Abstract
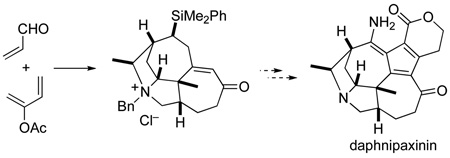
The aza-Cope–Mannich reaction and ring-closing metathesis are key steps in the assembly of intermediates containing rings A–D of Daphniphyllum alkaloids of the daphnicyclidin-type such as daphnipaxinin and oldhamine A.
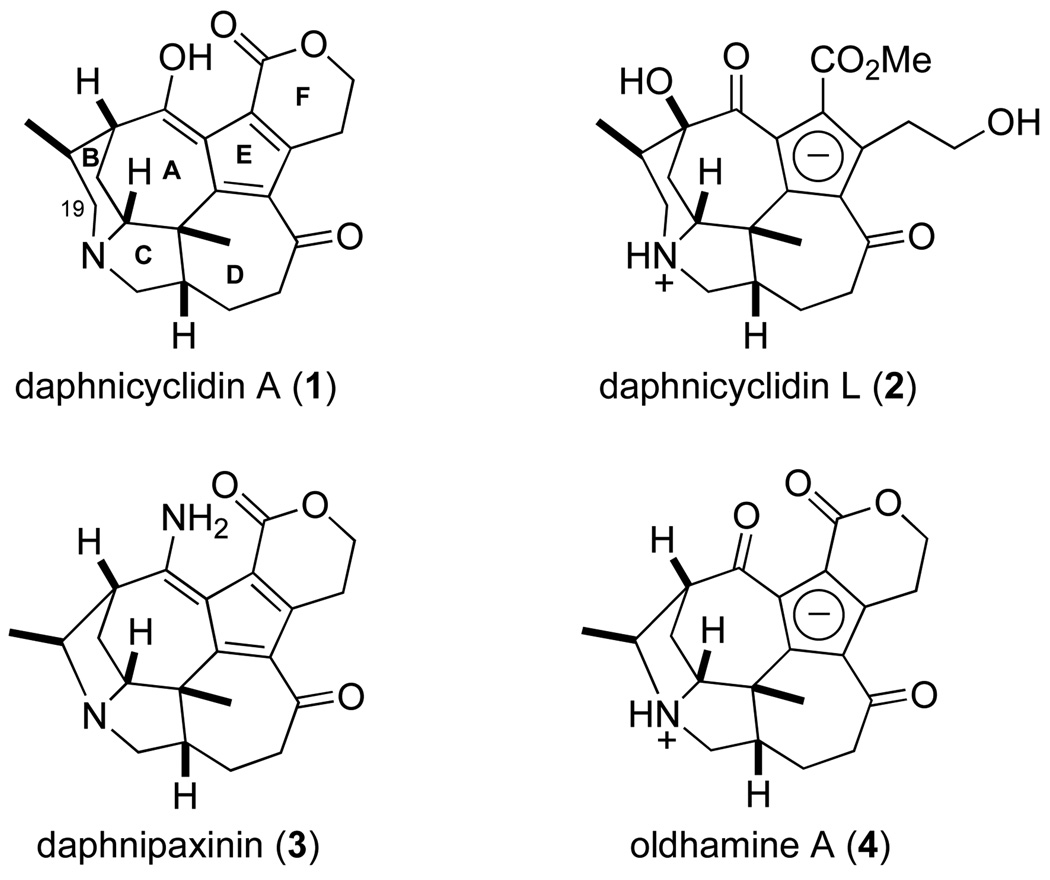
Kobayashi reported in 2001 the first members of a structurally unprecedented group of fused hexa- or pentacyclic Daphniphyllum alkaloids, daphnicyclidins A (1)–H.1 Daphnicyclidin-type alkaloids, which now number more than 15, have compact structures that are believed to derive via extensive structural modification of Daphniphyllum alkaloids generated from cyclization of squalene precursors.2 Four representative examples are depicted in Figure 1. These compounds illustrate several of the remarkable structural features of these alkaloids, in particular ring E, which is found in all but two members as either a fulvene or a cyclopentadienyl anion unit. Structures for Daphniphyllum alkaloids of the daphnicyclidin-type have been secured on the basis of mass spectrometric, spectroscopic and X-ray data,3 augmented by chemical correlations.2 Daphnipaxinin (3) is notable as the first diamino Daphniphyllum alkaloid isolated.4 Bioactivities of these alkaloids have been poorly studied, although several display moderate cytotoxicity,1,2 and others were isolated from plants used in traditional medicine; for example, daphnipaxinin (3) is found in D. paxianum, an herb used in Chinese folk medicine for the treatment of inflammation.4
Figure 1.
