Abstract
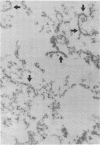
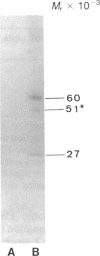
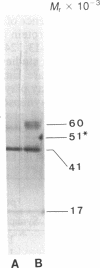
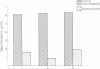
Rat superior cervical sympathetic ganglion was used to begin studying the regulation of molecular components of the synapse. Ganglionic postsynaptic densities (PSDs)exhibited a thin, disc-shaped profile electron microscopically, comparable to that described for brain. Moreover, the presumptive ganglionic PSD protein (PSDp) was phosphorylated in the presence of Ca2+ and calmodulin, bound 125I-labeled calmodulin, and exhibited a Mr of 51,000, all characteristic of the major PSD protein of brain. These initial studies indicated that ganglionic PSDp and the major PSD protein of brain are comparable, allowing us to study synaptic regulation in the well-defined superior cervical sympathetic ganglion. To obtain enough quantities of ganglionic PSDp, we used synaptic membrane fractions. During postnatal development, calmodulin binding to the ganglionic PSDp increased 411-fold per ganglion from birth to 60 days, whereas synaptic membrane protein increased only 4.5-fold. Consequently, different synaptic components apparently develop differently. Moreover, denervation of the superior cervical sympathetic ganglion in adult rats caused an 85% decrease in ganglionic PSDp-calmodulin binding, but denervation caused no change in synaptic membrane protein 2 weeks postoperatively. Our observations suggest that presynaptic innervation selectively regulates specific molecular components of the postsynaptic membrane structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. K., Zur Nedden D., Heintzelman M., Hunkapiller M., Zoon K. Biologic activity in a fragment of recombinant human interferon alpha. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1045–1047. doi: 10.1073/pnas.81.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen T. J., Keller C. H., LaPorte D. C., Edelman A. M., Storm D. R. Preparation of azidocalmodulin: a photoaffinity label for calmodulin-binding proteins. Proc Natl Acad Sci U S A. 1981 May;78(5):2782–2785. doi: 10.1073/pnas.78.5.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Chikaraishi D. M., Lewis E. J. Trans-synaptic increase in RNA coding for tyrosine hydroxylase in a rat sympathetic ganglion. Brain Res. 1985 Jul 22;339(1):151–153. doi: 10.1016/0006-8993(85)90635-3. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Effects of surgical decentralization and nerve growth factor on the maturation of adrenergic neurons in a mouse sympathetic ganglion. J Neurochem. 1972 May;19(5):1367–1377. doi: 10.1111/j.1471-4159.1972.tb01461.x. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I. A., Iversen L. L. Trans-synaptic regulation of growth and development of adrenergic neurones in a mouse sympathetic ganglion. Brain Res. 1971 Nov;34(2):229–240. doi: 10.1016/0006-8993(71)90278-2. [DOI] [PubMed] [Google Scholar]
- Black I. B., Hendry I., Iversen L. L. Differences in the regulation of tyrosine hydroxylase and dopa decarboxylase in sympathetic ganglia and adrenals. Nat New Biol. 1971 May 5;231(18):27–29. [PubMed] [Google Scholar]
- Bohn M. C., McEwen B., Luine V. N., Black I. B. Development and characterization of glucocorticoid receptors in rat superior cervical ganglion. Brain Res. 1984 Jun;316(2):211–218. [PubMed] [Google Scholar]
- Buc-Caron M. H., Nystrom P., Fischbach G. D. Induction of acetylcholine receptor synthesis and aggregation: partial purification of low-molecular-weight activity. Dev Biol. 1983 Feb;95(2):378–386. doi: 10.1016/0012-1606(83)90039-8. [DOI] [PubMed] [Google Scholar]
- Bursztajn S., Fischbach G. D. Evidence that coated vesicles transport acetylcholine receptors to the surface membrane of chick myotubes. J Cell Biol. 1984 Feb;98(2):498–506. doi: 10.1083/jcb.98.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Cohen R. S., Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980 Sep;86(3):831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Siekevitz P. Function of a calmodulin in postsynaptic densities. III. Calmodulin-binding proteins of the postsynaptic density. J Cell Biol. 1981 Jun;89(3):449–455. doi: 10.1083/jcb.89.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. S., Blomberg F., Berzins K., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. I. Overall morphology and protein composition. J Cell Biol. 1977 Jul;74(1):181–203. doi: 10.1083/jcb.74.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Banker G., Churchill L., Taylor D. Isolation of postsynaptic densities from rat brain. J Cell Biol. 1974 Nov;63(2 Pt 1):441–455. doi: 10.1083/jcb.63.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E., Fischbach G. D. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979 Oct;83(1):143–158. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring J. R., McGuire J. S., Jr, DeLorenzo R. J. Identification of the major postsynaptic density protein as homologous with the major calmodulin-binding subunit of a calmodulin-dependent protein kinase. J Neurochem. 1984 Apr;42(4):1077–1084. doi: 10.1111/j.1471-4159.1984.tb12713.x. [DOI] [PubMed] [Google Scholar]
