Abstract
Ca2+/Calmodulin protein kinase IIα (CaMKIIα) has a central role in regulating neuronal excitability. It is well established that CaMKIIα translocates to excitatory synapses following strong glutamatergic stimuli that induce NMDA-receptor (NMDAR)-dependent long-term potentiation in CA1 hippocampal neurons. We now show that CaMKIIα translocates to inhibitory but not excitatory synapses in response to more moderate NMDAR-activating stimuli that trigger GABAA-receptor (GABAAR) insertion and enhance inhibitory transmission. Such moderate NMDAR activation causes Thr286 autophosphorylation of CaMKIIα, which our results demonstrate is necessary and sufficient, under basal conditions, to localize CaMKIIα at inhibitory synapses and enhance surface GABAAR expression. Although stronger glutamatergic stimulation coupled to AMPA receptor insertion also elicits Thr286 autophosphorylation, accumulation of CaMKIIα at inhibitory synapses is prevented under these conditions by the phosphatase calcineurin. This preferential targeting of CaMKIIα to glutamatergic or GABAergic synapses provides neurons with a mechanism whereby activity can selectively potentiate excitation or inhibition through a single kinase mediator.
Ca2+/Calmodulin protein kinase IIα (CaMKIIα) is essential for NMDA receptor (NMDAR)-dependent potentiation of many excitatory synapses (1, 2). However, CaMKIIα also directly phosphorylates the inhibitory GABAA receptor (GABAAR) α1, β2, β3, and γ2 subunits (3–5). CaMKIIα activation increases GABAARs in synaptosomal preparations (6), and potentiates GABAAR-mediated currents in neurons of the spinal cord dorsal horn (7), cortex (8), cerebellum (9, 10), and hippocampus (7, 11). We recently reported that hippocampal inhibitory synapses are potentiated upon activation of NMDARs through a CaMKIIα-dependent insertion of GABAARs into the membrane (12). The similar role of CaMKIIα in NMDAR-dependent excitatory and inhibitory potentiation raises the question of how specificity in the modulation of excitatory or inhibitory synapses is maintained.
CaMKIIα translocates to excitatory synapses on dendritic spines following long-term potentiation (LTP) induction (13, 14), glutamate receptor activation (15–17), or sensory stimulation in vivo (18). However, it is not known whether CaMKIIα must similarly translocate to inhibitory synapses on dendritic shafts to modulate GABAergic transmission. If so, an understanding of the differential regulation of CaMKIIα targeting to inhibitory and excitatory synapses would provide important insight into the control of neuronal excitability.
Here we show that although strong activation of NMDARs induces translocation of CaMKIIα to excitatory synapses and enhances surface AMPAR levels, a weaker activation of NMDARs localizes CaMKIIα to inhibitory synapses. This differential translocation of CaMKIIα is dependent on the activation of calcineurin (CaN), which prevents CaMKIIα targeting to inhibitory synapses in response to strong stimuli. Analysis of CaMKIIα mutants further reveals that when autophosphorylated, CaMKIIα is constitutively localized at inhibitory, not excitatory, synapses, and that this is sufficient under basal conditions to elevate surface GABAAR levels. Our results demonstrate that CaMKIIα can translocate to inhibitory synapses, and as a result of distinct targeting mechanisms, this single molecule can selectively couple activity to the modulation of either glutamatergic or GABAergic synapses.
Results
NMDA Induces Monomeric-GFP-CaMKIIα Clustering.
We previously reported that brief stimulation of NMDARs with NMDA [20–50 μM, 1 min, with 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and glycine] triggers a CaMKIIα-dependent insertion of GABAARs in hippocampal neurons (12). This same stimulus induces phosphatase-dependent AMPAR removal (19). To understand how CaMKIIα functions at inhibitory synapses, we analyzed a mGFP–tagged CaMKIIα (mGFP-CaMKIIα) expressed in hippocampal neurons. NMDA treatment elicited a gradual clustering of mGFP-CaMKIIα throughout the dendritic shafts (Fig. 1 A–C and Movie S1) (control: n = 16 cells; NMDA: n = 16 cells). In NMDA-treated cells, puncta emerged above the pretreatment fluorescence (Movie S2), indicating that CaMKIIα accumulates in discrete dendritic regions in response to this stimulus.
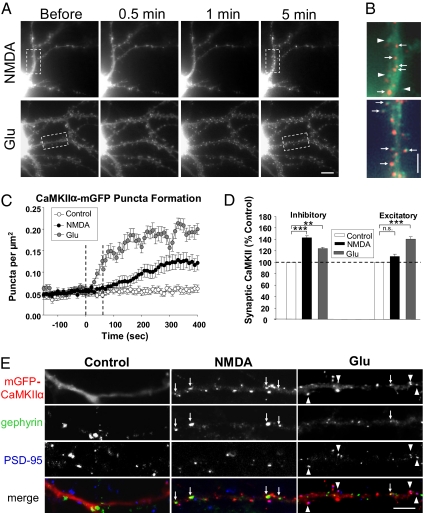
Fig. 1.