Representative Daphniphyllum alkaloids of the daphnicyclidin-type.
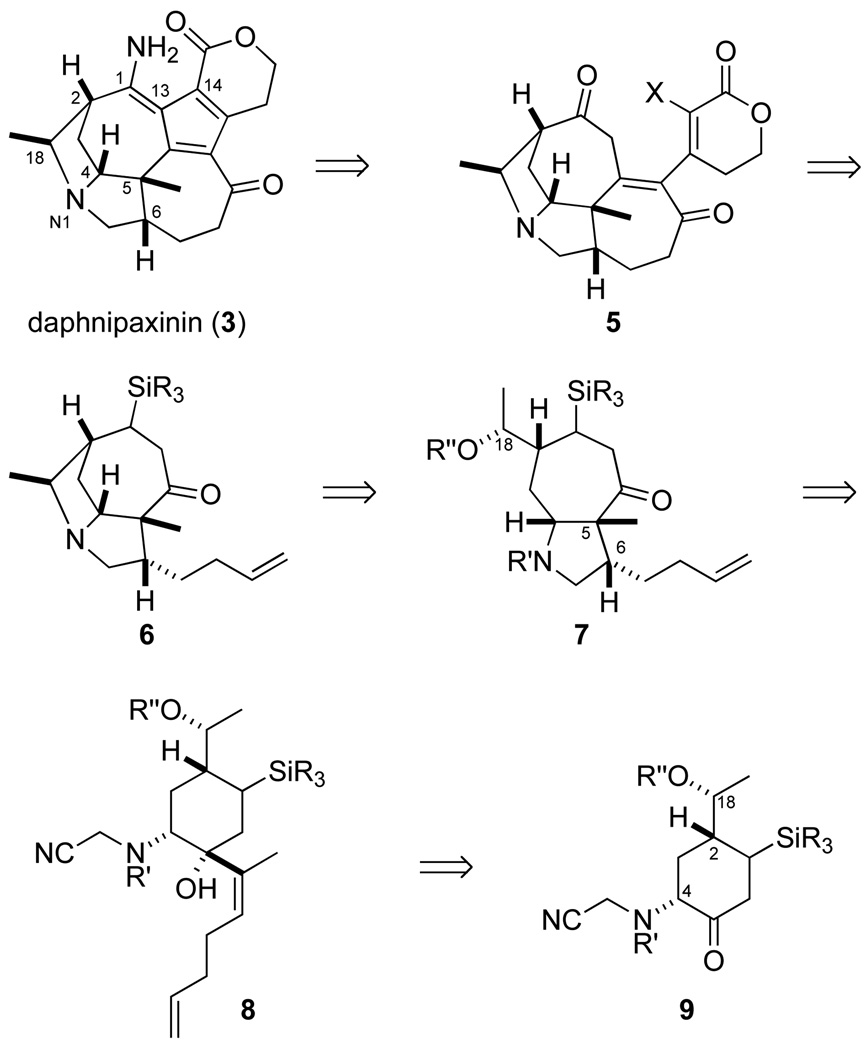
Stimulated by their unprecedented structures, their scarce natural supply,5 and their largely unexplored pharmacological profiles, we initiated chemical synthesis studies towards Daphniphyllum alkaloids of the daphnicyclidin-type. Ring B is a piperidine in most members of this group, whereas it is a pyrrolidine in daphnipaxinin (3) and oldhamine A (4). We chose to pursue a potentially general strategy in which ring B would be constructed at an advanced stage of the synthesis by forming a bond to C18 from either N1 to form a pyrrolidine ring (as suggested in Scheme 1) or the conjugate base of a cyanomethyl derivative of this amine to construct a piperidine ring. We planned to assemble rings A and C and the five stereocenters of daphnicyclidin-type alkaloids early in the synthesis by aza-Cope–Mannich rearrangement (8→7) of a formaldiminium ion derived from an aminocyclohexanol precursor such as 8.6 We report herein enantioselective construction of the tetracyclic A–D fragment of daphnipaxinin (3) and oldhamine A (4) by this approach.7
Scheme 1.
