Abstract
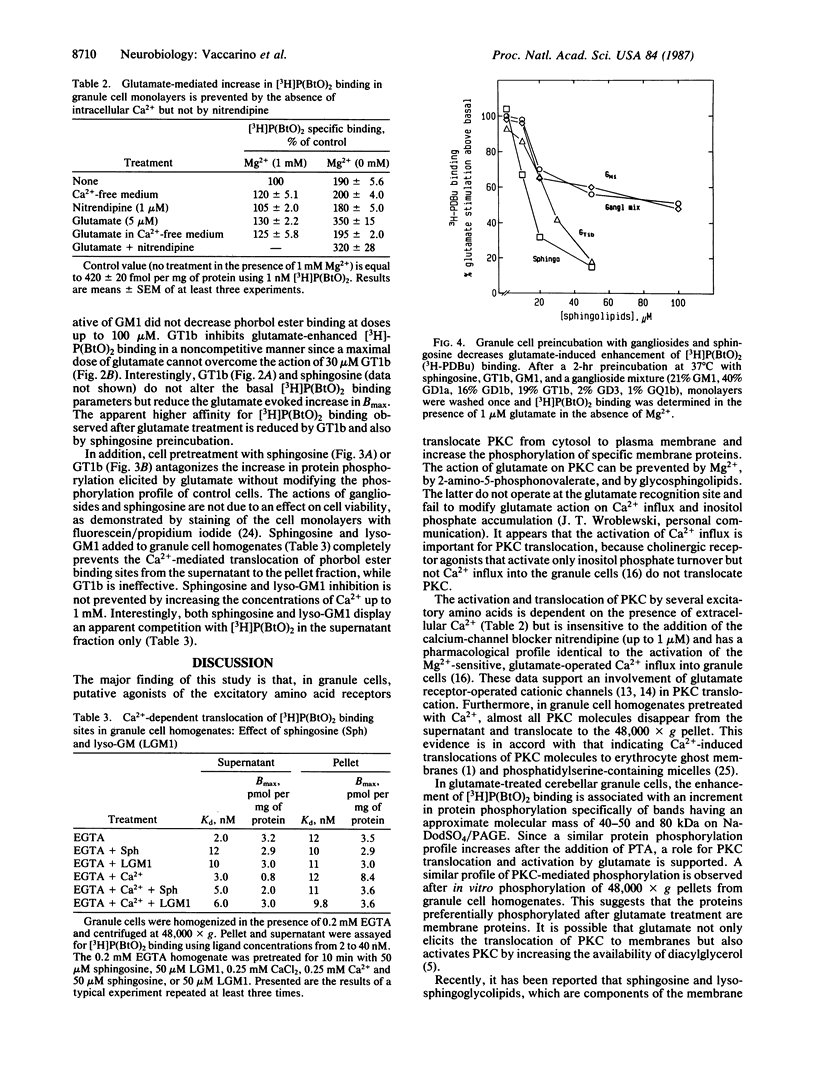
In primary cultures of cerebellar granule cells, protein kinase C (PKC) translocation and activation can be triggered by the stimulation of excitatory amino acid neurotransmitter receptors. Glutamate evokes a dose-related translocation of 4-beta-[3H]phorbol 12,13-dibutyrate ([3H]-P(BtO)2) binding sites from the cytosol to the neuronal membrane and stimulates the incorporation of 32P into a number of membrane proteins, particularly protein bands in the range of 80, 50, and 40 kDa. The glutamate-evoked PKC translocation is Mg2+ sensitive, is prevented by 2-amino-5-phosphonovalerate and phencyclidine, is not inhibited by nitrendipine (a voltage-dependent Ca2+-channel blocker) but is abolished by the removal of Ca2+ from the incubation medium, suggesting that glutamate-mediated Ca2+ influx is operative in the redistribution of PKC. Exposure of granule cells to the gangliosides trisialosylgangliotetraglycosylceramide (GT1b) or monosialosylgangliotetraglycosylceramide (GM1) inhibits the translocation and activation of PKC evoked by glutamate. These glycosphingolipids fail to interfere with glutamate binding to its high-affinity recognition site or with the [3H]P(BtO)2 binding, nor do they affect the Ca2+ influx. These gangliosides may prevent PKC translocation by interfering with the PKC binding to the neuronal membrane phosphatidylserine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Bell R. M. Protein kinase C activation by diacylglycerol second messengers. Cell. 1986 Jun 6;45(5):631–632. doi: 10.1016/0092-8674(86)90774-9. [DOI] [PubMed] [Google Scholar]
- Browning M. D., Huganir R., Greengard P. Protein phosphorylation and neuronal function. J Neurochem. 1985 Jul;45(1):11–23. doi: 10.1111/j.1471-4159.1985.tb05468.x. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Choi D. W. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987 Feb;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Maulucci-Gedde M., Kriegstein A. R. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987 Feb;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. K., Agranoff B. W. Receptor activation and inositol lipid hydrolysis in neural tissues. J Neurochem. 1987 Apr;48(4):999–1017. doi: 10.1111/j.1471-4159.1987.tb05618.x. [DOI] [PubMed] [Google Scholar]
- Gallo V., Ciotti M. T., Coletti A., Aloisi F., Levi G. Selective release of glutamate from cerebellar granule cells differentiating in culture. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987 Feb 6;235(4789):670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987 Feb 5;325(6104):522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kreutter D., Kim J. Y., Goldenring J. R., Rasmussen H., Ukomadu C., DeLorenzo R. J., Yu R. K. Regulation of protein kinase C activity by gangliosides. J Biol Chem. 1987 Feb 5;262(4):1633–1637. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Nichols R. A., Haycock J. W., Wang J. K., Greengard P. Phorbol ester enhancement of neurotransmitter release from rat brain synaptosomes. J Neurochem. 1987 Feb;48(2):615–621. doi: 10.1111/j.1471-4159.1987.tb04137.x. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Novelli A., Alho H., Guidotti A., Costa E. The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J Neurosci. 1986 Jul;6(7):1905–1911. doi: 10.1523/JNEUROSCI.06-07-01905.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli A., Nicoletti F., Wroblewski J. T., Alho H., Costa E., Guidotti A. Excitatory amino acid receptors coupled with guanylate cyclase in primary cultures of cerebellar granule cells. J Neurosci. 1987 Jan;7(1):40–47. doi: 10.1523/JNEUROSCI.07-01-00040.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettamanti G., Preti A., Cestaro B., Venerando B., Lombardo A., Ghidoni R., Sonnino S. Gangliosides, neuraminidase and sialyltransferase at the nerve endings. Adv Exp Med Biol. 1980;125:263–281. doi: 10.1007/978-1-4684-7844-0_25. [DOI] [PubMed] [Google Scholar]
- Vaccarino F. M., Alho H., Santi M. R., Guidotti A. Coexistence of GABA receptors and GABA-modulin in primary cultures of rat cerebellar granule cells. J Neurosci. 1987 Jan;7(1):65–76. doi: 10.1523/JNEUROSCI.07-01-00065.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., LeVine H., 3rd, May W. S., Jr, Cuatrecasas P., Sahyoun N. A model for intracellular translocation of protein kinase C involving synergism between Ca2+ and phorbol esters. Nature. 1985 Oct 10;317(6037):546–549. doi: 10.1038/317546a0. [DOI] [PubMed] [Google Scholar]
- Wroblewski J. T., Nicoletti F., Fadda E., Costa E. Phencyclidine is a negative allosteric modulator of signal transduction at two subclasses of excitatory amino acid receptors. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5068–5072. doi: 10.1073/pnas.84.14.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]




