokine IL-17 (Sutton et al., 2009). Moreover, during blood transfusion, it is known that the severity of GVHD correlates with the abundance of TCRγδ+ T cells and is inversely correlated with Treg cell abundance in donor blood (Pabst et al., 2007). Finally, bone marrow transplantation-mediated GVHD is exacerbated by TCRγδ+ T cells (Maeda et al., 2005). Taken together, these data reveal conflicting roles for TCRγδ+ T cells as both immune-regulators and mediators of inflammation.
Treg cells have been shown to play an important role in intestinal homeostasis in models of experimental colitis (Saurer and Mueller, 2009). Moreover, the severity of colitis in humans has been shown to be inversely correlated with the frequency of peripheral Treg cells (Takahashi et al., 2006). In the PI3KδD910A mouse strain and ‘r1ΔT/r2n’ mouse strain that lacks both Pik3r1 (encoding p85α, p55α, and p50α) and Pik3r2 (encoding p85β) in T cells, both Treg cell development and Treg cell function are diminished, which results in induction of inflammation, including colitis (Fruman and Bismuth, 2009; Oak et al., 2006; Patton et al., 2006). Also, CD28-deficient mouse strains show diminished production of IL-10, whereas strong activation of CD28 signaling by superagonistic anti-CD28 antibody enhances production of IL-10 from Treg cells (Beyersdorf et al., 2005; Toto et al., 2000).
In this study, we show that deletion of the phosphoinositide dependent protein kinase 1 (Pdk1) gene by Cd4-Cre (T cell specific deletion) impairs Treg cell activation as well as CD4+ T cell activation. Unexpectedly, the TCRγδ+ T cell population was dramatically increased in the colonic IEL population of the Pdk1 gene deleted mice. We found that TCRγδ+ T cells are constitutively activated by commensal bacteria and that this activation-mediated expansion of TCRγδ+ T cells is suppressed by wild type Treg cells. In vitro, Treg cells directly suppressed TCRγδ+ T cells through IL-10. Therefore, our data shows that suppression of intestinal TCRγδ+ T cells by Treg cells helps maintain normal intestinal homeostasis.
RESULTS
T cell specific deletion of the Pdk1 gene by Cd4-Cre induces spontaneous colitis
We have recently shown using the T cell specific deletion of the Pdk1 gene by Cd4-Cre (Tg(Cd4-Cre)1Cwi) that PDK1 plays an essential role in CD4+ T-cell activation, by transducing signals from the CD28 co-receptor leading to activation of NF-κB (Park et al., 2009). Therefore it was highly surprising to find that the T cell specific deletion of the Pdk1 gene by Cd4-Cre induced spontaneous colitis (Figure 1A and Figure S1) even though CD4+ T cell activation was dramatically reduced (Park et al., 2009). Pdk1 gene deleted mice (Pdk1flox/flox; Cd4-Cre) display a high incidence of rectal prolapse, unlike littermate controls in the same breeding cage (Figure 1B). This surprising observation suggests that spontaneous colitis is not caused by activation of conventional T cells and is not due to external factors such as intestinal flora. The prolapsed mice displayed severe inflammation in the intestine (Figure 1A) and young mice also showed abundant lymphocytic infiltration in both small and large intestine (data not shown). Interestingly, serum IL-12p40 in Pdk1flox/flox; Cd4-Cre mice was significantly higher (Figure 1C), similar to observations in Crohn’s disease (Sartor, 2006). IL-12p40 was also significantly increased throughout the colon of Pdk1flox/flox; Cd4-Cre mice (Figure 1D). IL-12p40 mRNA in the Pdk1flox/flox; Cd4-Cre colon was similarly increased, as were mRNA amounts of the pro-inflammatory cytokines IL-17A, IL-23p19 and TNF-α (Figure 1E–H). However, expression of IL-4, IFN-γ, IL-12p35, and TGF-β were not significantly increased in the colon (Figure 1I–L). Numerous recent reports have shown that IL-17A expression is linked with induction of inflammation (Bettelli et al., 2006; McGeachy et al., 2009). Moreover, the cytokine IL-23, which is a heterodimer of IL-12p40 and IL-23p19, is important for expansion and maintenance of IL-17-expressing T cells (Ahern et al., 2008; Awasthi and Kuchroo, 2009; Mangan et al., 2006; McGeachy et al., 2009; Veldhoen and Stockinger, 2006). Therefore, we hypothesized that IL-17-expressing T cells might be playing an important role in intestinal inflammation in Pdk1flox/flox; Cd4-Cre mice.
Figure 1.
Pdk1 gene deletion by Cd4-Cre induces spontaneous colitis. (A) Photograph of the colon of a rectally prolapsed Pdk1flox/flox; Cd4-Cre mouse (flox/flox) and its littermate control Pdk1+/flox; Cd4-Cre mouse (+/flox) (original magnification 100×). (B) Incidence of rectal prolapse (n = 15 mice per each group). (C) ELISA analysis of serum IL-12p40 (samples pooled, n = 8 – 9 mice per each group). (D) ELISA analysis for IL-12p40 level of ileum and colon explant cultures. (E–H), Relative mRNA of IL-12p40 (E), IL-23p19 (F), IL-17A (G), TNF-α (H), IL-4 (I), IFN-γ (J), IL-12p35 (K), and TGF-β (L) normalized to β-actin in colon (n = 3 – 4 mice per each group). Error bars (C–L), s.d. of triplicate samples. *P<0.05, **P<0.01.
TCRγδ+ T cell population is dramatically increased in spontaneous colitis
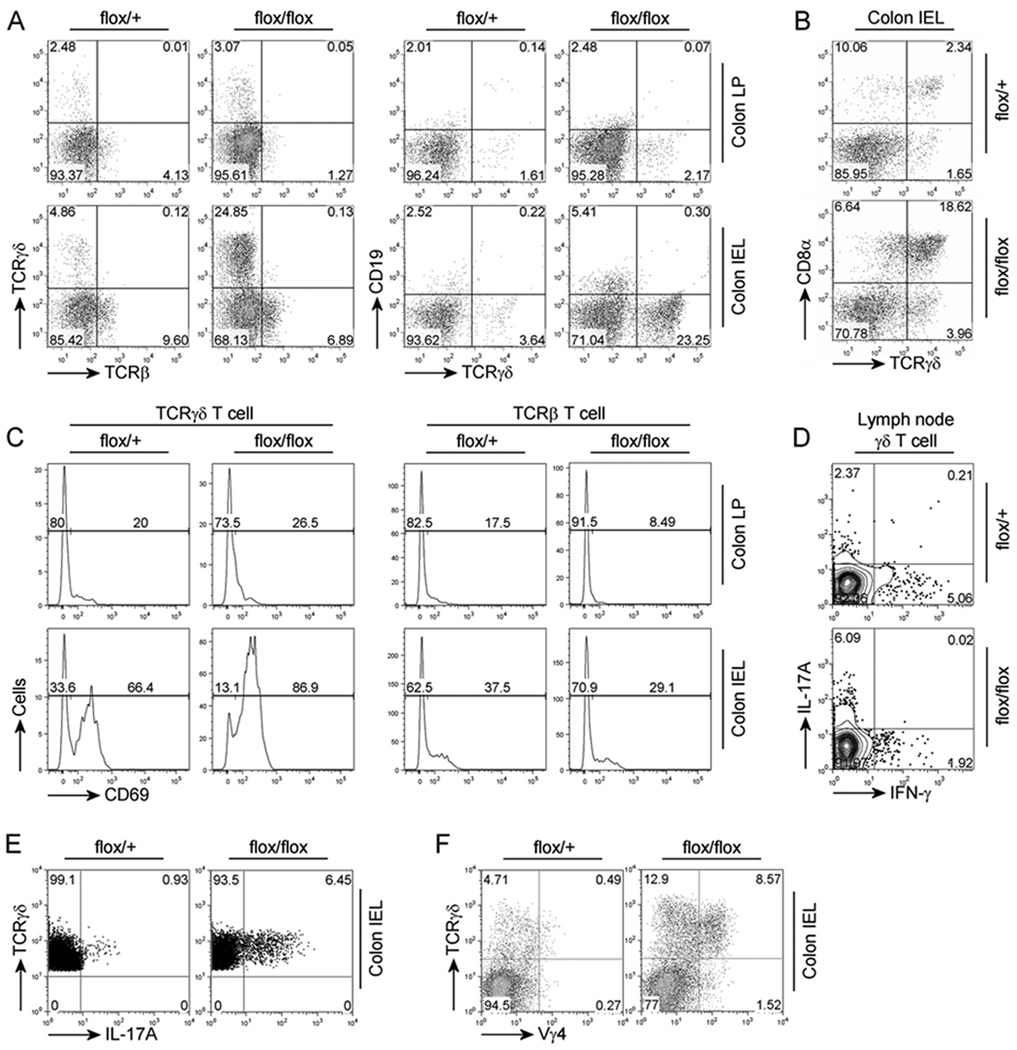
IL-17 is primarily expressed by Th17 T-cells, a recently characterized subset of CD4+ T cells (Awasthi and Kuchroo, 2009). As conventional T cells cannot be activated without PDK1 (Park et al., 2009), we wanted to determine which cell population was responsible for the increased amounts of IL-17A mRNA in the intestine of the Pdk1flox/flox; Cd4-Cre mice. We analyzed the T cell population in the intestinal epithelial cells (IEL) and lamina propria (LP) comparments using flow cytometry (Figure 2A). Interestingly, neither the number of conventional TCRβ+ T cells, nor the CD19+ B cell population, which has been implicated in spontaneous colitis thorough production of autoantibody (Bhan et al., 2000; Mizoguchi et al., 1996; Targan and Karp, 2005), were significantly affected in either the IEL or LP cells (Figure 2A). Instead the TCRγδ+ T cell population, in particular the CD8α+ TCRγδ+ T cell population, was dramatically increased in the IEL, but not LP, of Pdk1flox/flox; Cd4-Cre mice (Figure 2A and 2B). This was not due to PDK1 changes in TCRγδ+ T cells, as the Pdk1 gene was not deleted in these cells (Figure S2).