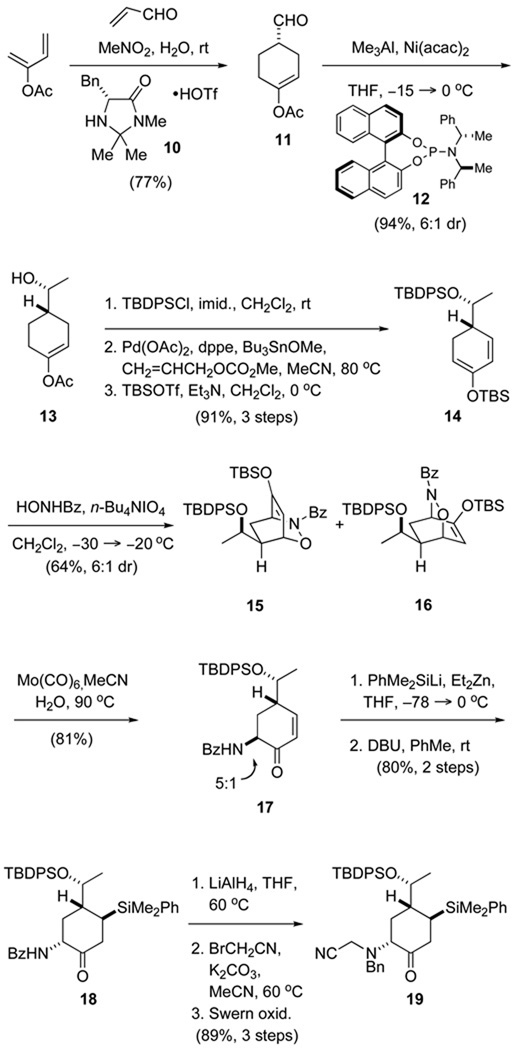
The method we devised to access a 2-aminocyclohexanone intermediate incorporating the C2, C4 and C18 stereocenters of daphnicyclidin-type Daphniphyllum alkaloids is summarized in Scheme 2. The synthesis began with (S)-cyclohexenecarboxyaldehyde 11 (91% ee), which was prepared by the organocatalytic Diels–Alder method of MacMillan.8 To effectively carry out the cycloaddition of 2-acetoxy-1,3-butadiene and acrolein, it was important to use the hydrotriflate salt of catalyst 10 (2.5 mol %) and water-saturated nitromethane as the reaction solvent.9 The C18 stereocenter was incorporated by nickel-catalyzed methylation of the aldehyde using 2 mol % of the (Rp,S,S)-phosphoramidite catalyst 12, following the general prescriptions of Woodward.10,11 In three conventional steps, product 13 was converted to siloxycyclohexadiene 14.12 The amino substituent was then introduced by a two-step sequence that began with hetero Diels–Alder reaction of diene 14 with (nitrosocarbonyl)benzene to give a 6:1 mixture of major adducts 15 and 16.13,14 Although these products could be isolated in pure form and processed separately to intermediate 18, it was more convenient to directly reduce the mixture of stereoisomeric cycloadducts with Mo(CO)6 to form cyclohexenone 17 in good yield, as a ca 5:1 mixture of benzoylamino epimers.15 The silyl substituent, which we envisioned as a precursor of a carbonyl group at C1, was introduced at this point by conjugate addition of a zincate reagent generated from phenyldimethylsilyllithium.16 Base-catalyzed epimerization of this product then delivered benzoylaminocyclohexanone 18 in 41% overall yield from siloxycyclohexadiene 14. In three additional steps, this intermediate was transformed to the N-benzyl,N-cyanomethyl congener 19 in high overall yield.
