Abstract
Diabetes mellitus (DM) is a major and growing concern in the United States, in large part because of an epidemic of obesity in America and its relation to type 2 DM. In affected patients, postprandial glucose may be an early indicator of glucose intolerance or a prediabetes condition, which may be a better predictor of cardiovascular risk than impaired fasting glucose level. Treating patients who have early signs of hyperglycemia, including elevated postprandial glucose level, with intensive glucose control that does not lead to weight gain, and ideally may be associated with weight reduction, may be vital to preventing or reducing later cardiovascular morbidity and mortality. Because hypoglycemia is an important complication of current DM treatments and may cause acute secondary adverse cardiovascular outcomes, not causing hypoglycemia is mandatory. Given that weight loss can significantly lower cardiovascular risk and improve other cardiovascular risk factors in patients with type 2 DM and that medications are available that can result in weight reduction without leading to hypoglycemia, the successful treatment of patients with type 2 DM should be individualized and should address the complete pathophysiologic process. This review is a hypothesis article that presents arguments against general approaches to the treatment of type 2 DM. An algorithm is presented in which the goal for managing patients with type 2 DM is to lower the blood glucose level as much as possible for as long as possible without causing hypoglycemia. In addition, body weight should ideally be improved, reducing cardiovascular risk factors and avoiding therapeutic inertia.
ACCORD = Action to Control Cardiovascular Risk in Diabetes; ADA = American Diabetes Association; ADVANCE = Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release and Controlled Evaluation; BMI = body mass index; BP = blood pressure; CHD = coronary heart disease; CVD = cardiovascular disease; DM = diabetes mellitus; DPP-4 = dipeptidyl peptidase 4; EASD = European Association for the Study of Diabetes; FPG = fasting plasma glucose; GLP-1 = glucagon-like peptide 1; HbA1c = hemoglobin A1c; HDL-C = high-density lipoprotein cholesterol; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; KORA = Cooperative Health Research in the Region of Augsburg; LDL-C = low-density lipoprotein cholesterol; LEAD = Liraglutide Effect and Action in Diabetes; MI = myocardial infarction; MONICA = MONItoring of trends and determinants in CArdiovascular disease; NHANES = National Health and Nutrition Examination Survey; PPG = postprandial glucose; PROACTIVE = PROspective pioglitAzone Clinical Trial In macroVascular Events; TC = total cholesterol; VADT = Veterans Administration Diabetes Trial
Data from the National Health Interview Survey during the past 10 years indicate that the prevalence of diabetes mellitus (DM) is increasing in the United States, with current estimates of 23.6 million children and adults having the disease.1 Among adults with DM, more than 80% are overweight/obese (ie, have a body mass index [BMI; calculated as weight in kilograms divided by height in meters squared] ≥25), indicating that overweight/obesity is a major problem in this patient population.2 National surveys show that the prevalence of DM is greater among people who have a high BMI,3 a fact that clearly supports the strong link between overweight/obesity and DM.
Both overweight/obesity and type 2 DM are independent risk factors of cardiovascular disease (CVD).3,4 Heart failure is 2 to 5 times more likely to occur in patients with DM than in patients without DM.5 In patients with DM, important predictors of all-cause and CVD mortality include hyperglycemia and other cardiovascular risk factors, such as smoking, elevated blood pressure (BP), and abnormal lipid levels.6 In patients with a prediabetes condition, the risk of a CVD event is modestly increased.7
A prediabetes condition also increases the risk of microvascular disease. Kim et al8 found the presence of microalbuminuria (urinary albumin excretion rate of 20-200 μg/min) in 6.0% of healthy patients, 11.8% of patients with impaired glucose tolerance (IGT), and 21.8% of patients with type 2 DM. Franklin et al9 showed that sensory peripheral neuropathy was evident in 3.9% of controls, 11.2% of patients with IGT, and 25.8% of patients with type 2 DM. Finally, in the MONItoring of trends and determinants in CArdiovascular disease (MONICA)/Cooperative Health Research in the Region of Augsburg (KORA) surveys, the prevalence of polyneuropathy in patients with DM, IGT, impaired fasting glucose (IFG), and normal glucose tolerance was 28.0%, 13.0%, 11.3%, and 7.4%, respectively (P<.05 for DM vs normal glucose tolerance, IFG, and IGT).10
This article discusses the need for the aggressive but safe treatment of patients with type 2 DM through careful glycemic control and weight loss. With this strategy, we can attempt to prevent the complications that are associated with this disease and its treatment, avoiding therapeutic nihilism.