Figure 2.
TCRγδ+ T cells are dramatically increased in PDK1 deficient mice. (A) Flow cytometry of IELs and lamina propria (LP) from colon. (B) Flow cytometry of IELs from colon. (C) Cytometry for analysis of CD69 expression on TCRγδ+ T cells in the colon IELs or in the colon LP. (D) Flow cytometry for analysis of IFN-γ and IL-17A-expressing TCRγδ+ T cells in the lymph node after stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. (E) Flow cytometry for analysis of IL-17A-expressing TCRγδ+ T cells in the colon IELs after stimulation with PMA and ionomycin. (F) Flow cytometry analysis for Vγ4-expressing TCRγδ+ T cells in the colon IELs. Numbers adjacent to outlined areas indicate percent cells in each (A–F).
Interestingly, surface expression of CD69, an early T cell activation marker, was higher in TCRγδ+ T-cells from the IEL compartment than in TCRγδ+ T cells in the LP (Figure 2C). In addition, the CD69-positive IEL TCRγδ+ T cells of Pdk1flox/flox; Cd4-Cre mice were increased relative to wild-type control mice. These results indicate that TCRγδ+ T cells in the colonic IEL compartment are frequently activated and that IEL TCRγδ+ T cells are more activated in Pdk1flox/flox; Cd4-Cre mice. The number of TCRγδ+ T cell was increased only slightly in the thymus and peripheral lymph nodes (Figure S3) suggesting that the dramatic increase of intestinal TCRγδ+ T-cells in the Pdk1flox/flox; Cd4-Cre mice was likely due to enhanced proliferation. Interestingly, in the lymph node, TCRγδ+ T cells from the Pdk1flox/flox; Cd4-Cre mice expressed more IL-17A than IFN-γ, in contrast to Pdk1+/flox; Cd4-Cre mice (Figure 2D). This data indicates that in the Pdk1flox/flox; Cd4-Cre mice the balance between IL-17A- and IFN-γ-expressing TCRγδ+ T cells was altered. More interestingly, IL-17A-expressing TCRγδ+ T cells were increased in the colonic IEL of Pdk1flox/flox; Cd4-Cre mice (Figure 2E). Vγ4 TCRγδ+ T cells usually represents a small proportion of the colonic IEL (Pereira et al., 1997). However, consistent with the observed elevation in TCRγδ+ T cell IL-17A production (Figure 2E) and IEL IL-17A (Figure 1G), we found that the Vγ4 TCRγδ+ T cell population was increased in the Pdk1flox/flox; Cd4-Cre mice (Figure 2F).
TCRγδ+ T cells are required for the development of spontaneous colitis
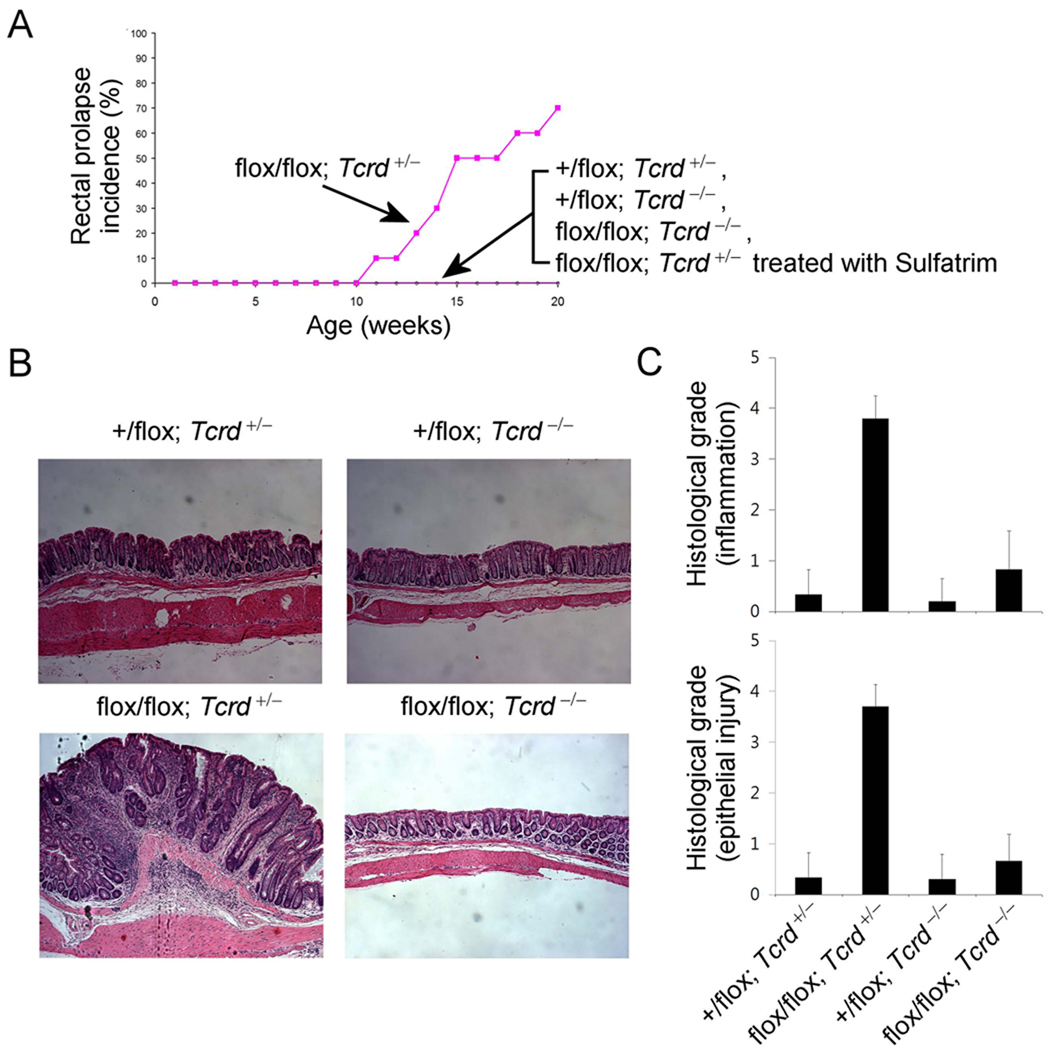
Based on the results presented above, the increase in the TCRγδ+ T cell population in colonic IELs may lead to spontaneous colitis. However, the data from several experimental colitis models, e.g. tissue injury induced by DSS or TNBS treatment, suggests that TCRγδ+ T cells are protective in such models (Chen et al., 2002; Inagaki-Ohara et al., 2004). In contrast, TCRγδ+ T cells are dramatically increased in Tcra−/− mice that develop spontaneous colitis (Mizoguchi et al., 1996). Furthermore, in human colitis such as Crohn’s disease and ulcerative colitis, activated TCRγδ+ T cells are increased in the colitic area (McVay et al., 1997; Yeung et al., 2000). Unlike injury-mediated colitis, TCRγδ+ T cells are involved in development of the spontaneous colitis in Tcra−/− mice (Mizoguchi et al., 1996; Nanno et al., 2008), and colitis induced by transfer of bone marrow cells from Tcrb−/− mice into Cd3ε transgenic mice (Simpson et al., 1997). Thus, it has been controversial whether TCRγδ+ cells participate in the development or prevention of colitis, even though division of function by TCR variants has been suggested (Bonneville, 2006). We therefore directly tested the effect of removing the TCRγδ+ T cell population from the Pdk1flox/flox; Cd4-Cre mice by crossing these mice with the Tcrd−/− mice. Remarkably, the double deficient mice (Tcrd−/−, Pdk1flox/flox; Cd4-Cre) did not show any rectal prolapse (Figure 3A and 3B) and showed dramatically reduced signs of colitis (Figure 3C), even though Tcrd+/−, Pdk1flox/flox; Cd4-Cre littermates showed rectal prolapse (Figure 3A). Treatment of the mice with Sulfatrim also abrogated colitis (Figure 3A) suggesting that the response of TCRγδ+ T cells to commensal bacteria is important for the activation and proliferation of the TCRγδ+ T cells.