Glutamatergic stimuli differentially elicit synaptic clustering of mGFP-CaMKIIα. (A) Live hippocampal neurons expressing mGFP-CaMKIIα were imaged every 10 s before and after 1 min NMDA (50 μM, with 10 μM CNQX) or glutamate (100 μM, Glu) application. Images are of mGFP-CaMKIIα 10 s before and 0.5, 1, and 5 min after agonist application. (Scale bar, 5 μm.) (B) Enlargement of boxed areas in A. Images taken 5 min posttreatment (red) were overlayed onto baseline images (green) to illustrate the CaMKIIα redistribution. Arrowheads highlight spines without mGFP-CaMKIIα puncta. (Scale bar, 2.5 μm.) (C) Quantification of mGFP-CaMKIIα puncta formation following agonist addition. Dashed lines indicate the duration of agonist application. (D) Synaptic localization of mGFP-CaMKIIα in transfected neurons. Transfected cells were treated with NMDA (50 μM) or glutamate (100 μM) for 1 min, fixed 5 min later, and labeled with antibodies to PSD-95 and gephyrin. Agonist-induced effects on synaptic mGFP-CaMKIIα are expressed as percent-change from untreated controls. n.s., not significant; **P < 0.01; ***P < 0.001. (E) Images depict mGFP-CaMKIIα (red) expressing neuronal dendrites, labeled with gephyrin (green), and PSD-95 (blue). Arrows highlight inhibitory synaptic mGFP-CaMKIIα; arrowheads indicate excitatory synaptic mGFP-CaMKIIα. (Scale bar, 5 μm.)
Studies have shown that brief (1–3 min) application of glutamate (50–100 μM/10 μM glycine) induces the accumulation of CaMKIIα at excitatory synapses (16, 17, 20, 21). Glutamate-induced CaMKIIα translocation also requires NMDAR activation as assessed by pharmacological and genetic disruption of receptor activation (16, 17). We also observed the emergence of mGFP-CaMKIIα puncta upon glutamate application (100 μM, 1 min; n = 8 cells) (Fig. 1 A and C and Movies S3 and S4). These puncta formed more quickly than those observed following NMDA treatment and apparently localized in spines (Fig. 1B), suggesting differential targeting of CaMKIIα in response to these two stimuli.
CaMKIIα Selectively Localizes to Inhibitory Synapses Following Moderate Glutamate Receptor Activation.
To define the location of mGFP-CaMKIIα puncta, cells were treated with agonist for 1 min, fixed 5 min later, and labeled with antibodies to identify PSD-95-positive excitatory and gephyrin-positive inhibitory synapses. NMDA increased the proportion of inhibitory synapses with colocalized mGFP-CaMKIIα to 143 ± 3% of control levels (n = 18; ***P < 0.001) (Fig. 1 D and E), but had no significant effect on mGFP-CaMKIIα at excitatory synapses (110 ± 4%, n = 18, P = 0.2) (Fig. 1 D and E). In contrast, glutamate enhanced excitatory synaptic mGFP-CaMKIIα (140 ± 4%; n = 9; ***P < 0.001) (Fig. 1 D and E) as reported previously, with a smaller increase at inhibitory synapses (124 ± 2%, n = 9; **P < 0.01) (Fig. 1 D and E).
To test whether endogenous CaMKIIα selectively localizes to inhibitory synapses, we labeled neurons with antibodies to CaMKIIα, PSD-95, and gephyrin. Under basal conditions, 26 ± 3% of PSD-95 puncta and 27 ± 3% of gephyrin puncta colocalized with CaMKIIα puncta. As with the transfected kinase, NMDA elicited an increase in endogenous CaMKIIα-containing inhibitory synapses 5 min after agonist treatment (154 ± 12% of control, n = 6; **P < 0.01) (Fig. 2 A and B) and had an insignificant effect on CaMKIIα-containing excitatory synapses (112 ± 10%, n = 6, P = 0.7) (Fig. 2 A and B). APV [(2R)-amino-5-phosphonovaleric acid] blocked NMDA-induced clustering of endogenous CaMKIIα at inhibitory synapses (102 ± 5%, n = 5, P = 0.7) (Fig. 2B), as reported for glutamate-induced clustering at excitatory synapses (16). Glutamate increased CaMKIIα-containing excitatory synapses (168 ± 10%, n = 4; *P < 0.05) (Fig. 2 A and B), with no significant change in endogenous CaMKIIα-containing inhibitory synapses (87 ± 10%, n = 9, P = 0.3) (Fig. 2 A and B). There was no change in the number of synapses or nonsynaptic CaMKIIα puncta following either treatment (Fig. S1 A and B). These results demonstrate that NMDAR activation couples to the selective accumulation of CaMKIIα at either inhibitory or excitatory synapses in a stimulus-specific manner.