HYPERGLYCEMIA: IFG, IGT, AND DM
The prevalence of DM has been increasing in recent years and is now an epidemic in the United States. Data from the National Health and Nutrition Examination Survey (NHANES) from 1999 and 2002 showed that the prevalence of IFG was 26.0% and that of DM was 9.3%.11 Between 2005 and 2006, in adults 20 years or older, the prevalence of IFG remained nearly steady at 25.7%, whereas that of DM increased to 12.9% and that of IGT, recently added to NHANES, was 13.8%.12
The addition of IGT to NHANES represents an increasing understanding of the roles of fasting plasma glucose (FPG) and postprandial glucose (PPG) on overall hyperglycemia exposure and control. Postprandial hyperglycemia, or IGT, plays an important role in the development of DM complications.13-18 Recent evidence is that, although hemoglobin A1c (HbA1c) is a direct function of both PPG and FPG in mild-to-moderate hyperglycemia, PPG is a major contributor to HbA1c, and as hyperglycemia worsens (eg, HbA1c >8.4%), FPG becomes a greater influence on HbA1c.19 Similarly, as HbA1c increases, there is a continuously greater risk of CVD and mortality.14 However, cardiovascular risk correlates with PPG even when the HbA1c level is only mildly or moderately elevated. Significantly greater cardiovascular events were noted in men with an HbA1c level of 7.6% and in women with an HbA1c level of 8.4% (compared with an HbA1c level of 7.3% in men and an HbA1c level of 7.5% in women; P<.01 for HbA1c within sex).20
Hyperglycemia is a continuous risk factor for CVD morbidity and mortality with no apparent HbA1c threshold. Microvascular and CVD risk increase in patients with a longer duration of DM and a higher HbA1c level.21-23 Initiation of intensive therapy soon after DM was diagnosed showed reductions in the risk of microvascular and macrovascular disease, with additional benefits seen in patients with an HbA1c level of 7% or lower.22,24,25 Patients with a longer duration of DM and preexisting complications did not experience the benefit of intensive therapy despite achieving an HbA1c level of approximately 7.0% in the Veterans Administration Diabetes Trial (VADT)26 and an HbA1c level of less than 6.5% in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release and Controlled Evaluation (ADVANCE) study27 and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study.28 In VADT, ADVANCE, and ACCORD, the mean duration of DM was 11.5, 8.0, and 10.0 years, respectively, with 40%, 32%, and 35% of enrolled patients, respectively, having a history of CVD.23 In the ACCORD study, the rate of fatal myocardial infarction (MI) increased.28 However, a subgroup analysis showed a protective effect of intensive therapy in patients with a shorter duration of DM or earlier atherosclerotic disease in VADT,29 in patients without prior cardiovascular events (primary prevention) or a baseline HbA1c level less than 8.0% in ACCORD, and reductions in cardiovascular outcomes in patients without preexisting microvascular or macrovascular disease in ADVANCE.23 Recent subset analyses from ACCORD show that, with the intensive treatment strategy, the lowest risk of death was associated with lower mean levels of HbA1c. As mean HbA1c levels increased from 6% to 9%, mortality risk increased steadily. The minority subgroup of individuals in the intensive treatment group who had HbA1c levels greater than 7% accounted for the excess risk accompanying that treatment regimen. Thus, attempting to lower the HbA1c level to less than 7% with intensive therapy in treatment-resistant patients may be detrimental.30-32
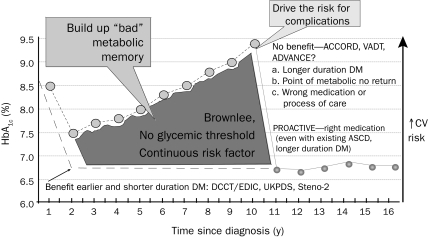
In summary, an HbA1c goal of less than 7% is still recommended; however, goals should be individualized for selected patients.23 In addition, unrecognized hypoglycemia and weight gain in the ACCORD trial were likely major issues regarding its adverse outcomes.23 Finally, data from 2 meta-analyses that included these studies, as well as the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROACTIVE) study,33 showed that lower glucose levels reduced MIs and cardiovascular events, albeit with no effect on all-cause mortality.34,35 Figure 1 is a hypothetical representation of the natural history of diabetic patients enrolled in cardiovascular outcomes studies.36
FIGURE 1.
Understanding cardiovascular (CV) outcome studies in type 2 diabetes mellitus (DM). These data highlight the importance of balancing the benefits and risks of antidiabetes medications when making treatment decisions using agents that minimize the risk of hypoglycemia and weight gain and possibly lead to weight loss. ACCORD = Action to Control Cardiovascular Risk in Diabetes; ADVANCE = Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release and Controlled Evaluation; ASCD = atherosclerotic cardiovascular disease; DCCT/EDIC = Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications; HbA1c = hemoglobin A1c; PROACTIVE = PROspective pioglitAzone Clinical Trial In macroVascular Events; UKPDS = United Kingdom Prospective Diabetes Study; VADT = Veterans Affairs Diabetes Trial.
From Diabetologia,36 modified with permission from Springer Publishing.
Improved glycemic control alone is insufficient to reduce the incidence of macrovascular disease in patients with type 2 DM. Other factors, including weight loss, dyslipidemia, and hypertension, are also important.37 The multidimensional pathophysiology of type 2 DM suggests that a multifactorial approach is needed for successful treatment.38 This includes reduction of insulin resistance and improvement in abnormal islet cell function, avoidance of hypoglycemia, decrease in body weight, and treatment of comorbidities.37,39
TYPE 2 DM AND OVERWEIGHT/OBESITY: EXAMINING THE EVIDENCE
Risk factors for coronary heart disease (CHD), such as hypercholesterolemia, high BP, and a high BMI, often occur in clusters. This clustering compounds risk and corresponds to a higher incidence of CHD.40 In the Framingham study, the composite risk score (high-density lipoprotein cholesterol [HDL-C] levels, BMI, systolic BP, triglyceride levels, glucose levels, and serum total cholesterol [TC] levels) increased with weight gain. There was a 20% higher risk of CHD in men and a 37% higher risk in women with a 2.25-kg (5-lb) weight increase (P≤.002).40 Similarly, in a meta-analysis of studies of type 2 DM, a 5-kg weight gain corresponded to a 30% higher CHD risk.41 The Heart Outcomes Prevention Evaluation study, which included patients with type 2 DM, showed that obesity (abdominal adiposity in particular) led to an increased risk of 23% for MI (P<.01), 38% for congestive heart failure (P<.03), and 17% for all-cause mortality (P<.05).42
Eeg-Olofsson et al3 evaluated the risk of CVD and mortality from CVD in overweight/obese patients with type 2 DM. In their analysis, the relative risks of CHD, stroke, CVD, and total mortality for a 5-unit increase in BMI at baseline were 15%, 11%, 13%, and 27%, respectively. In contrast, weight reduction in patients with type 2 DM may improve cardiovascular risk and mortality. Regardless of DM status, weight reduction alone has been associated with a reduction in cardiovascular risk in overweight/obese individuals (Figure 2).43 In a retrospective regression analysis in patients with type 2 DM, each 1 kg of weight loss was associated with 3 to 4 months of prolonged survival.44 Moreover, a prospective analysis of 12-year data from 4970 overweight individuals with DM enrolled in the American Cancer Society's Cancer Prevention Study I estimated the effects of intentional weight loss on mortality in overweight individuals with DM.45 On the basis of a questionnaire, 34% of the study cohort reported intentional weight loss, which was associated with a 25% reduction in total mortality and a 28% reduction in CVD and DM mortality.45
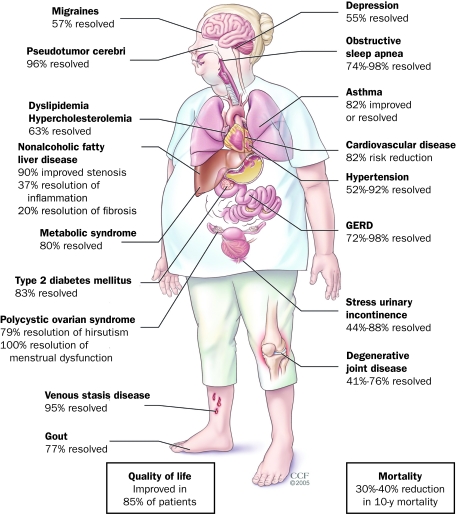
FIGURE 2.