Figure 3.
TCRγδ+ T cells are responsible for the development of spontaneous colitis. (A) Incidence of rectal prolapse (n=7–10 mice per group). Photograph (B) and histological grade (C) of colon from each genotype (original magnification 100×).
PDK1 affects Treg cell development
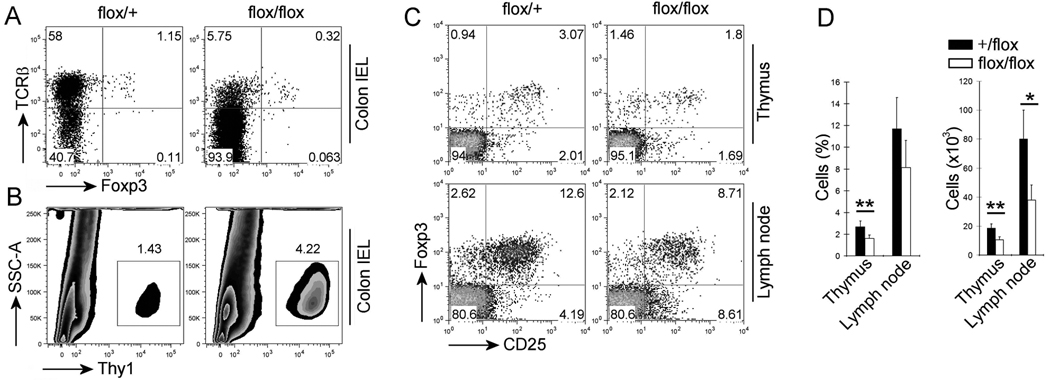
The results presented above indicated that intestinal TCRγδ+ T-cells, activated by commensal flora, mediate spontaneous colitis in Pdk1flox/flox; Cd4-Cre mice. This raises the question of how the dysregulated expansion of the TCRγδ+ T-cells is prevented in wild-type mice. As Treg cells play a key role in maintenance of immune homeostasis, we wanted to test the possibility that intestinal TCRγδ+ T-cells are normally suppressed by Treg cells. Therefore, we hypothesized that as in conventional T cells, PDK1 is also necessary for Treg cell function. Because Cd4-Cre deletes Pdk1 in CD4+CD25+Foxp3+ Treg cells, failure of PDK1 deficient Tregs to regulate intestinal TCRγδ+ T cells could lead to colitis. We therefore examined the Treg cell population in Pdk1flox/flox; Cd4-Cre mice. In the colon of Pdk1flox/flox; Cd4-Cre mice compared to wild-type control mice, the Treg cell population, as a percentage of the total Thy1+ T cell population, is decreased (Figure 4A). However, as the total Thy1+ T cell numbers is increased in Pdk1flox/flox; Cd4-Cre mice (Figure 4B), the absolute number of Treg cells was not significantly changed. In the thymus and peripheral lymph nodes of Pdk1flox/flox; Cd4-Cre mice both the percentage and absolute number of Treg cells were reduced (Figure 4C and 4D), but this reduction was less than that seen in Cd28−/− mice (Tai et al., 2005). This was consistent with our finding that while PDK1 is important for CD28 signaling to NF-κB, it probably does not abolish all other CD28 initiated pathways (Park et al., 2009). Therefore our data indicates that PDK1 only moderately affected Treg cell development.
Figure 4.
PDK1 significantly affects the Treg cell development. (A) Flow cytometry analysis of Treg cells in the IELs from colon and gating on Thy1+ cells. (B) Flow cytometry analysis of Thy1+ cells in the IELs from colon. (C) Flow cytometry analysis of Treg cells from thymus and peripheral lymph nodes and gating on CD4+ cells. (D) The number of Treg cells in thymus and lymph node (n = 5 mice). Open bars, flox/flox; filled bars, +/flox. Numbers adjacent to outlined areas indicate percent cells in each (A–C).
PDK1 is essential for Treg cell activation and function
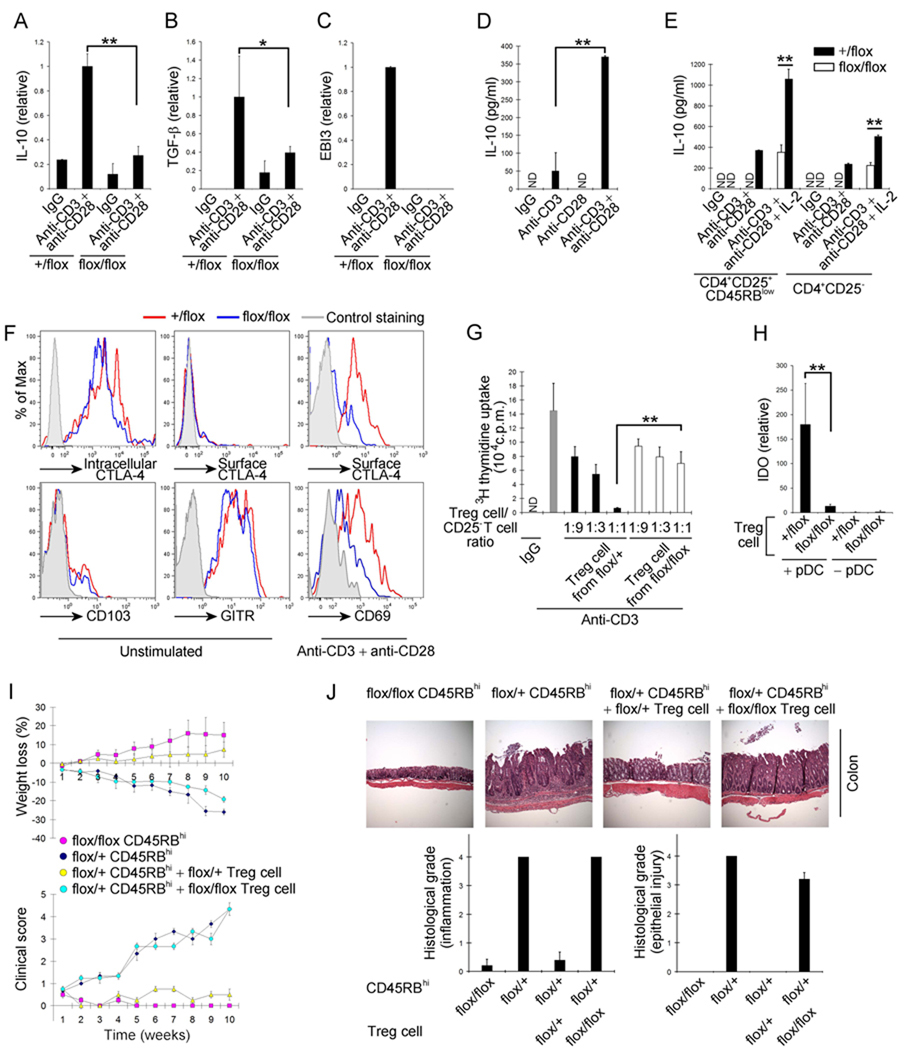
Treg cells produce cytokines including IL-10, TGF-β, and IL-35 (heterodimer of IL-12p35 and Ebi3) during the modulation of immune responses (Shevach, 2009). Therefore, we analyzed the ability of Pdk1flox/flox; Cd4-Cre Treg cells to produce these cytokines upon stimulation with anti-CD3and anti-CD28. While wild-type Treg cells increased mRNA amounts of IL-10, TGF-β, and Ebi3, PDK1 deficient Treg cells failed to up-regulate these cytokines (Figure 5A–C). Interestingly, in WT Treg cells the expression of IL-10, a key cytokine that regulates the inhibitory activity of Treg cells (Rubtsov et al., 2008), was dramatically increased upon CD28 co-ligation (Figure 5D) suggesting that CD28 signaling could have an important role in this Treg cell function. In addition, we found that compared to wild-type Treg cells, PDK1 deficient Treg cells produced less IL-10, as measured by both ELISA and intracellular cytokine staining upon stimulation with anti-CD3 and anti-CD28 (Figure 5E and Figure S4). We demonstrated above that CD28 co-ligation increased IL-10 production, and have previously reported that PDK1 is involved in membrane proximal signaling during CD3 and CD28-mediated T cell activation in CD4+ αβTCR T cell activation (Lee et al., 2005; Park et al., 2009). Consistent with these findings, phorbol 12-myristate 13-acetate (PMA) and ionomycin-mediated stimulation of Treg cells bypassed the requirement for PDK1, inducing equivalent IL-10 production in Pdk1flox/+; Cd4-Cre and Pdk1flox/flox; Cd4-Cre Treg cells (Figure S4). Even though the production of IL-10 from Treg cells is abrogated by Pdk1 deletion, the expression of most Treg cell markers (intracellular CTLA-4 and Foxp3, surface GITR and surface CD103) in resting Treg cells were not significantly affected (Figure 5F). These data are consistent with recent reports that found that extrinsic IL-10 expression, by macrophages, is necessary for the maintenance of Treg cell markers including Foxp3 expression (Murai et al., 2009) while Treg cell intrinsic IL-10 is not required for Foxp3 expression (Rubtsov et al., 2008). However, interestingly, while wild-type Treg cells also expresses the early T cell activation marker CD69 and CTLA-4 on the cell surface after activation with anti-CD3 and anti-CD28, Treg cells lacking Pdk1 show significantly reduced expression of cell surface CD69 and CTLA-4 (Figure 5F). CTLA-4 has been known to be involved in inhibition of T cell proliferation by contact, and in up-regulation of indoleamine 2,3-dioxygenase (IDO) in plasmacytoid dendritic cell (pDC) which is also important for immunomodulation (Puccetti and Grohmann, 2007). Consistent with this, PDK1 deficient Treg cells are only weakly inhibitory (Figure 5G) and mediate weak induction of IDO in pDC (Figure 5H). Therefore these in vitro data suggest that PDK1 is required for activation of Treg cells.