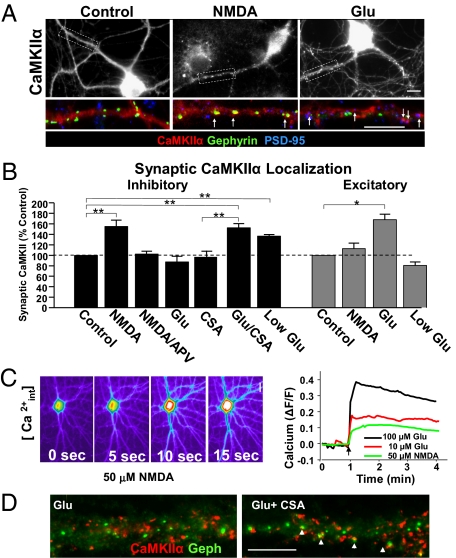
Fig. 2.
The magnitude of glutamate receptor activation dictates synaptic targeting of endogenous CaMKIIα. (A) Control, NMDA, and glutamate-treated (1 min) neurons were fixed after 5 min and labeled with antibodies to CaMKIIα (Upper images and red channel in enlarged images), gephyrin (green), and PSD-95 (blue). Arrows indicate synaptically-localized CaMKIIα. (Scale bars, 10 μm.) (B) Quantification of synaptic localization of endogenous CaMKIIα in neurons 5 min after glutamatergic stimuli. The fraction of PSD-95+ or gehyrin-positive synapses with colocalized CaMKIIα puncta in each condition is shown as the percentage of untreated control levels. APV represents NMDAR antagonist d-APV (50 μM); *P < 0.05; **P < 0.01. (C) Neurons loaded with Fluo-3-AM were imaged before and after addition of 50 μM NMDA, 100 μM glutamate, or 10 μM glutamate. Images were acquired every 3 s. Pseudocolored images depict the Ca2+ response in an NMDA-treated cell (Left). The time-course of fluorescence changes in proximal dendrites is plotted as ΔF/F (Right). (Scale bar, 10 μm.) (D) Images show Ab labeling of CaMKIIα (red) and gephyrin (green) in neurons 5 min after glutamate treatment (1 min, 100 μM) with or without 1 μM CSA. (Scale bar, 10 μm.).
What accounts for the differences in synaptic CaMKIIα targeting? Unlike NMDA, glutamate activates AMPA and metabotropic glutamate receptors (mGluRs) as well as NMDARs. Coactivation of mGluRs is not likely to be a factor, as CaMKIIα localization was unaffected when the mGluR antagonist MCPG was included with glutamate (Fig S2). AMPAR coactivation with glutamate may enhance depolarization, and thus more effectively activate NMDARs by relieving their voltage-dependent Mg2+ block. Imaging Ca2+ responses in hippocampal neurons loaded with the Ca2+ indicating dye Fluo-3-AM confirmed that 100 μM glutamate induced a larger Ca2+ elevation than that elicited by 50 μM NMDA (Fig. 2C). A lower dose of glutamate (10 μM, low glu), however, elicited a Ca2+ response comparable to that generated by NMDA application. As with NMDA, low glutamate increased CaMKIIα clustering at inhibitory (136 ± 2% of control, n = 5; **P < 0.01) (Fig. 2B) but not excitatory synapses (80 ± 6%, n = 5, P = 0.6) (Fig. 2B). Thus, the magnitude of NMDAR activation and Ca2+ influx is a critical determinant in the selective targeting of CaMKIIα to inhibitory synapses.
CaMKIIα Targeting to Inhibitory Synapses Is Inhibited by CaN.
Endogenous and transfected CaMKIIα exhibit similar targeting; although the transfected kinase weakly accumulates at inhibitory synapses following glutamate treatment, endogenous CaMKIIα does not. As mGFP-CaMKIIα transfection more than doubled the amount of immunocytochemically detected kinase (251 ± 36% of control levels), the introduced kinase may overwhelm an endogenous signaling pathway that regulates CaMKIIα translocation. Conditions that elevate CaMKIIα activity at excitatory synapses have been reported to induce CaN binding to GABAARs at inhibitory synapses, resulting in their dephosphorylation and depression of inhibition (22, 23). Thus, we examined whether CaN also regulates CaMKIIα translocation to inhibitory synapses with glutamate treatment. Although glutamate did not elicit clustering of endogenous CaMKIIα at inhibitory synapses (Fig. 2D), blocking CaN activity with cyclosporin A (CSA, 30-min pretreatment) revealed a glutamate-induced increase in CaMKIIα colocalization with gephyrin (Glu/CSA 152 ± 7% of CSA control, n = 5; **P < 0.01; CSA alone, 98 ± 9% of control, n = 5) (Fig. 2B). CaN therefore negatively regulates CaMKIIα clustering at inhibitory synapses.
Targeting to Inhibitory Synapses Is Dependent on the CaMKIIα Activation State.