Scheme 2.
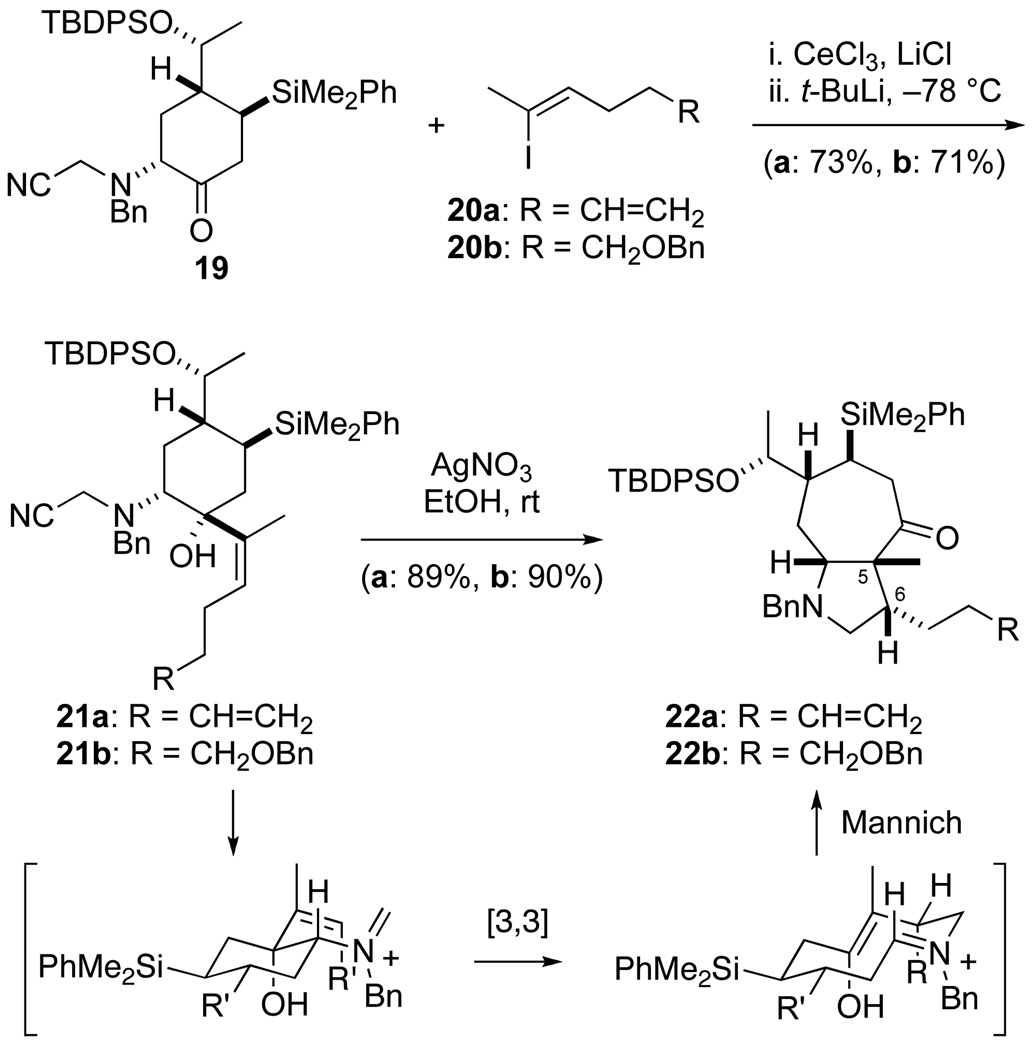
Aminoketone 19 can serve as a precursor for fashioning various cycloheptapyrrolidine intermediates by the aza-Cope–Mannich transformation. Considerable effort was invested to find useful conditions for the addition of vinyl organometallic nucleophiles to this hindered ketone. Premixing ketone 19 with CeCl3 ·2LiCl17 at room temperature, addition of vinyl iodide 20a, and subsequent dropwise addition of t-BuLi at −78 °C provided tertiary alcohol 21a in 73% yield. Exposing this product to 1.2 equiv of AgNO3 in ethanol at room temperature delivered cycloheptapyrrolidine 22a18 as a single stereoisomer in 89% yield. In related fashion, ketone 19 and vinyl iodide 20b were converted in two steps to cycloheptapyrrolidine 22b (64% overall yield). The aza-Cope–Mannich transformation to form 22a and 22b generated the A and C rings and C5 and C6 stereocenters of the daphnicyclidin-type alkloids in high yield and with complete stereocontrol.
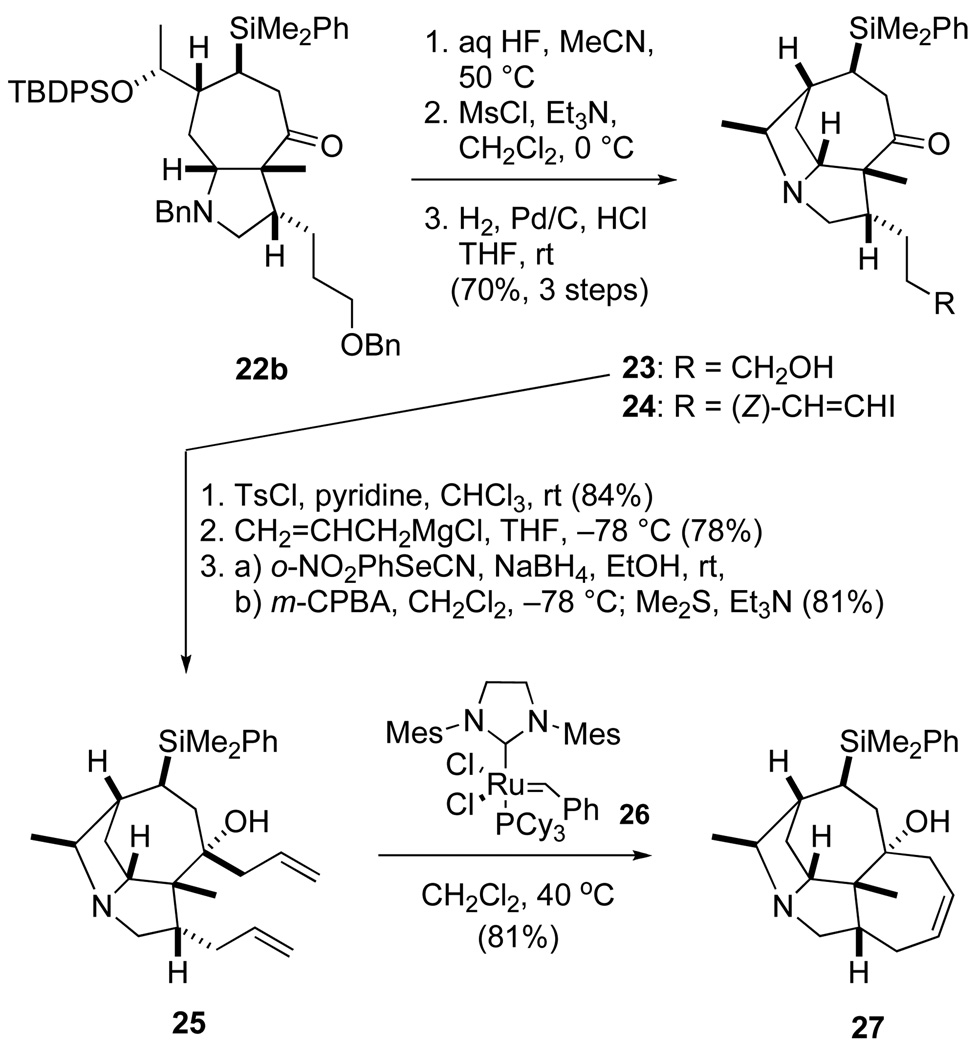
Several approaches for forming the B and D rings were investigated. In one approach, the B ring was formed first by sequential treatment of cycloheptapyrrolidine 22b with aqueous HF to cleave the