Figure 5.
PDK1 is necessary for the inhibitory function of Treg cells. (A–C), Relative mRNA amounts of IL-10 (A), TGF-β (B), and Ebi3 (C) in Treg cells after activation with anti-CD3 and anti-CD28. (D) ELISA analysis of IL-10 production by WT Treg cells after stimulation with either anti-CD3, anti-CD28, or anti-CD3 and anti-CD28. (E) ELISA analysis of IL-10 production by Treg cells after stimulation with anti-CD3 and anti-CD28 with or without IL-2. Open bars, flox/flox; filled bars, +/flox. (F) Flow cytometry analysis of intracellular CTLA-4, surface CTLA-4, CD103, GITR on Treg cells and of surface CTLA-4 and CD69 on Treg cells after activation with anti-CD3 and anti-CD28. All analyses were done after gating on CD4+CD25+Foxp3+. (G) In vitro inhibition of CD4+ T cell proliferation with Tregs cells. (H) Relative IDO mRNA expression in the pDC by Treg cells activated with anti-CD3 and anti-CD28. (I) Clinical score and weight changes of mice after transfer of CD4+CD25−CD45RBhigh cells with or without Treg cells. (J) Photograph of colon and histological grade of the colitis 10 weeks after the transfer (original magnification 100×). Error bars (A–E and G–H), s.d. of triplicate samples. *P<0.05, **P<0.01.
We also tested the ability of PDK1 deleted Treg cells to work in vivo by determining their ability to suppress experimental colitis induced upon adoptive transfer of CD4+CD45RBhigh T cells into a Rag1−/− mouse. Because PDK1 is necessary for conventional T cell activation, PDK1 deficient CD4+CD45RBhigh T cells could not induce colitis, whereas wild type CD4+CD45RBhigh T cells clearly induced colitis as measured by weight loss and increased clinical scores (Figure 5I). While transfer of wild type Treg cells could prevent the induced colitis, transfer of PDK1 deficient Treg cells failed to prevent colitis (Figure 5J). Therefore these experiments suggested that Treg cells required PDK1 for normal function both in vitro and in vivo.
TCRγδ+ T cells are regulated by Treg derived inhibitory cytokines
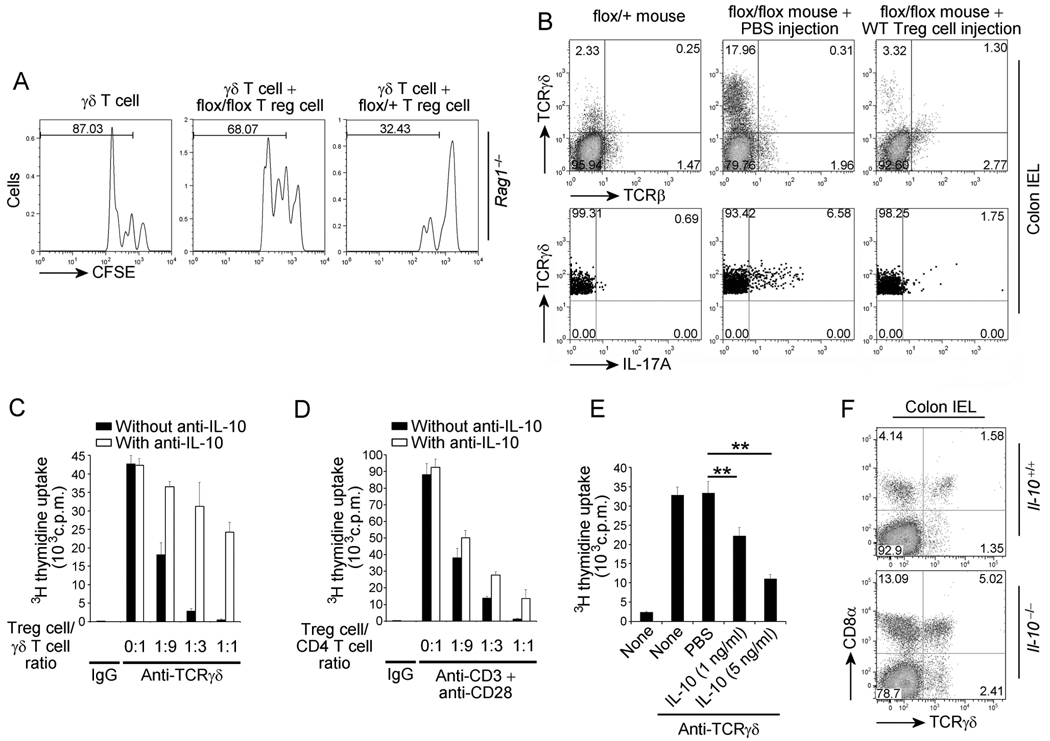
Interestingly, homeostatic proliferation of TCRγδ+ T cells following transfer into Rag1−/− mice is inhibited by wild-type Treg cells, but not by Treg cells from Pdk1flox/flox; Cd4-Cre mice (Figure 6A). Furthermore, TCRγδ+ T cell expansion was dramatically decreased after adoptive transfer of wild-type Treg cells into Pdk1flox/flox; Cd4-Cre mice (Figure 6B, top panel). The IL-17-expressing TCRγδ+ T cell population was also decreased following adoptive transfer of WT Treg cells (Figure 6B, bottom panel). Therefore these data indicated that Treg cells regulate TCRγδ+ T cell proliferation and function in the intestine and suggested that the main cause for the spontaneous colitis was the inability of PDK1 deficient Treg cells to suppress intestinal TCRγδ+ T cells. We also explored the possible mechanism by which Treg cells suppress TCRγδ+ T cell proliferation. Our results indicated that the suppression was not through contact inhibition (data not shown), but that CD4+CD25+ Treg cells could suppress expansion of TCRγδ+ T cells in vitro at Treg: TCRγδ+ T cell ratios similar to that seen for TCRαβ+ T cells (Figure 6C and 6D). Although it has previously been shown that IL-10 contributes little to Treg cell suppression of conventional CD4+ T cell proliferation in vitro (Thornton and Shevach, 1998), the role of IL-10 in the regulation of colitis remains controversial. Thus, while Treg cell intrinsic IL-10 production is not required for suppression of colitis in the transfer model, mice in which IL-10 is deleted only in Treg cells do, in fact, develop spontaneous colitis (Murai et al., 2009; Rubtsov et al., 2008). Furthermore, it has previously been shown that IL-10 efficiently suppresses TCRγδ+ T cell proliferation activated by Mycobacterium tuberculosis in vitro (Pechhold et al., 1994). Therefore, based on the decreased IL-10 production observed in the Pdk1flox/flox; Cd4-Cre mice, we investigated the contribution of IL-10 to Treg cell suppression of TCRγδ+ T cell proliferation. Surprisingly, use of neutralizing anti-IL10 antibody significantly inhibited Treg cell mediated suppression of TCRγδ+ T cell proliferation (Figure 6C) but had a relatively modest effect on proliferation of CD4+ TCRαβ+ T cell suppression (Figure 6D). Consistent with these findings, we found that recombinant IL-10 directly inhibited TCRγδ+ T cell proliferation in vitro (Figure 6E). The residual suppression of TCRγδ+ T cell proliferation observed upon administration of neutralizing anti-IL-10 antibody could be due to the production of other inhibitory cytokines, such as IL-35 (Collison et al., 2007). We found that recombinant IL-35, did indeed have an inhibitory effect on TCRγδ+ T cell proliferation in vitro (Figure S5). Interestingly, CD8α+ TCRγδ+ T cells are also dramatically increased in the IEL of IL-10 deficient mice (Figure 6F), which do develop spontaneous colitis. Given that TCRγδ T cells expand in the absence of IL-10, loss of Treg cell suppression of TCRγδ+ T cell proliferation in vitro by neutralization of IL-10, and inhibition of TCRγδ cell proliferation by its addition, strongly suggest that IL-10 is important for regulation of TCRγδ+ T cell expansion in colon IEL.