Comorbidities reduced after bariatric surgery and weight loss. The beneficial effect of bariatric surgery with resultant weight reduction is depicted on numerous organ systems. GERD = gastroesophageal reflux disease.
From Endocrine News,43 with permission from The Cleveland Clinic Center for Medical Art and Photography.
Weight reduction has also been associated with a decrease in other cardiovascular risk factors. In the weight loss arm of the Trials of Hypertension Prevention study, weight loss of 4.5 kg or more led to clinically important long-term reductions in BP and a 65% relative risk reduction for hypertension.46 Dietary composition may also affect metabolic markers and weight loss. In a 2-year study that compared the efficacy and safety of 3 weight-loss diets (low carbohydrate, non–calorie restricted; Mediterranean, restricted calorie; or low fat, restricted calorie) in 322 moderately obese individuals, the group consuming the low-carbohydrate diet had the greatest increases in HDL-C levels (P<.01) and the greatest decreases in triglyceride levels (P=.03). Low-density lipoprotein cholesterol (LDL-C) was not changed in any of the groups.47 In the subset of patients with DM (n=36), a Mediterranean, restricted-calorie diet led to significantly improved FPG levels (−32.8 mg/dL) compared with the low-fat diet (P<.001). Insulin levels decreased in all diet groups with no differences between them. Insulin resistance, as measured by homeostasis model assessment–insulin resistance, decreased to a significantly greater extent in patients assigned the Mediterranean diet than in those adhering to the low-fat diet (−2.3 and −0.3, respectively; P=.02 and P=.04 for the interaction among DM and the Mediterranean diet and time).47
The Action for Health in Diabetes (Look AHEAD) trial is a large (N=5000) 5-year, multicenter study to evaluate the effect of intensive lifestyle intervention, specifically diet and physical activity, compared with DM support and education in overweight/obese individuals aged 45 to 75 years with type 2 DM.48 The data reported here reflect the results after 1 year of follow-up. Patients in the intensive lifestyle intervention group had significantly greater weight reduction (8.6% of body weight) than patients in the DM support and education group (0.7% of body weight; P<.001).49 This was accompanied by significantly improved glycemic control, HbA1c reduction (intensive lifestyle intervention, 7.3%-6.6%, P<.001; DM support and education, 7.3%-7.2%, not significant), and significantly improved cardiovascular risk factors (systolic and diastolic BP, triglyceride and HDL-C levels, and urine albumin-to-creatinine ratio; all P<.01).49
The American Diabetes Association (ADA) has published a joint statement with the North American Association for the Study of Obesity and the American Society for Clinical Nutrition recommending a moderate weight loss (5% of body weight) to improve insulin action, decrease FPG level, and reduce the need for antidiabetes medications.50 Additional benefits of weight loss include improvement of other cardiovascular risk factors (eg, decreasing BP, improving serum lipid levels, and reducing markers of inflammation).50 In addition, a joint statement from the ADA and the American Heart Association recommends structured programs that emphasize lifestyle changes, which include reducing fat and total energy intake with increased regular physical activity to produce long-term weight loss of between 5% and 7% of initial weight and improvements in BP.51
According to the ADA, a beneficial initial weight loss goal is approximately 2 BMI units or approximately 4 to 8 kg (8-16 lb).52 Weight loss of 2 to 5 kg (5-10 lb) may improve glucose tolerance, BP, and lipid levels.52 Weight loss and weight management programs for patients with type 2 DM should be individualized.
HYPOGLYCEMIA: THE POTENTIALLY LIMITING FACTOR IN GLYCEMIC MANAGEMENT OF TYPE 2 DM
Hypoglycemia is a serious concern that limits the feasibility of intense glucose control in real-world practice. Several factors may increase the risk of hypoglycemia in patients with type 2 DM: use of insulin secretagogues, missed meals, advanced age, duration of disease, and unawareness of hypoglycemia.53 On the basis of the United Kingdom Hypoglycemia Study, the rates of mild, moderate, and severe hypoglycemia in patients with type 2 DM were comparable for patients treated with sulfonylureas and with insulin for less than 2 years.54 Fear of iatrogenic hypoglycemia when managing patients with type 2 DM may result in suboptimal glycemic control that increases the risk of macrovascular and microvascular complications.55 Hypoglycemia in patients with type 2 DM may be associated with increased symptoms of chest pain and electrocardiographic abnormalities and may account for sudden death.56-58 The acute severity of hypoglycemia can be exemplified in an average of 380,000 emergency department visits each year based on an estimate of approximately 5 million visits between 1993 and 2005.59 Hypoglycemia is also associated with detrimental effects on cognitive function and mood changes.60
A case-control study presented at the 2009 annual meeting of the European Association for the Study of Diabetes (EASD) evaluated the effect of hypoglycemia in patients with type 2 DM. This report studied all patients seen in Veterans Affairs hospitals between 2000 and 2004 who had 2 or more years of Veterans Affairs care and no prior history of MI, acute coronary syndromes, or cardiac surgery.61 The study showed a 65% increase in the odds of MI with hypoglycemia within the previous 2 weeks, even after adjustment for potential confounding cofactors. Furthermore, a lower but still slightly elevated risk of MI of approximately 20% was seen with hypoglycemic events within the previous 6 months.