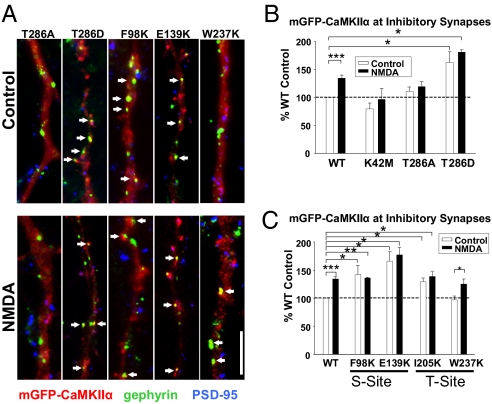
Elevated intracellular Ca2+ induces calmodulin to bind to CaMKIIα, displacing the pseudosubstrate/autoinhibitory domain from the substrate-binding site (S-site) (Fig. S3). This process places the kinase in an open conformation, allowing T286 autophosphorylation and causing CaMKIIα to remain active independent of Ca2+ (24). To identify the molecular events underlying CaMKIIα translocation to inhibitory synapses, we examined the targeting of mutant CaMKIIα constructs with altered activation properties. Although WT-CaMKIIα localization at inhibitory synapses increased to 136 ± 6% of control after NMDA treatment (n = 14; ***P < 0.001) (Fig. 3 A and B), K42M, a kinase-inactive form able to bind to NMDA receptors (25), exhibited no significant change in localization with NMDA treatment (K42M control: 79 ± 10% of WT control, P = 0.1; NMDA: 96 ± 19%, n = 6, P = 0.6) (Fig. 3B). A T286A mutant, which translocates to excitatory synapses (16, 20, 25), did not relocate to inhibitory synapses in response to NMDA (T286A:control: 109 ± 6%; NMDA: 116 ± 9%, n = 8, P = 0.3) (Fig. 3 A and B). The phosphomimetic T286D mutant, on the other hand, constitutively localized at inhibitory but not excitatory synapses in unstimulated neurons (inhibitory: 163 ± 19%, n = 4; *P < 0.05) (Fig. 3 A and B) (excitatory: 116 ± 40%, n = 4) (Fig. S4). NMDA treatment did not further increase T286D at inhibitory synapses (NMDA: 180 ± 3%, n = 4; *P < 0.05) (Fig. 3 A and B). These results suggest that kinase activity and T286 phosphorylation play an essential role in targeting CaMKIIα to inhibitory synapses.
Fig. 3.
NMDA-induced targeting to inhibitory synapses is dependent on the CaMKIIα activation state. (A) Representative dendrites of control and NMDA-treated neurons transfected with mutant CaMKIIα constructs (red) and immunolabeled for gephyrin (green), and PSD-95 (blue). Arrows indicate synaptically localized mGFP-CaMKIIα puncta. (Scale bar, 5 μm.) (B and C) NMDA effects on the proportion of inhibitory synapses with colocalized mutant mGFP-CaMKIIα puncta are presented as percentage of WT control values. *P < 0.05; **P < 0.01; ***P < 0.001.
We next characterized additional CaMKIIα mutants: F98K and E139K located in the S-site, and I205K and W237K residing in the T286-interacting domain (T-site). Each disrupts CaMKIIα translocation to excitatory synapses by reducing its binding to NMDARs (25); we also found that all four were defective in glutamate-induced translocation to excitatory synapses (Fig. S4). In response to NMDA, W237K localization increased at inhibitory synapses similar to WT (W237K control: 97 ± 6%; NMDA: 125 ± 9% of WT control, n = 5; *P < 0.05) (Fig. 3 A and B). However, F98K, E139K, and I205K all showed enhanced basal targeting to inhibitory synapses with no further change in response to NMDA (F98K:control: 143 ± 25%, NMDA: 136 ± 1%; E139K: control: 166 ± 35%, NMDA: 177 ± 13%; I205K:control: 128 ± 24%, NMDA: 139 ± 8%; n = 5; *P < 0.05; **P < 0.01) (Fig. 3 A and B). Like WT-CaMKIIα, none of the CaMKIIα mutants tested displayed significant redistribution to excitatory synapses following NMDA (Fig. S4).
T286 Phosphorylation and CaMKIIα Targeting.
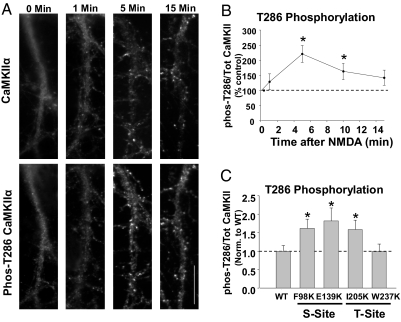
The T286D mutant suggests that phosphorylation of T286 is sufficient to localize CaMKIIα at inhibitory synapses. To test whether T286 is phosphorylated on the endogenous kinase under conditions that localize it to inhibitory sites, we treated neurons with NMDA, fixed at various time-points, and labeled with antibodies against CaMKIIα and phospho-T286-CaMKIIα. The ratio of immunofluorescence labeling for p-T286-CaMKIIα to total CaMKIIα revealed that NMDA enhanced T286 phosphorylation over time with the peak occurring ≈5 min after treatment (Fig. 4 A and B), coinciding with the peak of mGFP-CaMKIIα puncta formation (Fig. 1C). In contrast, glutamate caused a significant increase in p-T286 at just 1 min after treatment (159 ± 14% of control, n = 6; *P < 0.05).