Figure 6.
Treg cells regulate proliferation of TCRγδ+ T cells. (A) Flow cytometry for analysis of homeostatic proliferation of CFSE-labeled IEL TCRγδ+ T cells in Rag1−/− mice with or without Treg cells. (B) Flow cytometry of IELs after adoptive transfer of WT Treg cells(top panel) and flow cytometry for analysis of IL-17-expressing TCRγδ+ T cells in the IELs of Pdk1flox/flox; Cd4-Cre mice (flox/flox) with or without adoptive transfer of WT Treg cells, after stimulation with PMA and ionomycin (bottom panel). (C) Treg cell-mediated in vitro inhibition of TCRγδ+ T cell proliferation activated with plate-bound anti-TCRγδ with or without anti-IL-10 neutralizing antibody. (D) Treg cell-mediated in vitro inhibition of CD4+ T cell proliferation activated by anti-CD3 and anti-CD28 with or without IL-10 neutralizing antibody. (E) Inhibition of TCRγδ+ T cell proliferation by IL-10 after activation with plate-bound anti-TCRγδ. (F) Flow cytometry for TCRγδ+ T cells in the IELs of Il-10−/− or Il-10+/+ mice. Error bars (C, D and E), s.d. of triplicate samples. *P<0.05, **P<0.01. Numbers adjacent to outlined areas indicate percent cells in each (A, B, and F).
Functional Treg cell adoptive transfer prevents spontaneous colitis
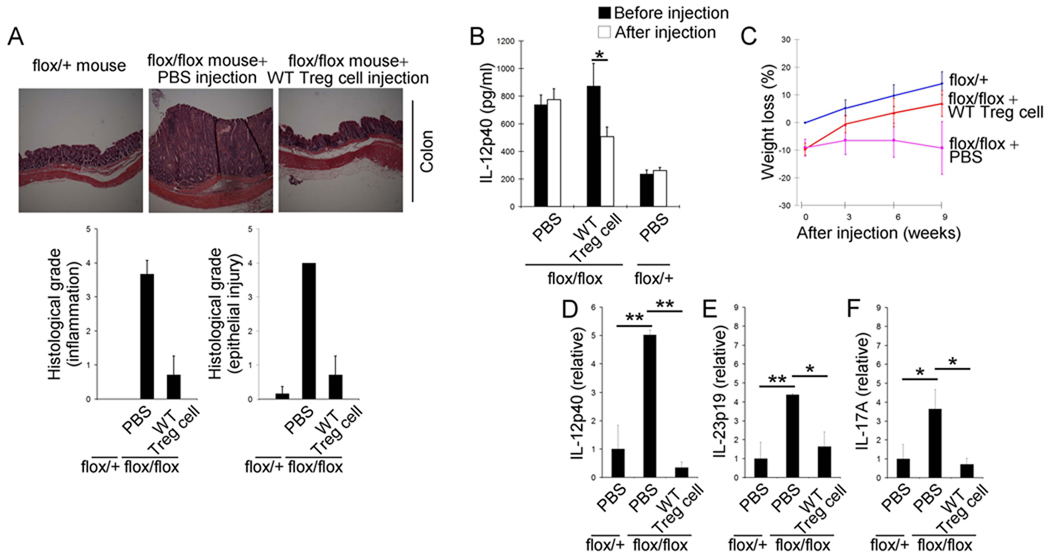
To determine if the spontaneous colitis observed in Pdk1flox/flox; Cd4-Cre mice was due to a defect in Treg cell function, versus an intrinsic dysregulation of TCRγδ+ T cells, we tested whether disease could be prevented by adoptive transfer of wild type Treg cells. Transfer of wild type Treg cells into Pdk1flox/flox; Cd4-Cre mice blocked both the development of spontaneous colitis (Figure 7A), and elevation of serum IL-12p40 (Figure 7B). The average weight of Pdk1flox/flox; Cd4-Cre mice was less than littermate controls, and this weight difference increased as the mice aged (Figure 7C). However, this progressive weight loss was prevented upon the transfer of wild type Treg cells (Figure 7C). Moreover, the increase in amounts of inflammatory cytokines including IL-12p40 (Figure 7D), IL-23p19 (Figure 7E), and IL-17A (Figure 7F) did not occur in Pdk1flox/flox; Cd4-Cre mice receiving WT Treg cells. Therefore the majority of the inflammation indices were reduced upon transfer of wild type Treg cells. Hence these studies demonstrate that regulation of TCRγδ+ T cells in the IEL by Treg cells is important for intestinal homeostasis (Figure S6).
Figure 7.
Treg are essential for TCRγδ+ T cell regulation to prevent spontaneous colitis. (A) Photograph of colon and histological grade of the colitis 9 weeks after injection of WT Treg cells or PBS into Pdk1flox/flox; Cd4-Cre mice (flox/flox) (n=7–12 mice per group) (original magnification 100×). (B) ELISA analysis of serum IL-12p40 3 weeks after injection of WT Treg cells or PBS into Pdk1flox/flox; Cd4-Cre mice (flox/flox) (n=4–5 mice per group). (C) Relative weight changes of Pdk1flox/flox; Cd4-Cre mice (flox/flox) after injection of WT Treg cells or PBS and of Pdk1+/flox; Cd4-Cre mice (+/flox) (n=7–12 mice per group). (D–F) Relative mRNA amounts of IL-12p40 (D), IL-23p19 (E), and IL-17A (F) in colon (n=3–4 mice per each group). Error bars (D–F), s.d. of triplicate samples. *P<0.05, **P<0.01.
DISCUSSION
The regulation of intestinal inflammatory homeostasis is a complex biological process, breakdown of which results in human disease states including inflammatory bowel diseases and even colon cancer. It is known that Rag1−/− mice do not display hyperinflammation against normal commensal bacteria, which suggests that the immune system without T- and B- cells is capable of regulating commensal bacteria without sequelae. However, Rag1−/− mice cannot mount efficient defenses against pathogenic bacterial infection in the intestine. This indicates the necessity of a highly regulated system for efficient defense against pathogenic bacterial infection, without upsetting normal immune homeostasis.
The intestinal IEL compartment interfaces with intestinal commensal bacteria and the main T cell population in the IEL are TCRγδ+ T cells. TCRγδ+ T cells are believed to be a fast acting T cell population because they can be activated without professional APCs. TCRγδ+ T cells have been shown to be critical for defense against intestinal bacteria because the Tcrd−/− mouse and TCRγδ+ T cell depleted mouse are susceptible to dissemination of pathogenic bacteria from the intestine (Egan et al., 2005; Mixter et al., 1994). However, under normal conditions, TCRγδ+ T cells should be tolerant of commensal bacteria.