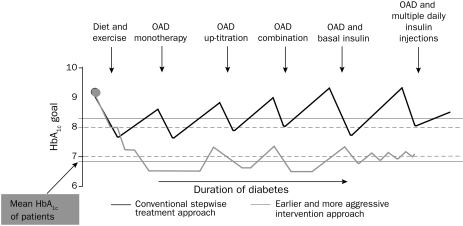
Earlier and more aggressive intervention when a patient is not experiencing severe hypoglycemia may improve the glycemic profile by avoidiance of prolonged periods of hyperglycemia (Figure 3).62 By transitioning earlier to more intense glucose treatment, rather than waiting for an increase in HbA1c and then intensifying glucose control, periods of glycemic exposure may be avoided. Intensive glucose control has shown benefits (eg, reducing the risk of nonfatal MI), but it may also increase the risk of severe hypoglycemia.26-28,63 A meta-analysis of the effect of intensive glucose control on cardiovascular outcomes in patients with type 2 DM found that other treatment-related factors (such as weight loss) may have had a potential effect.34
FIGURE 3.
Earlier and more aggressive intervention may improve patients' chances of reaching treatment goal. This figure depicts a conceptual approach to treatment of patients with type 2 diabetes mellitus. Compared with conventional therapy, early and aggressive management of hyperglycemia is more likely to result in attainment of glycemic controls. HbA1c = hemoglobin A1c; OAD = oral antidiabetes agents.
From Int J Clin Pract,62 with permission from Blackwell Publishing Ltd.
PHARMACOLOGICAL TREATMENT CONSIDERATIONS: BENEFITS AND RISKS OF THE INCRETIN-BASED AGENTS IN MANAGING TYPE 2 DM
Glucagon-like peptide 1 (GLP-1) receptor agonists can effectively reduce glucose and body weight with potential beneficial effects on other cardiovascular risk factors such as BP and lipids.64 Dipeptidyl peptidase 4 (DPP-4) inhibitors also lower glucose levels but have neutral effects on weight.64
Exenatide, a GLP-1 receptor agonist, is approved by the US Food and Drug Administration as a twice-daily subcutaneous injection as an adjunct to diet and exercise to improve glycemic control in adults with type 2 DM. Exenatide can be used as monotherapy or in combination with sulfonylureas, thiazolidinediones, and metformin. The concurrent use of exenatide with insulin has not been studied and therefore cannot be recommended.65 A once-weekly formulation has been submitted to the US Food and Drug Administration for regulatory review. Liraglutide, another GLP-1 receptor agonist, is approved for use as a once-daily subcutaneous injection as an adjunct to diet and exercise to improve glycemic control in adults with type 2 DM. It is not recommended as first-line therapy for patients whose DM is inadequately controlled with diet and exercise. It can be used as monotherapy or in combination with metformin, a sulfonylurea, or a thiazolidinedione. Concurrent use of liraglutide and insulin has not been studied. Liraglutide has also been approved in the European Union for adjunctive use in patients with type 2 DM and inadequate glycemic control with metformin, a sulfonylurea, or a combination of metformin and a sulfonylurea or metformin and a thiazolidinedione.66 Both exenatide and liraglutide have a mechanism of action and effects that are similar to those of native GLP-1. These incretin-based therapies enhance glucose-dependent insulin secretion; reduce body weight; suppress inappropriate glucagon secretion; regulate gastric emptying; suppress appetite, resulting in reduced food intake; and promote β-cell neogenesis and proliferation in animal models.67 The glucose-lowering effects of GLP-1 receptor agonists are glucose dependent, which ensures that insulin secretion is coupled to glycemia and helps to reduce the risk of hypoglycemia.68,69
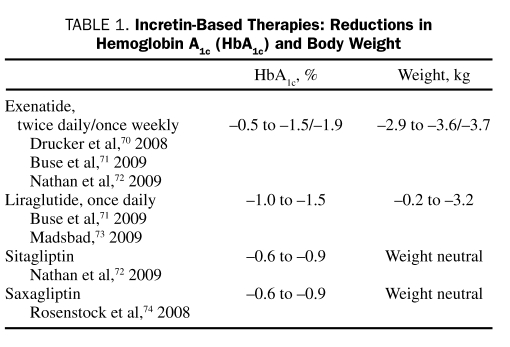
Exenatide and liraglutide have been shown to reduce HbA1c levels and body weight (Table 1).70-74 In an open-label, open-ended trial, exenatide reduced HbA1c levels and weight in 68% of patients (N=217) who were treated for up to 3.5 years.75 In comparator studies, exenatide lowered HbA1c levels (−1.04% to −1.11%) and body weight (−2.3 to −2.5 kg), whereas insulin analogues reduced HbA1c levels (−0.89% to −1.11%) but resulted in weight gain (+1.8 to +2.9 kg).76,77
TABLE 1.
Incretin-Based Therapies: Reductions in Hemoglobin A1c (HbA1c) and Body Weight
Three retrospective or noncontrolled studies have evaluated the effects of exenatide on cardiovascular risk factors, such as blood lipids. The addition of exenatide to the regimen in patients with metabolic syndrome and multiple cardiovascular risk factors resulted in a significant improvement from baseline levels in TC (−10.8 mg/dL; P=.0007), LDL-C (−11.8 mg/dL; P<.0001), HDL-C (+8.5 mg/dL; P<.0001), and triglycerides (−44.4 mg/dL; P=.0003) in a 3-year study.75 After a 16-week treatment period, adults with metabolic syndrome had significant improvements (P<.001) in TC (−7.4 mg/dL) and triglyceride levels (−16.7 mg/dL).78 In an additional study, after 16 weeks of treatment with exenatide in patients with the metabolic syndrome, there were significant reductions in TC (−8.5 mg/dL; P<.002) and LDL-C (−5.8 mg/dL; P<.004) levels and nonsignificant reductions in triglyceride levels (−5.4 mg/dL).79
Nausea, vomiting, and diarrhea are the most commonly reported adverse events with exenatide, occurring in 44%, 13%, and 13%, of patients, respectively. However, the incidence of nausea, vomiting, and dyspepsia with monotherapy is 8%, 4%, and 3%, respectively.65 This may be due to the avoidance of nausea associated with combination therapies or to more careful admonitions to patients to stop eating when they feel full, which reduces or eliminates the gastrointestinal upset due to slower gastric emptying. Nausea between meals, which may be due to an unduly sensitive hypothalamic effect, decreases over time with continued therapy65 but may account for most of the low (1%) dropout rates in published studies of the drug.