Fig. 4.
Phosphorylation of Thr286 is required for CaMKIIα targeting to inhibitory synapses. (A) Neurons treated with NMDA, were rapidly fixed at several time points and labeled with antibodies to CaMKIIα and phospho-T286-CaMKIIα. (Scale bar, 10 μm.) (B) Time-course of T286 phosphorylation, plotted as the ratio of p-T286 to total CaMKIIα labeling and normalized to the time 0 value. *P < 0.05. (C) Phosphorylation of T286 in neurons transfected with mutant CaMKIIα constructs was determined by labeling cells with p-T286 and CaMKIIα antibodies. The ratio of p-T286 to total CaMKIIα is shown normalized to WT levels. *P < 0.05.
We next examined whether S- and T-site mutants constitutively localized at inhibitory synapses exhibit basally elevated T286 phosphorylation. Cells expressing F98K, E139K, and I205K showed enhanced p-T286 in unstimulated neurons relative to WT-transfected neurons (F98K: 1.6 ± 0.3-fold WT levels; E139K: 1.8 ± 0.4; I205K: 1.6 ± 0.3, n > 15 cells in each condition;*P < 0.05) (Fig. 4C). W237K, which is not constitutively found at inhibitory sites, did not have increased phosphorylation of T286 (1.0 ± 0.2, n = 25 cells, P = 0.99) (Fig. 4C), indicating that this mutation does not induce CaMKIIα activation in our experimental conditions.
To establish whether autophosphorylation of the mutant kinases accounts for their constitutive targeting to inhibitory synapses, we generated a double mutant with the S-site E139K and the T286A autophosphorylation mutations. This double mutant showed no increase in basal or NMDA-elicited colocalization with gephyrin puncta (WT NMDA: 140 ± 12% of WT control; *P < 0.05; E139K/T286A control 108 ± 6%, E139K/T286A NMDA: 109 ± 4; n = 6) (Fig. S5). Thus, phosphorylation of T286 is critical for the CaMKIIα targeting to inhibitory synapses.
Stimuli That Increase CaMKIIα at Inhibitory Synapses Enhance Surface GABAAR Expression.
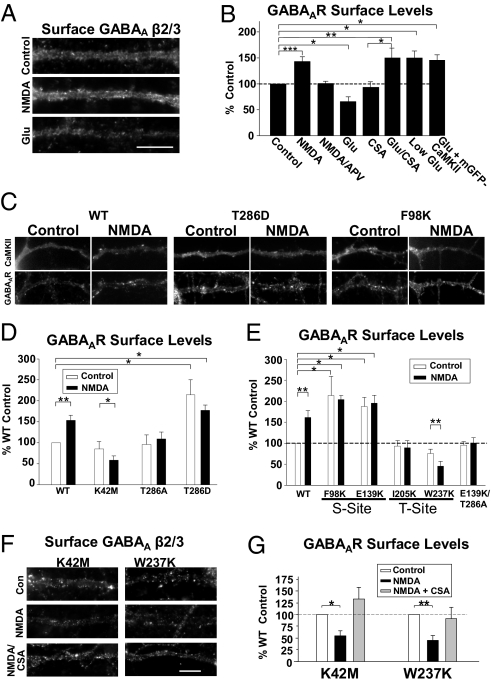
Synapse-specific CaMKIIα targeting likely has important effects on the surface expression of GABAARs and AMPARs. We labeled treated and untreated neurons with an antibody to extracellular epitopes on the AMPAR GluR2 or GABAAR β2/3 subunits under nonpermeabilizing conditions to detect surface receptors. In NMDA-treated neurons surface AMPARs decreased, but surface GABAARs increased, likely through receptor insertion, as we reported previously (12) (GluR2: 62 ± 7% of control; GABAA β2/3: 143 ± 9%, n = 10; ***P < 0.001) (Fig. 5 A and B and Fig. S6). APV eliminated the NMDA-induced increase in GABAARs (101 ± 4%, n = 3) (Fig. 5B). Glutamate had the opposite effect of NMDA: surface AMPARs increased and surface GABAARs decreased (GluR2: 150 ± 16%; GABAA β2/3: 65 ± 9%, n = 4; *P < 0.05) (Fig. 5 A and B and Fig. S6). Therefore, these two NMDAR-activating stimuli oppositely induce changes in inhibitory and excitatory receptor expression. More significantly, conditions that increase CaMKIIα at each type of synapse are associated with increased surface levels of receptors at those sites. Furthermore, in the presence of the CaN inhibitor CSA, or with mGFP-CaMKIIα overexpression, glutamate produced an increase in surface GABAARs (CSA alone 91 ± 13% of control; glu/CSA: 146 ± 18%, n = 6; **P < 0.01; glu + mGFP-CaMKIIα: 146 ± 11%, n = 5; *P < 0.05) (Fig. 5B) in parallel with the targeting of CaMKIIα to inhibitory synapses under these conditions (Figs. 1D and 2B). Furthermore, 10 μM glutamate (low glu), which increases CaMKIIα at inhibitory synapses (Fig. 2B) and decreases surface AMPARs (26), enhanced surface GABAARs (150 ± 13%, n = 5; *P < 0.05) (Fig. 5B). Therefore, all conditions that increased clustering of CaMKIIα at inhibitory synapses also enhanced surface GABAAR levels.