Recognition of bacterial protein or non-protein antigen in the IEL compartment can lead to the activation of TCRγδ+ T cells (Hayday and Tigelaar, 2003; Nanno et al., 2007). According to our data, most of TCRγδ+ T cell in the IEL are constitutively activated, as indicated by the expression of the activation marker CD69, whereas TCRγδ+ T cells in LP are negative for the CD69 marker. Our study has demonstrated that TCRγδ+ T cells can cause the development of spontaneous colitis and interestingly. Pdk1flox/flox; Cd4-Cre mice have more activated TCRγδ+ T cells in the IEL compartment, whereas the majority of the TCRγδ+ T cells in the LP compartment still remain negative for the expression of CD69. Moreover, the TCRγδ+ T cell population in the LP compartment is not increased in colitic mice, but the TCRγδ+ T cell population in the IEL compartment is. Finally, antibiotic treatment abrogates this increase in TCRγδ+ T cells in the IEL compartment in mice in which the Pdk1 gene is deleted. Thus TCRγδ+ T cells are activated and expanded in the IEL compartment due to recognition of commensal bacteria, and Pdk1flox/flox; Cd4-Cre mice cannot control the activation and expansion of these TCRγδ+ T cells.
IL-17 is increased in the colitic area and this may contribute to the development of colitis (Fujino et al., 2003; Yen et al., 2006). For TCRγδ+ T cells, it is known that IL-17A is mainly produced by the Vγ4 subset of TCRγδ+ T cells (Martin et al., 2009). Consistent with this, we find expansion of the Vγ4 TCRγδ+ T cell population and enrichment of this population amongst the IL-17A-expressing TCRγδ+ T cell population and amongst the IEL TCRγδ+ T cell population as a whole. These data indicate that abnormal expansion of a potential proinflammatory TCRγδ+ T cell variant may contribute to the disease development such as colitis. IL-17 has a complex role in colitis (O'Connor et al., 2010). Although it has recently been reported that IL-17 has a protective role in the adoptive transfer model of experimental colitis that is mediated by CD4+ T cells (O'Connor et al., 2009), IL-17 is an important mediator of spontaneous colitis in Il-10 gene deficient mice (Yen et al., 2006). Thus, IL-17 production by TCRγδ T cells in Pdk1flox/flox; Cd4-Cre mice may contribute to the development of intestinal inflammation in these mice.
PDK1 is regulated by the PI3K signaling pathway and activation of this signaling cascade triggers activation of several transcription factors including NF-κB, NFATc, and AKT (Mora et al., 2004; Nirula et al., 2006; Park et al., 2009). The effect of deleting the Pdk1 gene in Treg cells is similar to the effect of mutating or deleting PI3K in mice (Fruman and Bismuth, 2009; Oak et al., 2006; Patton et al., 2006). We found that Treg cells do not efficiently produce IL-10 without activation, and that CD28 ligation dramatically increased IL-10 production from activated Treg cells. PDK1 deficient Treg cells also failed to induce IL-10 in response to CD3 and CD28 ligation, but could induce IL-10 when triggered using PMA and ionomycin which acts downstream of PDK1. In addition, expression of other cytokine genes such as TGF-β and Ebi3 (subunit of IL-35) was impaired by Pdk1 gene deletion in Treg cells. Thus, PDK1 is involved in Treg cell activation, particularly with regard to cytokine production.
As TCRγδ+ T cells recognize antigen without APCs (Hayday and Tigelaar, 2003; Nanno et al., 2007), TCRγδ+ T cell may not recognize the same antigens as Treg cells. Previous reports have shown that intestinal dendritic cells take up bacterial antigen and present these antigens to Treg cells for their expansion and activation (Smits et al., 2005; Strauch et al., 2005). Therefore, the production of inhibitory cytokines such as IL-10 by activated Treg cells may help regulate TCRγδ+ T cells. As Treg cell specific Il-10 gene deletion leads to development of colitis(Rubtsov et al., 2008), it is possible that Treg cell mediated production of IL-10 may be crucial to suppression of TCRγδ+ T cells. Indeed, we found that Treg cell IL-10 was necessary for suppressing TCRγδ+ T cell proliferation in vitro and that in the absence of IL-10, TCRγδ+ T cells underwent significant expansion in vivo. Thus these data may help explain the unclear role of IL-10 in colitis. In the transfer model of colitis, mediated by TCRαβ+ T cells, Treg cell production of IL-10 has little bearing on disease. However, loss of Treg cell IL-10, which leads to development of colitis without the massive lymphoproliferation characteristic of Treg cell deficient animals, likely affects the ability of Treg cells to suppress TCRγδ+ T cell proliferation. Therefore, while Treg cell extrinsic IL-10 production is necessary for maintenance of Treg cell encodedFOXP3 expression and IL-10 independent suppression of CD4+ T cells in the transfer model of colitis (Murai et al., 2009), Treg cell produced IL-10 likely is crucial to suppression of TCRγδ+ T cells and prevention of spontaneous colitis in the Pdk1flox/flox; Cd4-Cre mouse.
In summary, even though conventional TCRαβ+ T cells cannot be activated in Cd4-Cre-mediated PDK1 deleted mice, these mice developed spontaneous colitis. The spontaneous colitis was prevented by deletion of the Tcrd gene, demonstrating the active role of TCRγδ+ T cells in this process. Moreover, transfer of WT Treg cellss blocked spontaneous colitis and inhibited TCRγδ+ T cell expansion in the colonic intraepithelial compartment of these mice. Furthermore, colitis was prevented by treatment with antibiotics, suggesting TCRγδ+ T cells are continuously activated by commensal bacteria and can induce spontaneous colitis if the TCRγδ+ T cells are not regulated by Treg cells. Our in vitro data demonstrate that Treg cells can suppress TCRγδ+ T cell proliferation through IL-10 and that PDK1 deleted Treg cells are deficient in IL-10 production. Our in vivo experimental model shows that functional Treg cells are important for suppressing TCRγδ+ T cell responses against commensal bacteria. Thus, suppression of TCRγδ+ T cells by Treg cells occurs in vivo and is important for maintaining normal intestinal homeostasis.
EXPERIMENTAL PROCEDURES
Mice
Pdk1flox/flox mice(Hashimoto et al., 2006) were first bred with Cd4-Cre transgenic mice(Lee et al., 2001) and offspring were bred with Pdk1flox/flox mice to generate Pdk1flox/flox; Cd4-Cre and Pdk1+/flox; Cd4-Cre. For making of PDK1 and TCRδ double deficient mice, Pdk1flox/flox; Cd4-Cre mice were bred with TCRδ deficient (Tcrd−/−) mice. All mice were kept in specific pathogen–free conditions in the animal care facility at Columbia University (New York, New York) or Yale University (New Haven, Connecticut). All mouse experiments were approved by Institutional Animal Care and Use Committee of Columbia University and Yale University.
Antibodies and reagents
Fluorescein isothiocyanate-conjugated anti-mouse TCRβ (H57-597), allophycocyanin-conjugated anti-mouse CD19 (1D3), phycoerythrin-conjugated anti-mouse CD4 (GK1.5), allophycocyanin-conjugated anti-mouse CD8α (53-6.7), fluorescein isothiocyanate-conjugated anti-mouse CD8α (53-6.7), allophycocyanin-conjugated anti-mouse CD8a (53-6.7), fluorescein isothiocyanate-conjugated anti-mouse TCRγδ (UC7-13D5), allophycocyanin-conjugated anti-mouse IL-17 (eBio17B7), phycoerythrin-conjugated anti-mouse thy1 (53-2.1), peridinin chlorophyll protein–conjugated anti-mouse CD3ε (17A2), peridinin chlorophyll protein–Cy5.5-conjugated anti-mouse CD25 (PC61.5), and alexa 647-conjugated anti-mouse Foxp3 (FJK-16s) were purchased from eBioscience. IL-10 was purchased from eBioscience. IL-35 (IL-35 2A and IL-35 GS) was produced in HEK293 cells through transfection of pIg-IL-35-2A or pIg-IL-35-GS. pIg-NEO was used for the control.