Hypoglycemia occurs in 4% to 5% of patients receiving exenatide monotherapy and increases in incidence when exenatide is combined with a sulfonylurea or a sulfonylurea and metformin.65
On the basis of postmarketing data, exenatide use has been associated with acute pancreatitis. Patients with type 2 DM have approximately a 3-fold greater risk of pancreatitis than those without the disease.80 No definitive association has been established between the risk of acute pancreatitis and any of the evaluated antidiabetes agents, including exenatide, sitagliptin, metformin, and glyburide.81 After initiating exenatide and after dose increases, clinicians are advised to observe patients carefully for signs and symptoms of pancreatitis. Exenatide therapy should be discontinued promptly in patients with abdominal pain until pancreatitis is ruled out as a cause.65
In a similar context, there have been postmarketing reports of altered renal function, including increased serum creatinine values, renal impairment, worsened chronic renal failure, and acute renal failure in patients treated with exenatide. Reversibility of altered renal function has been observed in many cases with supportive treatment and discontinuation of treatment with exenatide and other potentially offending agents. Although exenatide has not been shown to be nephrotoxic in preclinical and clinical studies, it should not be used in patients with a creatinine clearance less than 30 mL/min.65
A series of phase 3 clinical trials has been conducted with liraglutide. Known as the Liraglutide Effect and Action in Diabetes (LEAD) program, results show that liraglutide significantly improves glycemic control, as measured by HbA1c, when used as monotherapy, compared with a sulfonylurea, glitazone, or both.73 In addition, treatment with liraglutide has been associated with body weight reductions of −1.0 to −3.0 kg and improvements in serum lipoprotein levels.73
The most common adverse events reported with liraglutide are nausea, dyspepsia, and diarrhea.71,74 Cases of acute pancreatitis have been reported in patients treated with liraglutide; however, a causal relationship has not been established.74 Liraglutide causes thyroid C-cell tumors at clinically relevant exposures in rodents. It is unknown whether liraglutide causes C-cell tumors, including medullary thyroid carcinoma, in humans because human relevance could not be determined from nonclinical studies.66
MISCELLANEOUS AGENTS
Pramlintide is an amylin analogue indicated as adjunctive therapy for patients with type 2 DM and inadequate glycemic control with insulin with or without a sulfonylurea or metformin. Pramlintide significantly lowered HbA1c levels (−0.7%) in patients treated with insulin glargine with or without oral antidiabetes agents compared with placebo (−0.36%; P<.05) after 16 weeks of therapy. In addition, pramlintide was associated with a weight loss of −1.6 kg compared with a weight gain of +0.7 kg with placebo (P<.0001).82 The most common adverse events were mild-to-moderate nausea (31% pramlintide vs 10% placebo) and mild-to-moderate hypoglycemia (44% pramlintide vs 47% placebo).82
Ranolazine, a first-in-class antianginal drug with cardioprotective properties, has recently been shown to induce glucose-lowering effects. In a recent trial of 171 patients with type 2 DM and non–ST-segment elevation acute coronary syndromes, ranolazine resulted in an absolute HbA1c reduction of 1.2% in patients with a baseline HbA1c level of 8% to 10%. No cases of serious hypoglycemia were reported.83
Colesevelam has been shown to reduce HbA1c levels by −0.5% in controlled trials in patients with type 2 DM receiving metformin or a sulfonylurea, with effects persisting for 52 weeks or more. Gastrointestinal disturbances are the most common adverse events reported; the incidence of hypoglycemia has been low (approximately 3%).84
Bromocriptine is approved as a quick-release formulation for the management of patients with type 2 DM. Daily doses of 1.6 to 4.8 mg result in absolute reductions in HbA1c levels of −0.5% to −0.6%. Nausea is the most common adverse event reported, with the incidence decreasing after the initial titration period. Dizziness is also not uncommon.85 Additional safety concerns with bromocriptine include hypotension and reports of cardiac valve fibrosis.
The α-glucosidase inhibitors work by decreasing the rate of absorption of glucose from the gastrointestinal tract. Therapy with these agents results in decreases in HbA1c of approximately −0.5% to −0.8%.72 As a result of the high incidence of gastrointestinal adverse effects, use of these agents remains limited.72
The glinides act by stimulating insulin secretion. Repaglinide therapy results in reductions in HbA1c of approximately −1.5% (similar to sulfonylurea therapy), whereas the glycemic effects of nateglinide are more modest.72 The risk of hypoglycemia with these agents is somewhat less than that seen with some sulfonylureas.72
Treatment algorithms have been published that specifically address treating DM and pathophysiologic factors as a whole. The ADA/EASD algorithm recognizes the role of GLP-1 receptor agonists, especially in patients with concerns about hypoglycemia and/or weight.72 More recently, the Association of Clinical Endocrinologists/American College of Endocrinology issued new treatment algorithms for patients with type 2 DM.86 These algorithms, which emphasize safety and quality of glycemic control as their first priorities, have moved sulfonylureas to a lower priority because of their propensity for hypoglycemia, weight gain, and limited duration of effectiveness. In addition, these priorities will result in earlier and more frequent use of incretin-based therapies, such as the GLP-1 receptor agonists and the DPP-4 inhibitors.86 Metformin remains a cornerstone of therapy because of its efficacy and safety: metformin lowers HbA1c levels as well as or better than any other oral agent, hypoglycemia is not usually an issue, it is weight neutral, it costs significantly less than thiazolidinediones or incretin-based therapy, and it is recommended as a first-line agent in many if not most guidelines for the treatment of obese individuals with type 2 DM who have normal renal function.