Fig. 5.
Stimuli that target CaMKIIα to inhibitory synapses increase surface GABAAR expression. (A) Neurons treated with NMDA or glutamate for 1 min were labeled for surface-expressed GABAAR β2/3 subunits. (Scale bar, 10 μm.) (B) Changes in surface receptor labeling intensity are shown as a percentage of untreated control levels. *P < 0.05; **P < 0.01; ***P < 0.001. (C) Neurons transfected with WT or mutant mGFP-CaMKIIα were treated with NMDA and labeled for surface GABAAR β2/3 subunits. Representative images show dendritic mGFP-CaMKIIα and GABAAR staining. (D and E) Quantification of dendritic GABAAR β2/3 surface staining of WT and mutant mGFP-CaMKIIα–transfected neurons. Changes in fluorescence values are presented as the percent of WT control neurons. *P < 0.05; **P < 0.01. (F) NMDA induced a significant decrease in surface GABAAR levels in K42M- and W237K-transfected neurons that is blocked by the CaN inhibitor CSA. Representative dendrites show surface GABAAR labeling. (Scale bar, 10 μm.) (G) Surface GABAAR expression is presented as the percent of control levels for each mutant. *P < 0.05; **P < 0.01.
CaMKIIα Kinase Activity and T-Site Required for GABAAR Insertion.
WT-CaMKIIα–transfected cells also showed an increase in GABAAR surface expression following NMDA application (153 ± 12% of control; n = 18; **P < 0.01) (Fig. 5 C and D) equivalent to nontransfected cells (Fig. 5 A and B). In contrast, GABAAR expression was reduced in K42M-expressing cells after NMDA application (control: 85 ± 18%, P = 0.3; NMDA: 58 ± 10%, n = 10; *P < 0.05) (Fig. 5D).
The T286A mutant prevented NMDA-induced potentiation of GABAAR expression (control: 95 ± 22%; NMDA: 108 ± 16%, n = 5, P = 0.5) (Fig. 5 C and D). In contrast, the T286D, F98K, and E139K mutants all constitutively localized at inhibitory synapses and increased the basal levels of surface GABAARs with no further increase observed following NMDA (T286D:control: 212 ± 40%, NMDA: 181 ± 10%, n = 6; *P < 0.05; F98K:control: 213 ± 45%, NMDA: 203 ± 10%; E139K:control: 187 ± 21%, NMDA: 195 ± 18%; n = 4; *P < 0.05) (Fig. 5 C–E). The E139K/T286A double mutant, in agreement with its inability to localize to inhibitory synapses, did not cause any change in surface GABAARs with or without NMDA stimulation (control: 95 ± 8%; NMDA: 100 ± 12%, n = 6) (Fig. 5E).
The T-site mutants functioned differently. Cells expressing I205K, whose localization at inhibitory synapses is constitutively elevated, did not exhibit enhanced GABAAR levels with or without NMDA treatment (control: 91 ± 13%; NMDA: 99 ± 13%; n = 4) (Fig. 5E). W237K-expressing cells exhibited an NMDA-induced decrease in surface GABAARs, despite this kinase's ability to target to inhibitory synapses following NMDA (control: 75 ± 10%, P = 0.6; NMDA: 45 ± 11%, n = 9; **P < 0.01) (Fig. 5 C and E). Thus, NMDAR-dependent trafficking of GABAARs to inhibitory synapses depends upon a unique role of the CaMKIIα T-site.
Cells expressing the K42M or W237K constructs displayed a surprising decrease in receptor levels in response to NMDA. We tested whether CaN activity mediates this reduction of GABAARs as with glutamate stimulation (Fig. 5 A and B). Consistent with this possibility, the NMDA-mediated decrease in surface GABAARs was blocked by CSA (K42M NMDA + CSA: 132 ± 25% of control, n = 4; W237K NMDA + CSA: 91 ± 23%, n = 4) (Fig. 5 F and G). Therefore, in response to moderate NMDAR activation, CaN and CaMKIIα are both active at inhibitory synapses, but CaMKIIα-mediated GABAAR insertion normally dominates.