TBDPS group, and methanesulfonyl chloride to activate the alcohol for intramolecular alkylation by the tertiary amine. Hydrogenolysis of the resulting quaternary ammonium salt cleaved both benzyl groups to deliver tricyclic aminoketone 23 in 70% overall yield. Although this product could be elaborated to a β-ketophosphonate derivative, all attempts to form the seven-membered D ring by intramolecular Horner–Wadsworth–Emmons reaction failed.19 Alternatively, 23 could be converted to (Z)-vinyl iodide 24, but attempted ring closure by lithium halogen exchange or under Nozaki–Hiyama–Kishi conditions20 resulted in protodeiodination of the starting material. Our first success in fashioning this ring was achieved by first transforming amino ketone 23 to diene 25 by a sequence of three standard reactions. Ring-closing metathesis of amino diene 25 proceeded in good yield using the Grubbs second-generation catalyst 2621 to form tetracyclic amine 27 in 81% yield.
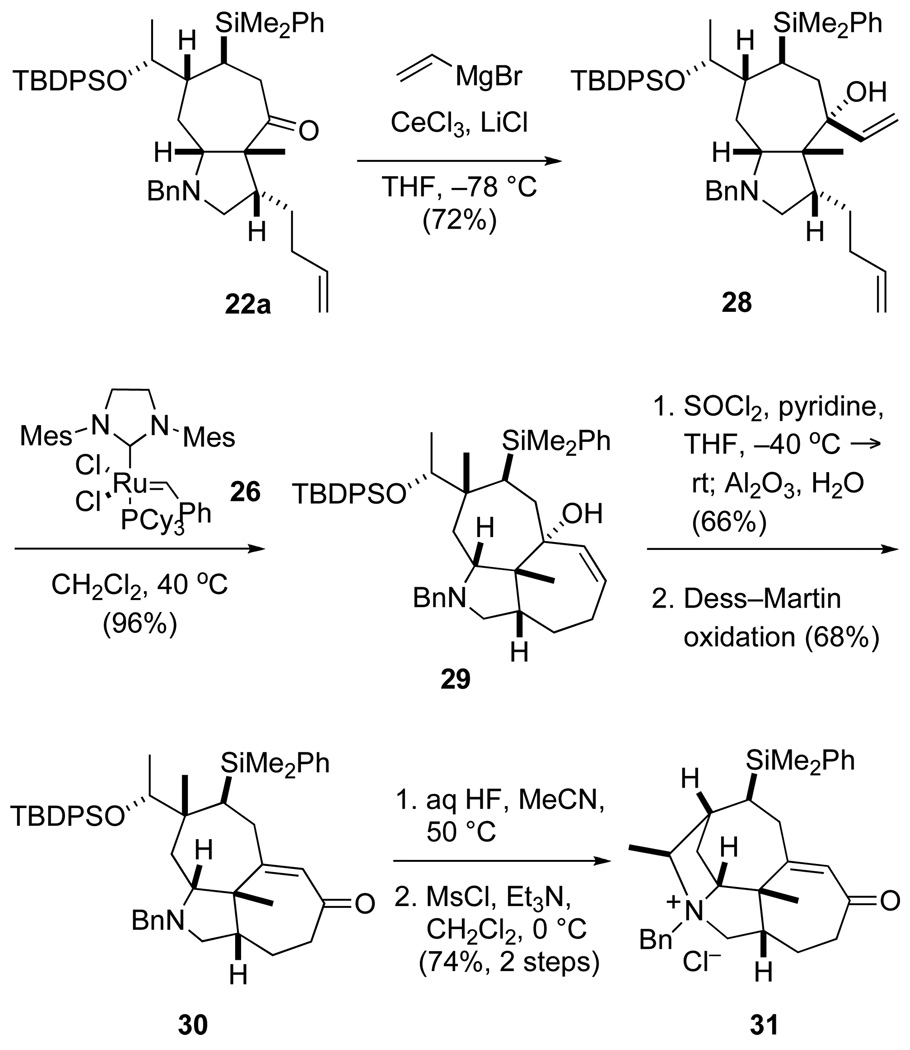
Alternatively, the seven-membered D ring could be fashioned prior to forming ring B (Scheme 5). Exposure of cycloheptapyrrolidine 22a to an excess of vinylmagnesium bromide and CeCl3·2LiCl in THF at −78 °C provided a single