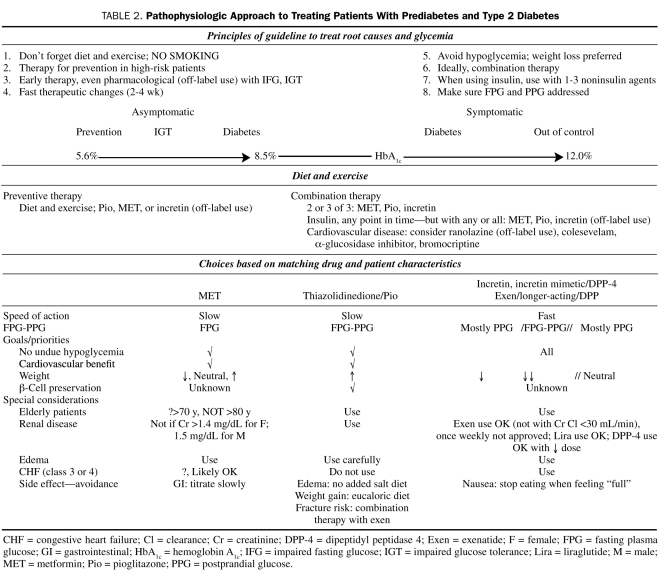
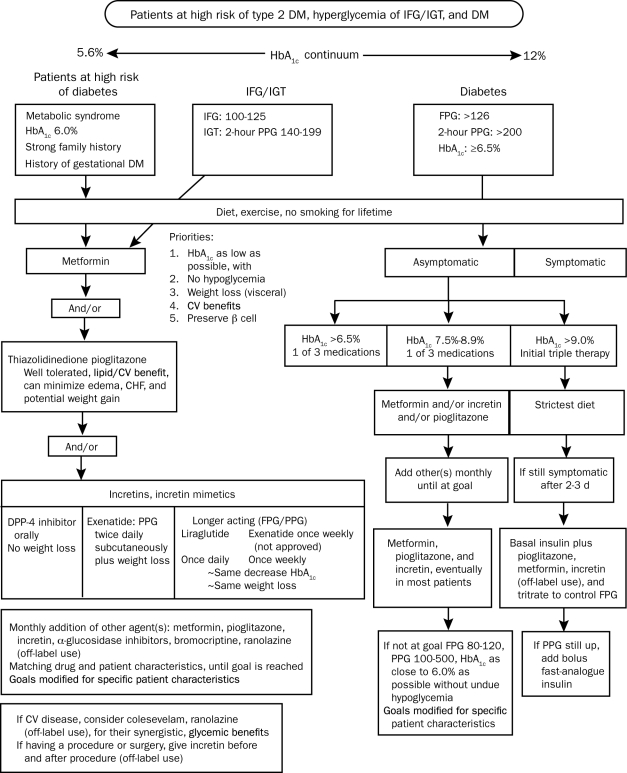
Additional guidelines are available that help clinicians with treatment decisions. Table 2 and Figure 4 reflect one of the author's (S.S.S.) thoughts on a pathophysiologic approach to treatment, as well as a recommended algorithm for patients along the diabetes continuum. Treatments such as metformin and GLP-1 receptor agonists are unique in their ability to not result in weight gain and to exhibit a low incidence of hypoglycemia. These treatment guidelines stress the need for diet and exercise and the judicious use of medical therapies in an effort to address the many aspects of the pathophysiologic process involved in causing hyperglycemia, as well as avoiding weight gain and hypoglycemia and, ideally, losing weight.
TABLE 2.
Pathophysiologic Approach to Treating Patients With Prediabetes and Type 2 Diabetes
FIGURE 4.
Recommended algorithm for high-risk patients and patients with prediabetes and type 2 diabetes mellitus (DM). CHF = congestive heart failure; CV = cardiovascular; DPP-4 = dipeptidyl peptidase 4; FPG = fasting plasma glucose; HbA1c = glycosylated hemoglobin; IFG = impaired fasting glucose; IGT = impaired glucose tolerance; PPG = postprandial glucose.
CONCLUSION
A close pathophysiologic link exists among type 2 DM, overweight/obesity, and CVD. An ideal approach to DM is one that controls hyperglycemia as early as possible and maintains glycemic control for as long as possible without causing hypoglycemia and maintaining or reducing weight. Even a modest weight reduction can improve glycemic control, BP, lipids, and other cardiovascular risk factors. Therapies that lower glucose level, reduce weight, and may also have potential benefits on other cardiovascular risk factors should be considered in the treatment of patients with type 2 DM.
CLINICAL PEARLS
Type 2 DM develops as a result of a number of pathophysiologic defects that result in hyperglycemia as the hallmark manifestation of the disease. The degree of hyperglycemia and the degree of postprandial hyperglycemia correlate with CVD morbidity and mortality.
Overweight/obesity and type 2 DM are independent risk factors for CVD, and 80% of adult patients with type 2 DM are overweight/obese.
Patients with type 2 DM frequently have other risk factors for CVD, including smoking, elevated BP, and hyperlipidemia.
Effective management of patients with type 2 DM includes control of hyperglycemia (avoiding hypoglycemia) and control of overweight/obesity, as well as correction of other cardiovascular risk factors present, to improve long-term clinical outcomes.
