Abstract
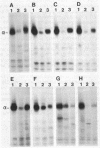
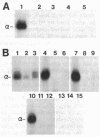
Anti-peptide antibodies that distinguish between the rat brain sodium channel subtypes referred to as RI and RII were prepared and used to determine their relative expression in nerve and muscle tissues. Sodium channels purified from rat brain are approximately 18% RI and 80% RII. In brain, the RII subtype is preferentially expressed with RI/RII ratios ranging from 0.07 in the hippocampus to 0.17 in the cerebral cortex. The RI subtype is preferentially expressed in more caudal areas of the central nervous system with values of RI/RII of 0.98 for medulla oblongata and 2.2 for spinal cord. Expression of additional unidentified sodium channel subtype(s) is detected in midbrain, medulla, and spinal cord, and expression of unidentified sodium channel subtypes predominates over expression of RI and RII in retina and optic nerve. The RI and RII subtypes are primarily expressed in the central nervous system and are not detected in significant numbers in skeletal or cardiac muscle, sympathetic ganglia, adrenal medulla, sciatic nerve, or cauda equina. The RII subtype appears first in development of both brain and spinal cord but declines in adult spinal cord as the RI subtype increases. The strict regional expression of these two sodium channel subtypes suggests that they may have distinct functional properties or physiological roles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S. Voltage-regulated sodium channel molecules. Annu Rev Physiol. 1984;46:517–530. doi: 10.1146/annurev.ph.46.030184.002505. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Tanaka J. C., Furman R. E. Molecular characteristics and functional reconstitution of muscle voltage-sensitive sodium channels. J Cell Biochem. 1984;26(3):135–146. doi: 10.1002/jcb.240260302. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Weigele J. B., Chalikian D. M., Murphy L. E. Muscle surface membranes: preparative methods affect apparent chemical properties and neurotoxin binding. Biochim Biophys Acta. 1979 Jan 5;550(1):59–76. doi: 10.1016/0005-2736(79)90115-9. [DOI] [PubMed] [Google Scholar]
- Baumgold J., Zimmerman I., Bambrick L. Appearance of [3H]saxitoxin binding sites in developing rat brain. Brain Res. 1983 Sep;285(3):405–407. doi: 10.1016/0165-3806(83)90040-8. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Morrow C. S., Hartshorne R. P. Neurotoxin binding to receptor sites associated with voltage-sensitive sodium channels in intact, lysed, and detergent-solubilized brain membranes. J Biol Chem. 1979 Nov 25;254(22):11379–11387. [PubMed] [Google Scholar]
- Catterall W. A. The molecular basis of neuronal excitability. Science. 1984 Feb 17;223(4637):653–661. doi: 10.1126/science.6320365. [DOI] [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Cyclic AMP-dependent phosphorylation of the alpha subunit of the sodium channel in synaptic nerve ending particles. J Biol Chem. 1984 Jul 10;259(13):8210–8218. [PubMed] [Google Scholar]
- Goldin A. L., Snutch T., Lübbert H., Dowsett A., Marshall J., Auld V., Downey W., Fritz L. C., Lester H. A., Dunn R. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7503–7507. doi: 10.1073/pnas.83.19.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984 Feb 10;259(3):1667–1675. [PubMed] [Google Scholar]
- Lombet A., Kazazoglou T., Delpont E., Renaud J. F., Lazdunski M. Ontogenic appearance of Na+ channels characterized as high affinity binding sites for tetrodotoxin during development of the rat nervous and skeletal muscle systems. Biochem Biophys Res Commun. 1983 Feb 10;110(3):894–901. doi: 10.1016/0006-291x(83)91046-x. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B., Strichartz G. R. A new method for labelling saxitoxin and its binding to non-myelinated fibres of the rabbit vagus, lobster walking leg, and garfish olfactory nerves. J Physiol. 1976 Oct;261(2):477–494. doi: 10.1113/jphysiol.1976.sp011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., Rossie S., Catterall W. A. A large intracellular pool of inactive Na channel alpha subunits in developing rat brain. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4847–4851. doi: 10.1073/pnas.82.14.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Miledi R. Partial purification and functional expression of brain mRNAs coding for neurotransmitter receptors and voltage-operated channels. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7994–7998. doi: 10.1073/pnas.81.24.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth B. R., Hafemann D. R. Tetrodotoxin binding as a marker for functional differentiation of various brain regions during chick and mouse development. J Neurochem. 1975 Feb;24(2):261–270. doi: 10.1111/j.1471-4159.1975.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Schmidt J. W., Catterall W. A. Glycosylation is required for maintenance of functional sodium channels in neuroblastoma cells. J Biol Chem. 1983 Apr 25;258(8):5117–5123. [PubMed] [Google Scholar]
- Wollner D. A., Catterall W. A. Antigenic differences among the voltage-sensitive sodium channels in the peripheral and central nervous systems and skeletal muscle. Brain Res. 1985 Apr 1;331(1):145–149. doi: 10.1016/0006-8993(85)90724-3. [DOI] [PubMed] [Google Scholar]