Discussion
We find that CaMKIIα can selectively translocate to inhibitory, as well as excitatory synapses, with distinct stimuli driving redistribution of the kinase to excitatory or inhibitory synapses. This previously unknown ability of CaMKIIα to localize at inhibitory synapses appears to be a key step in CaMKIIα-dependent GABAergic potentiation, as the process is necessary and sufficient to trigger events that increase surface GABAAR levels. The synapse-selective translocation of CaMKIIα provides a mechanism by which activity can couple to the potentiation of inhibitory synapses without producing CaMKIIα-dependent LTP at excitatory synapses.
The strength of Ca2+-elevating stimuli plays a key part in determining the local targeting of CaMKIIα. Similar signaling differences have been associated with the induction of excitatory LTP and long-term depression, and are generally attributed to the differential activation of the Ca2+-dependent effectors CaMKIIα and CaN (27). Our data shows that the balance of activation of these two regulators contributes to the modulation of inhibitory synapses. Although CaMKIIα dominates in response to more moderate stimuli and CaN is more effective with stronger stimuli, each effector is active under both conditions. CaN inhibition reveals CaMKIIα-mediated GABAAR insertion at inhibitory synapses during strong stimulation (Fig. 5B), and kinase inactivation by K42M- CaMKIIα expression reveals CaN-dependent GABAAR removal during moderate stimulation (Fig. 5 F and G). Furthermore, the interaction of these two effectors contributes to the specificity of CaMKIIα targeting to inhibitory synapses. With moderate stimuli, sufficient CaN is activated to depress excitatory synapses, particularly as CaMKIIα does not translocate to spines under these conditions. In the shafts, levels of activated CaMKIIα, concentrated at inhibitory synapses by translocation, are high enough to outcompete the actions of CaN. With stronger stimuli, however, CaN appears to be activated in shafts to a level enabling it to dominate over CaMKIIα, in part by preventing translocation of the kinase. The ability of the phosphatase to bind to GABAARs (23) and localize at inhibitory synapses with the strong stimulus (Fig. S7) may enhance this competition, perhaps by competing for binding sites. Further studies are necessary to more closely examine the activation levels of CaN and CaMKIIα in spines versus shafts to better understand the functional interaction between the two.
CaMKIIα relies on distinct targeting mechanisms at excitatory and inhibitory synapses. For example, the Ca2+-independent T286D mutant constitutively localizes at inhibitory but not excitatory synapses [Fig. 3 and Fig. S4 (17)], suggesting an additional event is required to enable targeting to glutamatergic synapses. Furthermore, mutations in the S- and T-sites reduce the targeting of CaMKIIα to excitatory synapses by interfering with kinase binding to NR2B (25). However, three of these mutants, F98K, E139K, and I205K, exhibited enhanced localization at inhibitory synapses under control conditions, establishing that these sites do not serve as binding domains at inhibitory synapses. This constitutive targeting is consistent with their elevated levels of T286 phosphorylation (Fig. 4). One mutant that did not basally localize at inhibitory synapses, W237K, did not have increased T286 phosphorylation and only targeted to synapses following NMDA. These data suggest, and our analysis of the E139K/T286A double mutant confirms, that phosphorylation of T286 is necessary and sufficient for translocation to inhibitory synapses.
The functional importance of CaMKIIα translocation to inhibitory synapses is illustrated by the profound effects the expression of CaMKIIα mutants had on GABAAR trafficking (Fig. 5). The S-site mutants, F98K and E139K, both autophosphorylated and present at inhibitory synapses under basal conditions, elevated baseline GABAAR surface levels. This finding suggests that localization of the active kinase at the synapse is sufficient to initiate events that enhance surface GABAAR levels. However, the T-site mutant I205K, which is also autophosphorylated and localized at inhibitory synapses, failed to enhance surface GABAAR levels. The other T-site mutant W237K, although capable of translocating to inhibitory synapses with NMDA treatment, did not elevate surface GABAARs. Thus, residues within the T-site, although not involved in interactions necessary for CaMKIIα targeting to inhibitory synapses, do play a role in mediating GABAAR trafficking to the surface, potentially as a binding site for a critical protein interaction.
CaMKIIα translocation to inhibitory synapses is likely to be an important mechanism for controlling inhibitory synaptic strength. Furthermore, the different requirements for translocation to excitatory and inhibitory synapses provide a way for neurons to use this single pathway to potentiate both types of synapses and yet maintain stimulus-dependent specificity in the expression of synaptic plasticity. Although activation of NMDARs by broad pharmacological stimuli differs from synaptic stimulation, the ability of NMDARs, through activation of CaMKIIα and CaN, to simultaneously and oppositely modulate inhibitory and excitatory synapses, identifies a promising mechanism for future studies of heterosynaptic plasticity and the regulation of excitability in the hippocampus.
Materials and Methods
Details are presented in SI Materials and Methods.
Cell Cultures and Treatments.
Dissociated hippocampal cultures were prepared from P0 rat pups, as described previously (12), and plated on 12-mm polylysine-coated coverslips. Experiments were performed at 14 to 21 d in vitro.
Immunocytochemistry.