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC) National Diabetes Fact Sheet, 2007: General information. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf Accessed October 19, 2010
- 2. Bays HE, Chapman RH, Grandy S, SHIELD Investigators' Group The relationship of body mass index to diabetes mellitus, hypertension and dyslipidaemia: comparison of data from two national surveys. Int J Clin Pract. 2007;61(5):737-747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. 2009;52(1):65-73 [DOI] [PubMed] [Google Scholar]
- 4. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229-234 [DOI] [PubMed] [Google Scholar]
- 5. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241(19):2035-2038 [DOI] [PubMed] [Google Scholar]
- 6. Wei M, Gaskill SP, Haffner SM, Stern MP. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality: the San Antonio Heart Study. Diabetes Care. 1998;21(7):1167-1172 [DOI] [PubMed] [Google Scholar]
- 7. Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753-759 [DOI] [PubMed] [Google Scholar]
- 8. Kim YI, Kim CH, Choi CS, et al. Microalbuminuria is associated with the insulin resistance syndrome independent of hypertension and type 2 diabetes in the Korean population. Diabetes Res Clin Pract. 2001;52(2):145-152 [DOI] [PubMed] [Google Scholar]
- 9. Franklin GM, Kahn LB, Baxter J, Marshall JA, Hamman RF. Sensory neuropathy in non-insulin-dependent diabetes mellitus: the San Luis Valley Diabetes Study. Am J Epidemiol. 1990;131(4):633-643 [DOI] [PubMed] [Google Scholar]
- 10. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, KORA Study Group Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31(3):464-469 [DOI] [PubMed] [Google Scholar]
- 11. Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the US population: National Health and Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29(6):1263-1268 [DOI] [PubMed] [Google Scholar]
- 12. Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and prediabetes in the US population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32(2):287-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DECODE Study group. European Diabetes Epidemiology Group Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354(9179):617-621 [PubMed] [Google Scholar]
- 14. Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141(6):413-420 [DOI] [PubMed] [Google Scholar]
- 15. Shaw JE, Hodge AM, de Courten M, Chitson P, Zimmet PZ. Isolated post-challenge hyperglycaemia confirmed as a risk factor for mortality. Diabetologia. 1999;42(9):1050-1054 [DOI] [PubMed] [Google Scholar]
- 16. Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose: the Funagata Diabetes Study. Diabetes Care. 1999;22(6):920-924 [DOI] [PubMed] [Google Scholar]
- 17. Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men: 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998;21(3):360-367 [DOI] [PubMed] [Google Scholar]
- 18. Barrett-Connor E, Ferrara A. Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men: the Rancho Bernardo Study. Diabetes Care. 1998;21(8):1236-1239 [DOI] [PubMed] [Google Scholar]
- 19. Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 2006;12(suppl 1):42-46 [DOI] [PubMed] [Google Scholar]
- 20. Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91(3):813-819 [DOI] [PubMed] [Google Scholar]
- 21. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. United Kingdom Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853 [PubMed] [Google Scholar]
- 23. Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32(1):187-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589 [DOI] [PubMed] [Google Scholar]
- 26. Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139 [DOI] [PubMed] [Google Scholar]
- 27. Patel A, MacMahon S, Chalmers J, et al. Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release and Controlled Evaluation (ADVANCE) Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560-2572 [DOI] [PubMed] [Google Scholar]
- 28. Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reaven PD, Moritz TE, Schwenke DC, et al. Veterans Affairs Diabetes Trial Intensive glucose-lowering therapy reduces cardiovascular disease events in Veterans Affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes. 2009;58(11):2642-2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Circulation. 2010;122:844-846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riddle MC, Ambrosius WT, Brillon DJ, et al. Action to Control Cardiovascular Risk in Diabetes Investigators Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33(5):983-990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boyko EJ. ACCORD glycemia results continue to puzzle. Diabetes Care. 2010;33(5):1149-1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROACTIVE (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet. 2005;366(9493):1279-1289 [DOI] [PubMed] [Google Scholar]
- 34. Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373(9677):1765-1772 [DOI] [PubMed] [Google Scholar]
- 35. Mannucci E, Monami M, Lamanna C, Gori F, Marchionni N. Prevention of cardiovascular disease through glycemic control in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2009;19(9):604-612 [DOI] [PubMed] [Google Scholar]
- 36. del Prato S. Megatrials in type 2 diabetes: from excitement to frustration? Diabetologia. 2009;52(7):1219-1226 [DOI] [PubMed] [Google Scholar]
- 37. Schwartz S. Targeting the pathophysiology of type 2 diabetes: rationale for combination therapy with pioglitazone and exenatide. Curr Med Res Opin. doi: 10.1185/03007990802390795. [published online ahead of print September 30, 2010] doi: 10.1185/03007990802390795. [DOI] [PubMed] [Google Scholar]
- 38. DeFronzo RA. Banting Lecture: from the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. American Diabetes Association Standards of medical care. Diabetes Care. 2010;33(suppl 1):S11-S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson PW, Kannel WB, Silbershatz H, D'Agostino RB. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159(10):1104-1109 [DOI] [PubMed] [Google Scholar]
- 41. Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr. 2003;22(5):331-339 [DOI] [PubMed] [Google Scholar]
- 42. Dagenais GR, Yi Q, Mann JF, Bosch J, Pogue J, Yusuf S. Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J. 2005;149(1):54-60 [DOI] [PubMed] [Google Scholar]
- 43. Ruttimann J. Possible pitfalls of bariatric surgery. Endocrine News. 2008:16-18 [Google Scholar]
- 44. Lean ME, Powrie JK, Anderson AS, Garthwaite PH. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med. 1990;7(3):228-233 [DOI] [PubMed] [Google Scholar]
- 45. Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care. 2000;23(10):1499-1504 [DOI] [PubMed] [Google Scholar]
- 46. Stevens VJ, Obarzanek E, Cook NR, et al. Trials for the Hypertension Prevention Research Group Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134(1):1-11 [DOI] [PubMed] [Google Scholar]