allylic alcohol product 28 in 72% yield. Cyclization of this diene in refluxing CH2Cl2 in the presence of catalyst 26 provided tricyclic amine 29 in nearly quantitative yield. Although one-step procedures for converting tertiary allylic alcohol 29 to enone 30 failed, this transformation could be realized in a step-wise fashion. Thus, exposing allylic alcohol 29 to thionyl chloride and pyridine, followed by hydrolysis of the resulting transposed allylic chloride upon adding alumina and water and subsequent Dess–Martin oxidation22 provided enone 30 in 45% yield. The B ring could then be constructed in good yield by the previously described two-step sequence.
Scheme 5.
In summary, an enantioselective synthesis of an advanced tetracyclic intermediate containing all of the stereocenters found in daphnipaxinin and oldhamine A has been developed. A high-yielding aza-Cope–Mannich reaction was utilized to generate the A and C ring cycloheptapyrrolidine core and install both the C5 quaternary carbon stereocenter as well as the adjacent C6 stereocenter. An intramolecular amino alkylation/RCM sequence was then used to form the B and D rings, respectively. Efforts toward construction of the remaining E and F rings to complete an enantioselective synthesis of daphnipaxinin are currently underway.
Supplementary Material
Scheme 3.
Acknowledgment
This research was supported by the NIH Neurological Disorders & Stroke Institute (NS-12389), and by postdoctoral fellowship support for TBD (HL-078104), JME (GM-082113), CCK (Alexander von Humboldt Foundation), and JRM (CA-139868). NMR, mass spectra, and the X-ray analysis were obtained at UC Irvine using instrumentation acquired with the assistance of NSF and NIH Shared Instrumentation grants. We thank Dr. Joe Ziller, University of California, Irvine, for the single-crystal X-ray analysis and Dr. John Greaves, University of California, Irvine, for mass spectrometric analyses.
Footnotes
Supporting Information Available: Experimental details and procedures, tabulated NMR data for compounds 27 and 31, copies of 1H and 13C NMR spectra of new compounds, and an X-ray crystallographic file (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kobayashi J, Inaba Y, Shiro M, Yoshida N, Morita H. J. Am. Chem. Soc. 2001;123:11402–11408. doi: 10.1021/ja016955e. [DOI] [PubMed] [Google Scholar]
- 2. Kobayashi J, Kubota T. Nat. Prod. Rep. 2009;26:936–962. doi: 10.1039/b813006j. and other reviews cited therein.