Flow cytometry and ELISA analysis
Cells were isolated from the thymi, lymph nodes, and intestine of Pdk1+/flox; Cd4-Cre or Pdk1flox/flox; Cd4-Cre mice. The cells were stained with indicated antibodies and analyzed on a FACSCalibur instrument. Live cells were gated for analysis. Intracellular Foxp3 was stained with BD Cytofix-Cytoperm™ Plus and Alexa 647-conjugated anti-mouse Foxp3 antibody. Secreted-IL-10 amount was analyzed by IL-10 specific ELISA (eBioscience). Briefly, 5 × 104 cells per well were plated in 96 well plates coated with either anti-mouse CD3ε (5 µg/ml), anti-mouse CD28 (5 µg/ml), or both anti-mouse CD3ε (5 µg/ml) and anti-mouse CD28 (5 µg/ml). And then, the cells were incubated at 37 °C and 5% CO2. 48 hr later, the culture medium was analyzed.
Colon Organ Culture
In brief, 1 cm × 1 cm standardized segments of all three parts of the colon were washed in cold PBS supplemented with penicillin and streptomycin (Invitrogen). These segments were cultured in 24-well flat-bottom culture plates (Falcon) in serum-free RPMI 1640 medium (Invitrogen) supplemented with penicillin and streptomycin. After 24 hr, supernatant fluid was collected and was used for estimation of IL-12p40 secretion.
Isolation of RNA and Quantitative Reverse Transcriptase PCR
For quantitative RT-PCR, tissue was chopped and homogenized with a sterile syringe piston in TRIZOL (Invitrogen), and total RNA was isolated according to manufacturer's instructions. DNA was digested with DNase I and the DNaseI was heat inactivated. RNA was reverse transcribed by Superscript II (Invitrogen), and cDNAs were used for PCR with Quantitect SYBR Green reagents (Qiagen, Valencia, CA) on a Stratagene MX3000 bioanalyzer (La Jolla, CA). The abundance of each cytokine mRNA was normalized to β-actin expression and compared to the same mRNAs in WT intestines to calculate the fold induction. Sequences of primers available upon request.
IEL preparation
IELs were prepared as previously reported (Weigmann et al., 2007) with slight modification. Briefly, intestine was washed with ice-cold PBS and cut into the small pieces that were incubated with IEL preparation buffer containing 1× HBSS containing 5% FBS, 1mM DTT, and 5 mM EDTA at 37 °C shaking incubator for 20 min twice. The supernants were collected and filtered by 40 µM cell strainer. Cells were washed with 1× HBSS three times. For flow cytometry analysis, lymphocytes were gated.
Functional assessment of Treg cells in vivo
CD4+CD25−CD45RBhigh or CD4+CD25+CD45RBlow T cells were isolated from Pdk1+/flox; Cd4-Cre or Pdk1flox/flox; Cd4-Cre by using a MoFlow (Dako) or FACSAria instrument (BD Biosciences) on the basis of the staining. At day 0, Rag1 deficient (Rag1−/−) mice were injected intravenously with 4× 105 CD4+CD25−CD45RBhigh T cells alone or in combination with 1× 105 CD4+CD25+CD45RBlow T cells. Clinical scores were monitored every week for signs of colitis. The clinical score was defined as follows: Wasting: 0, no wasting; 1, 0.1–10% loss of initial total body weight; 2, >10% loss of initial total body weight; diarrhea: 0, none; 1, soft stool; 2, watery and/or bloody; hunching, bristled fur, and skin lesions: 0, unchanged; 1, any positive sign; rectum prolapse: 0, absent; 1, present. 8–12 wk after transfer, mice were killed and colons were dissected, fixed in 4% buffered formalin, and embedded in paraffin for histological analysis.
In vitro suppression assays
5× 104 CD4+CD25− cells were cultured in 96-well plates with 105 irradiated T cell–depleted splenocytes and 0.5 µg/ml anti-CD3ε (BD Biosciences). CD4+CD25+CD45RBlow cells from lymph nodes of Pdk1+/flox; Cd4-Cre or Pdk1flox/flox; Cd4-Cre mice were added to the culture in various ratios as indicated. The T cell proliferation was analyzed by pulsing the cells for 12 hrs with 1 µCi [3H]thymidine after 60 hr of stimulation. For suppression of TCRγδ+ T cells, Treg cells were preactivated with anti-CD3 and anti-CD28.
Histology and scoring
The specimens were fixed with 4% neutral-buffered formalin and embedded in paraffin. Sections were cut, deparaffinized, and stained with hematoxylin and eosin. The stained intestinal sections were graded by a blinded scorer. Histological grading was based on observed inflammation (0–4) and observed epithelial injury (0–4). Clinical scores of observed inflammation were defined as follows: 0, no increase inflammation; 1, low level of inflammation with mildly increased inflammatory cells in the lamina propria; 2, moderately increased inflammation in the lamina propria (multiple foci); 3, high level of inflammation with evidence of wall thickening by inflammation; 4, maximal severity of inflammation with transmural leukocyte infiltration and/or architectural distortion. Grading scores of observed epithelial injury was defined as follows: 0, normal or no inflammation by neutrophils; 1, occasional epithelial lesion (focal and superficial or rare cryptitis); 2, foci of cryptitis, including rare crypt abscess; 3, multiple crypt abscess and/or focal ulceration; 4, grade 3 + extensive ulceration.
Intracellular IL-17 Staining and flow cytometry
For intracellular IL-17 staining, freshly isolated IELs were cultured for 5 hr in complete media (RPMi1640 containing 5% FBS) supplemented with 50 ng/ml PMA, 500 ng/ml ionomycin, and 1 µl/ml BD GolgiPlug (BD Pharmingen) in 96-well plates. Cell were harvested and processed with the Intracellular Cytokine Staining kit (BD Pharmingen). Cells were stained with anti-TCRγδ and anti-IL-17. The stained cells were analyzed by FACSCalibur flow cytometers (Becton-Dickenson).
Homeostatic proliferation of TCRγδ+ T cell in intestinal intraepithelial cell
TCRγδ+ T cells were isolated from lymph nodes of Pdk1flox/flox; Cd4-Cre mice by using a MoFlow (Dako) or FACSAria (BD Biosciences) instrument on the basis of the staining. The isolated TCRγδ+ T cells were stained with 5 µM of CFSE for 5 min and then the labeling was stopped by addition of FBS. After wash with PBS, the cells were injected into Rag1−/− mice and 5 days later from the injection, IELs were prepared from the mice and the proliferation was analyzed by flow cytometry.
Adoptive transfer of WT Treg cells to rescue the spontaneous colitis
WT Treg cells (CD4+CD25+CD45RBlow) were isolated from lymph nodes of Pdk1+/flox; Cd4-Cre mice by using a MoFlow (Dako) or FACSAria (BD Biosciences) instrument on the basis of the staining. Pdk1flox/flox; Cd4-Cre mice (10 – 12 week old mice) were injected intravenously three times with the isolated WT Treg cells (1× 105 per mouse) every 3 weeks. After 3 weeks from the last injection of Treg cells, colon tissue samples were collected for histological analysis and mRNA expression analysis.
Statistical analyses
All statistical analyses were performed using the two-tailed Student’s t-test with unequal variance.