- 47. Shai I, Schwarzfuchs D, Henkin Y, et al. Dietary Intervention Randomized Controlled Trial (DIRECT) Group Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229-241 [DOI] [PubMed] [Google Scholar]
- 48. Look AHEAD Protocol Review Committee Protocol: action for health in diabetes: Look AHEAD Clinical Trial. Seventh revision, April 29, 2009. http://www.lookaheadtrial.org/public/LookAHEADProtocol.pdf Accessed October 19, 2010
- 49. Look AHEAD Research Group ; Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30(6):1374-1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klein S, Sheard NF, Pi-Sunyer X, et al. American Diabetes Association. North American Association for the Study of Obesity. American Society for Clinical Nutrition Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27(8):2067-2073 [DOI] [PubMed] [Google Scholar]
- 51. Buse JB, Ginsberg HN, Bakris GL, et al. American Heart Association. American Diabetes Association Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30(1):162-172 [DOI] [PubMed] [Google Scholar]
- 52. American Diabetes Association Management. In: Burant CF, ed. Medical Management of Type 2 Diabetes. 6th ed. Alexandria, VA: American Diabetes Association; 2008:33-85 [Google Scholar]
- 53. Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med. 2008;25(3):245-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. United Kingdom Hypoglycemia Study Group Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50(6):1140-1147 [DOI] [PubMed] [Google Scholar]
- 55. Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia. 2002;45(7):937-948 [DOI] [PubMed] [Google Scholar]
- 56. Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26(5):1485-1489 [DOI] [PubMed] [Google Scholar]
- 57. Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract. 2010;16(2):244-248 [DOI] [PubMed] [Google Scholar]
- 58. Tu E, Twigg SM, Semsarian C. Sudden death in type 1 diabetes: the mystery of the ‘dead in bed’ syndrome. Int J Cardiol. 2010;138(1):91-93 [DOI] [PubMed] [Google Scholar]
- 59. Ginde AA, Pallin DJ, Camargo CA., Jr Trends and disparities in U.S. emergency department visits for hypoglycemia, 1993-2005. Diabetes Care. 2008;31(3):511-513 [DOI] [PubMed] [Google Scholar]
- 60. Frier BM. Hypoglycaemia and cognitive function in diabetes. Int J Clin Pract Suppl. 2001. September;(123):30-37 [PubMed] [Google Scholar]
- 61. Hypoglycemia associated with increased risk of MI among US veterans with diabetes [press release]. Vienna, Austria: European Association for the Study of Diabetes (EASD) Annual Meeting; October 5, 2009. http://www.the-heart.org/article/1010567.do Accessed July 2, 2010 [Google Scholar]
- 62. del Prato S, Felton AM, Munro N, Nesto R, Zimmet P, Zinman B, Global Partnership for Effective Diabetes Management Improving glucose management: ten steps to get more patients with type 2 diabetes to glycaemic goal. Int J Clin Pract. 2005;59(11):1345-1355 [DOI] [PubMed] [Google Scholar]
- 63. Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151(6):394-403 [DOI] [PubMed] [Google Scholar]
- 64. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298(2):194-206 [DOI] [PubMed] [Google Scholar]
- 65. Byetta (exenatide BID) [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc; 2009. http://pi.lilly.com/us/byetta-pi.pdf Accessed October 19, 2010 [Google Scholar]
- 66. Victoza (liraglutide) [package insert]. Princeton, NJ: Novo Nordisk Inc; 2010. http://www.gahec.org/pharmupd/Victoza.ppt#303,23, Victoza® / liraglutide Trial Information Accessed October 19, 2010 [Google Scholar]
- 67. Stonehouse A, Okerson T, Kendall D, Maggs D. Emerging incretin based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev. 2008;4(2):101-109 [DOI] [PubMed] [Google Scholar]
- 68. Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88(7):3082-3089 [DOI] [PubMed] [Google Scholar]
- 69. Schnabel CA, Wintle M, Kolterman O. Metabolic effects of the incretin mimetic exenatide in the treatment of type 2 diabetes. Vasc Health Risk Manag. 2006;2(1):69-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Drucker DJ, Buse JB, Taylor K, et al. DURATION-1 Study Group Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240-1250 [DOI] [PubMed] [Google Scholar]
- 71. Buse JB, Rosenstock J, Sesti G, et al. LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39-47 [DOI] [PubMed] [Google Scholar]
- 72. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association. European Association for Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Madsbad S. Liraglutide Effect and Action in Diabetes trial. Expert Rev Endocrinol Metab. 2009;4(2):119-129 [DOI] [PubMed] [Google Scholar]
- 74. Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naïve patients with type 2 diabetes. Diabetes Obes Metab. 2008;10(5):376-386 [DOI] [PubMed] [Google Scholar]
- 75. Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24(1):275-286 [DOI] [PubMed] [Google Scholar]
- 76. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, GWAA Study Group Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559-569 [DOI] [PubMed] [Google Scholar]
- 77. Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50(2):259-267 [DOI] [PubMed] [Google Scholar]
- 78. Bhushan R, Elkind-Hirsch KE, Bhushan M, Butler WJ, Duncan K, Marrioneaux O. Exenatide use in the management of metabolic syndrome: a retrospective database study. Endocr Pract. 2008;14(8):993-999 [DOI] [PubMed] [Google Scholar]
- 79. Bhushan R, Elkind-Hirsch KE, Bhushan M, Butler WJ, Duncan K, Marrioneaux O. Improved glycemic control and reduction of cardiometabolic risk factors in subjects with type 2 diabetes and metabolic syndrome treated with exenatide in a clinical practice setting. Diabetes Technol Ther. 2009;11(6):353-359 [DOI] [PubMed] [Google Scholar]
- 80. Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32(5):834-838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25(4):1019-1027 [DOI] [PubMed] [Google Scholar]
- 82. Riddle M, Frias J, Zhang B, et al. Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care. 2007;30(11):2794-2799 [DOI] [PubMed] [Google Scholar]
- 83. Chisholm JW, Goldfine AB, Dhalla AK, et al. Effect of ranolazine on A1C and glucose levels in hyperglycemic patients with non-ST elevation acute coronary syndrome. Diabetes Care. 2010;33(6):1163-1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Levy P, Jellinger PS. The potential role of colesevelam in the management of prediabetes and type 2 diabetes. Postgrad Med. 2010;122(3)(suppl 1):1-8 [DOI] [PubMed] [Google Scholar]
- 85. Scranton R, Cincotta A. Bromocriptine—unique formulation of a dopamine agonist for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2010;11(2):269-279 [DOI] [PubMed] [Google Scholar]
- 86. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540-559 [DOI] [PubMed] [Google Scholar]