- Hendry I. A., Campbell J. Morphometric analysis of rat superior cervical ganglion after axotomy and nerve growth factor treatment. J Neurocytol. 1976 Jun;5(3):351–360. doi: 10.1007/BF01175120. [DOI] [PubMed] [Google Scholar]
- Hendry I. A., Iversen L. L., Black I. B. A comparison of the neural regulation of tyrosine hydroxylase activity in sympathetic ganglia of adult mice and rats. J Neurochem. 1973 Jun;20(6):1683–1689. doi: 10.1111/j.1471-4159.1973.tb00284.x. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Montgomery P. R. Subcellular localization of the 52,000 molecular weight major postsynaptic density protein. Brain Res. 1982 Feb 11;233(2):265–286. doi: 10.1016/0006-8993(82)91202-1. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lynch G., Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984 Jun 8;224(4653):1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- McGuinness T. L., Lai Y., Greengard P. Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J Biol Chem. 1985 Feb 10;260(3):1696–1704. [PubMed] [Google Scholar]
- Molinoff P. B., Brimijoin S., Axelrod J. Induction of dopamine- -hydroxylase and tyrosine hydroxylase in rat hearts and sympathetic ganglia. J Pharmacol Exp Ther. 1972 Jul;182(1):116–129. [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Increase in tyrosine hydroxylase activity after reserpine administration. J Pharmacol Exp Ther. 1969 Sep;169(1):74–79. [PubMed] [Google Scholar]
- Ostberg A. J., Raisman G., Field P. M., Iversen L. L., Zigmond R. E. A quantitative comparison of the formation of synapses in the rat superior cervical sympathetic ganglion by its own and by foreign nerve fibres. Brain Res. 1976 May 14;107(3):445–470. doi: 10.1016/0006-8993(76)90137-2. [DOI] [PubMed] [Google Scholar]
- Peters A., Kaiserman-Abramof I. R. The small pyramidal neuron of the rat cerebral cortex. The synapses upon dendritic spines. Z Zellforsch Mikrosk Anat. 1969 Sep 22;100(4):487–506. doi: 10.1007/BF00344370. [DOI] [PubMed] [Google Scholar]
- Purves D. Competitive and non-competitive re-innervation of mammalian sympathetic neurones by native and foreign fibres. J Physiol. 1976 Oct;261(2):453–475. doi: 10.1113/jphysiol.1976.sp011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G., Field P. M., Ostberg A. J., Iversen L. L., Zigmond R. E. A quantitative ultrastructural and biochemical analysis of the process of reinnervation of the superior cervical ganglion in the adult rat. Brain Res. 1974 May 10;71(1):1–16. doi: 10.1016/0006-8993(74)90187-5. [DOI] [PubMed] [Google Scholar]
- Siekevitz P. The postsynaptic density: a possible role in long-lasting effects in the central nervous system. Proc Natl Acad Sci U S A. 1985 May;82(10):3494–3498. doi: 10.1073/pnas.82.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen A. J. Postnatal development of ganglionic neurons in the absence of preganglionic input: morphological observations on synapse formation. Brain Res. 1981 Jan;227(1):49–58. doi: 10.1016/0165-3806(81)90093-6. [DOI] [PubMed] [Google Scholar]
- Smolen A., Raisman G. Synapse formation in the rat superior cervical ganglion during normal development and after neonatal deafferentation. Brain Res. 1980 Jan 13;181(2):315–323. doi: 10.1016/0006-8993(80)90615-0. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Wallace R. W., Whitaker J. N., Cheung W. Y. Immunocytochemical localization of calmodulin and a heat-labile calmodulin-binding protein (CaM-BP80) in basal ganglia of mouse brain. J Cell Biol. 1980 Jan;84(1):66–76. doi: 10.1083/jcb.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Sachs L., Carlin R. K., Siekevitz P. Characteristics of a Ca2+/calmodulin-dependent binding of the Ca2+ channel antagonist, nitrendipine, to a postsynaptic density fraction isolated from canine cerebral cortex. Brain Res. 1986 Nov;387(2):167–184. doi: 10.1016/0169-328x(86)90008-2. [DOI] [PubMed] [Google Scholar]