Supplementary Material
ACKNOWLEDGEMENTS
We thank M. Kasuga (Kobe University) for the PDK1 conditional knockout mice and C. Wilson (University of Washington) for Cd4-Cre mice. Supported by the National Institutes of Health (R01-AI59440).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Awasthi A, Kuchroo VK. Th17 cells: from precursors to players in inflammation and infection. Int Immunol. 2009;21:489–498. doi: 10.1093/intimm/dxp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A, Mota-Santos T, Itohara S, Degermann S, Heusser C, Tonegawa S, Coutinho A. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. 1990;172:239–244. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Beyersdorf N, Hanke T, Kerkau T, Hunig T. Superagonistic anti-CD28 antibodies: potent activators of regulatory T cells for the therapy of autoimmune diseases. Ann Rheum Dis. 2005;64 Suppl 4:iv91–iv95. doi: 10.1136/ard.2005.042564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan AK, Mizoguchi E, Smith RN, Mizoguchi A. Spontaneous chronic colitis in TCR alpha-mutant mice; an experimental model of human ulcerative colitis. Int Rev Immunol. 2000;19:123–138. doi: 10.3109/08830180009048393. [DOI] [PubMed] [Google Scholar]
- Bonneville M. Selection of intraepithelial gammadelta cells: the Holy GrIEL at last? Nat Immunol. 2006;7:791–792. doi: 10.1038/ni0806-791. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- Egan CE, Dalton JE, Andrew EM, Smith JE, Gubbels MJ, Striepen B, Carding SR. A requirement for the Vgamma1+ subset of peripheral gammadelta T cells in the control of the systemic growth of Toxoplasma gondii and infection-induced pathology. J Immunol. 2005;175:8191–8199. doi: 10.4049/jimmunol.175.12.8191. [DOI] [PubMed] [Google Scholar]
- Elson CO. Commensal bacteria as targets in Crohn's disease. Gastroenterology. 2000;119:254–257. doi: 10.1053/gast.2000.9159. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Bismuth G. Fine tuning the immune response with PI3K. Immunol Rev. 2009;228:253–272. doi: 10.1111/j.1600-065X.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Kido Y, Uchida T, Asahara S, Shigeyama Y, Matsuda T, Takeda A, Tsuchihashi D, Nishizawa A, Ogawa W, et al. Ablation of PDK1 in pancreatic beta cells induces diabetes as a result of loss of beta cell mass. Nat Genet. 2006;38:589–593. doi: 10.1038/ng1774. [DOI] [PubMed] [Google Scholar]
- Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- Hayday AC, Saito H, Gillies SD, Kranz DM, Tanigawa G, Eisen HN, Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985;40:259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Inagaki-Ohara K, Chinen T, Matsuzaki G, Sasaki A, Sakamoto Y, Hiromatsu K, Nakamura-Uchiyama F, Nawa Y, Yoshimura A. Mucosal T cells bearing TCRgammadelta play a protective role in intestinal inflammation. J Immunol. 2004;173:1390–1398. doi: 10.4049/jimmunol.173.2.1390. [DOI] [PubMed] [Google Scholar]
- Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H, Nakamura T, Shimizu M, Kawabata D, Yukawa N, et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009;60:2294–2303. doi: 10.1002/art.24687. [DOI] [PubMed] [Google Scholar]
- Lee KY, D'Acquisto F, Hayden MS, Shim JH, Ghosh S. PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science. 2005;308:114–118. doi: 10.1126/science.1107107. [DOI] [PubMed] [Google Scholar]
- Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Reddy P, Lowler KP, Liu C, Bishop DK, Ferrara JL. Critical role of host gammadelta T cells in experimental acute graft-versus-host disease. Blood. 2005;106:749–755. doi: 10.1182/blood-2004-10-4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay LD, Li B, Biancaniello R, Creighton MA, Bachwich D, Lichtenstein G, Rombeau JL, Carding SR. Changes in human mucosal gamma delta T cell repertoire and function associated with the disease process in inflammatory bowel disease. Mol Med. 1997;3:183–203. [PMC free article] [PubMed] [Google Scholar]
- Mixter PF, Camerini V, Stone BJ, Miller VL, Kronenberg M. Mouse T lymphocytes that express a gamma delta T-cell antigen receptor contribute to resistance to Salmonella infection in vivo. Infect Immun. 1994;62:4618–4621. doi: 10.1128/iai.62.10.4618-4621.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann GM, Tonegawa S, Nagler-Anderson C, Bhan AK. Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med. 1996;183:847–856. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanno M, Kanari Y, Naito T, Inoue N, Hisamatsu T, Chinen H, Sugimoto K, Shimomura Y, Yamagishi H, Shiohara T, et al. Exacerbating role of gammadelta T cells in chronic colitis of T-cell receptor alpha mutant mice. Gastroenterology. 2008;134:481–490. doi: 10.1053/j.gastro.2007.11.056. [DOI] [PubMed] [Google Scholar]
- Nanno M, Shiohara T, Yamamoto H, Kawakami K, Ishikawa H. gammadelta T cells: firefighters or fire boosters in the front lines of inflammatory responses. Immunol Rev. 2007;215:103–113. doi: 10.1111/j.1600-065X.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- Nirula A, Ho M, Phee H, Roose J, Weiss A. Phosphoinositide-dependent kinase 1 targets protein kinase A in a pathway that regulates interleukin 4. J Exp Med. 2006;203:1733–1744. doi: 10.1084/jem.20051715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor W, Jr, Zenewicz LA, Flavell RA. The dual nature of T(H)17 cells: shifting the focus to function. Nat Immunol. 2010;11:471–476. doi: 10.1038/ni.1882. [DOI] [PubMed] [Google Scholar]
- Oak JS, Deane JA, Kharas MG, Luo J, Lane TE, Cantley LC, Fruman DA. Sjogren's syndrome-like disease in mice with T cells lacking class 1A phosphoinositide-3-kinase. Proc Natl Acad Sci U S A. 2006;103:16882–16887. doi: 10.1073/pnas.0607984103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst C, Schirutschke H, Ehninger G, Bornhauser M, Platzbecker U. The graft content of donor T cells expressing gamma delta TCR+ and CD4+foxp3+ predicts the risk of acute graft versus host disease after transplantation of allogeneic peripheral blood stem cells from unrelated donors. Clin Cancer Res. 2007;13:2916–2922. doi: 10.1158/1078-0432.CCR-06-2602. [DOI] [PubMed] [Google Scholar]
- Park SG, Schulze-Luehrman J, Hayden MS, Hashimoto N, Ogawa W, Kasuga M, Ghosh S. The kinase PDK1 integrates T cell antigen receptor and CD28 coreceptor signaling to induce NF-kappaB and activate T cells. Nat Immunol. 2009;10:158–166. doi: 10.1038/ni.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, Leung E, Rowan WC, Sancho S, Walker LS, Vanhaesebroeck B, Okkenhaug K. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- Pechhold K, Wesch D, Schondelmaier S, Kabelitz D. Primary activation of V gamma 9-expressing gamma delta T cells by Mycobacterium tuberculosis. Requirement for Th1-type CD4 T cell help and inhibition by IL-10. J Immunol. 1994;152:4984–4992. [PubMed] [Google Scholar]
- Pereira P, Lafaille JJ, Gerber D, Tonegawa S. The T cell receptor repertoire of intestinal intraepithelial gammadelta T lymphocytes is influenced by genes linked to the major histocompatibility complex and to the T cell receptor loci. Proc Natl Acad Sci U S A. 1997;94:5761–5766. doi: 10.1073/pnas.94.11.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- Saurer L, Mueller C. T cell-mediated immunoregulation in the gastrointestinal tract. Allergy. 2009;64:505–519. doi: 10.1111/j.1398-9995.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Shiohara T, Moriya N, Hayakawa J, Itohara S, Ishikawa H. Resistance to cutaneous graft-vs.-host disease is not induced in T cell receptor delta gene-mutant mice. J Exp Med. 1996;183:1483–1489. doi: 10.1084/jem.183.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Hollander GA, Mizoguchi E, Allen D, Bhan AK, Wang B, Terhorst C. Expression of pro-inflammatory cytokines by TCR alpha beta+ and TCR gamma delta+ T cells in an experimental model of colitis. Eur J Immunol. 1997;27:17–25. doi: 10.1002/eji.1830270104. [DOI] [PubMed] [Google Scholar]
- Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, Zaat BA, Yazdanbakhsh M, Wierenga EA, van Kooyk Y, Kapsenberg ML. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Strauch UG, Obermeier F, Grunwald N, Gurster S, Dunger N, Schultz M, Griese DP, Mahler M, Scholmerich J, Rath HC. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005;54:1546–1552. doi: 10.1136/gut.2004.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Nakamura K, Honda K, Kitamura Y, Mizutani T, Araki Y, Kabemura T, Chijiiwa Y, Harada N, Nawata H. An inverse correlation of human peripheral blood regulatory T cell frequency with the disease activity of ulcerative colitis. Dig Dis Sci. 2006;51:677–686. doi: 10.1007/s10620-006-3191-2. [DOI] [PubMed] [Google Scholar]
- Targan SR, Karp LC. Defects in mucosal immunity leading to ulcerative colitis. Immunol Rev. 2005;206:296–305. doi: 10.1111/j.0105-2896.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toto P, Feliciani C, Amerio P, Suzuki H, Wang B, Shivji GM, Woodley D, Sauder DN. Immune modulation in pemphigus vulgaris: role of CD28 and IL-10. J Immunol. 2000;164:522–529. doi: 10.4049/jimmunol.164.1.522. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Stockinger B. TGFbeta1, a "Jack of all trades": the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 2006;27:358–361. doi: 10.1016/j.it.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Videla S, Vilaseca J, Guarner F, Salas A, Treserra F, Crespo E, Antolin M, Malagelada JR. Role of intestinal microflora in chronic inflammation and ulceration of the rat colon. Gut. 1994;35:1090–1097. doi: 10.1136/gut.35.8.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MM, Melgar S, Baranov V, Oberg A, Danielsson A, Hammarstrom S, Hammarstrom ML. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut. 2000;47:215–227. doi: 10.1136/gut.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.