- 3.Alkaloids 1, 2 and 4 have been characterized by single crystal X-ray analysis: (a) reference 2, Gan X, Bai H, Chen Q, Ma L, Hu L. Chem. Biodivers. 2006;3:1255–1259. doi: 10.1002/cbdv.200690127. Tan C, Di Y, Wang Y, Wang Y, Mu S, Gao S, Zhang Y, Kong N, He H, Zhang J, Fang X, Li C, Lu Y, Hao X. Tetrahedron Lett. 2008;49:3376–3379.
- 4.Yang SP, Yue JM. Org. Lett. 2004;6:1401–1404. doi: 10.1021/ol049756j. [DOI] [PubMed] [Google Scholar]
- 5.For example, daphnicyclidin (3) and oldhamine A (4) were isolated in 0.00024% and 0.00007%, respectively, from their dried plant sources.
- 6.For a recent brief review and leading references, see Overman LE. Tetrahedron. 2009;65:6432–6446. doi: 10.1016/j.tet.2009.05.067.
- 7.An enantiocontrolled route to a BCD ring fragment of daphnicyclidin A was described recently, see: Ikeda S, Shibuya M, Kanoh N, Iwabuchi Y. Org. Lett. 2009;11:1833–1836. doi: 10.1021/ol9003405.
- 8.Ahrendt KA, Borths CJ, MacMillan DWC. J. Am. Chem. Soc. 2000;122:4243–4244. [Google Scholar]
- 9.Kowalski MD. Ph.D. Dissertation. Irvine: University of California; 2008. [Google Scholar]
- 10.Biswas K, Prieto O, Goldsmith PJ, Woodward S. Angew. Chem. Int. Ed. 2005;44:2232–2234. doi: 10.1002/anie.200462569. [DOI] [PubMed] [Google Scholar]
- 11.The expected10 configuration of the major alcohol epimer was confirmed by X-ray analysis of a subsequent product.
- 12.Minami I, Takahashi K, Shimizu I, Kimura T, Tsuji J. Tetrahedron. 1986;42:2971–2977. [Google Scholar]
- 13.(a) Boger DL, Weinreb SM. Hetero Diels–Alder Methodology in Organic Synthesis. San Diego: Academic; 1987. [Google Scholar]; (b) Streith J, Defoin A. Synthesis. 1994:1107–1117. [Google Scholar]
- 14.A minor amount of a third stereoisomeric adduct that derives from the inseparable minor alcohol epimer of intermediate 13 was also produced.
- 15.Cabanal-Duvillard I, Berrien JF, Ghosez L, Husson H–P, Royer J. Tetrahedron. 2000;56:3763–3769. [Google Scholar]
- 16.Crump RANC, Fleming I, Urch CJ. J. Chem. Soc., Perkin Trans. 1. 1994:701–706. [Google Scholar]
- 17. Krasovskiy A, Kopp F, Knochel P. Angew. Chem., Int. Ed. 2006;45:497–500. doi: 10.1002/anie.200502485.. (b) We employed a simpler procedure for preparing this reagent (see Supporting Information); for a related procedure for preparing a cerium acetylide, see: Trost BM, Waser J, Meyer A. J. Am. Chem. Soc. 2008;130:16424–16434. doi: 10.1021/ja806724x.
- 18.Aza-Cope–Mannich product 22a was elaborated to a tricyclic pyrrolidinium salt by cleavage of TBDPS group and intramolecular quaternization by the sequence illustrated in Schemes 4 and 5; see Supporting Information for details. Single crystal X-ray analysis of this derivative confirmed the relative and absolute configuration of 22a. Crystallographic data for this compound were deposited with the Cambridge Crystallographic Data Centre: CCDC 751136.
- 19.For a pertinent example where this reaction also failed to form a seven-membered ring, see: Dudley GB, Danishefsky SJ. Org. Lett. 2001;3:2399–2402. doi: 10.1021/ol016222z.
- 20.For a review, see: Fürstner A. Chem. Rev. 1999;99:991–1045. doi: 10.1021/cr9703360.
- 21.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 22.Dess DB, Martin JC. J. Org. Chem. 1983;48:4155–4156. [Google Scholar]
Scheme 4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.