Cells were treated as described and briefly fixed in 4% PFA. After blocking, cells were incubated with primary Ab for 1 h. Cells were washed in TBS and incubated with fluorescently-conjugated Abs. After washing, coverslips were mounted for imaging.
Cell Transfections and Live Imaging.
Neurons were transfected at 11 to 13 d in vitro with mGFP-CaMKIIα constructs. After 48 h, cells were treated, fixed, and immunolabeled for PSD-95 and gephyrin, for p-T286-CaMKIIα and CaMKIIα, or for surface AMPARs or GABAARs. For live imaging, coverslips were perfused with Hepes-buffered artificial cerebrospinal fluid and imaged every 10 s. For Ca2+ imaging, cells loaded with Fluo-3-AM were imaged every 3 s for 5 min.
Image Acquisition and Analysis.
Images were acquired with a Hamamatsu Orca ER camera on an inverted Nikon fluorescent microscope. Surface receptor analysis was performed as described previously (12). For colocalization analysis, CaMKIIα and synaptic marker images were overlayed and colocalized puncta per unit length were identified and counted by an individual blind to experimental conditions. For analysis of p-T286-CaMKIIα, p-T286 Ab images were background subtracted and divided by subtracted CaMKIIα Ab images. Data are presented as the ratio of p-T286 to total CaMKIIα fluorescence intensity.
Statistics.
Except where specified, n values refer to each experiment performed using an independent culture in which 7 to 12 cells were imaged. Statistical comparisons were made by Student's t test. All values presented are mean ± SEM.
Supplementary Material
Acknowledgments
We thank Jennifer B. Beattie for exceptional technical assistance and Dr. Scott Nawy, Dr. Alberto Pereda, Dr. Diana Pettit, and Tanya Casimiro for helpful input on the manuscript. This work was supported by National Institutes of Health Grants NS 049661 (to R.C.C.) and NS052644 (to K.U.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010346107/-/DCSupplemental.
References
- 1.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 2.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 3.McDonald BJ, Moss SJ. Differential phosphorylation of intracellular domains of gamma-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. J Biol Chem. 1994;269:18111–18117. [PubMed] [Google Scholar]
- 4.McDonald BJ, Moss SJ. Conserved phosphorylation of the intracellular domains of GABA(A) receptor beta2 and beta3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology. 1997;36:1377–1385. doi: 10.1016/s0028-3908(97)00111-1. [DOI] [PubMed] [Google Scholar]
- 5.Houston CM, Lee HH, Hosie AM, Moss SJ, Smart TG. Identification of the sites for CaMK-II-dependent phosphorylation of GABA(A) receptors. J Biol Chem. 2007;282:17855–17865. doi: 10.1074/jbc.M611533200. [DOI] [PubMed] [Google Scholar]
- 6.Churn SB, et al. Calcium/calmodulin-dependent kinase II phosphorylation of the GABAA receptor alpha1 subunit modulates benzodiazepine binding. J Neurochem. 2002;82:1065–1076. doi: 10.1046/j.1471-4159.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang RA, Cheng G, Kolaj M, Randić M. Alpha-subunit of calcium/calmodulin-dependent protein kinase II enhances gamma-aminobutyric acid and inhibitory synaptic responses of rat neurons in vitro. J Neurophysiol. 1995;73:2099–2106. doi: 10.1152/jn.1995.73.5.2099. [DOI] [PubMed] [Google Scholar]
- 8.Aguayo LG, Espinoza F, Kunos G, Satin LS. Effects of intracellular calcium on GABA-A receptors in mouse cortical neurons. Pflug Arch Eur J Phyl. 1998;435:382–387. doi: 10.1007/s004240050527. [DOI] [PubMed] [Google Scholar]
- 9.Kano M, Kano M, Fukunaga K, Konnerth A. Ca(2+)-induced rebound potentiation of gamma-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase II. Proc Natl Acad Sci USA. 1996;93:13351–13356. doi: 10.1073/pnas.93.23.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houston CM, Smart TG. CaMK-II modulation of GABA(A) receptors expressed in HEK293, NG108-15 and rat cerebellar granule neurons. Eur J Neurosci. 2006;24:2504–2514. doi: 10.1111/j.1460-9568.2006.05145.x. [DOI] [PubMed] [Google Scholar]
- 11.Wei J, Zhang M, Zhu Y, Wang JH. Ca(2+)-calmodulin signalling pathway up-regulates GABA synaptic transmission through cytoskeleton-mediated mechanisms. Neuroscience. 2004;127:637–647. doi: 10.1016/j.neuroscience.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- 14.Otmakhov N, et al. Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. J Neurosci. 2004;24:9324–9331. doi: 10.1523/JNEUROSCI.2350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strack S, Choi S, Lovinger DM, Colbran RJ. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J Biol Chem. 1997;272:13467–13470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- 16.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 17.Thalhammer A, et al. CaMKII translocation requires local NMDA receptor-mediated Ca2+ signaling. EMBO J. 2006;25:5873–5883. doi: 10.1038/sj.emboj.7601420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gleason MR, et al. Translocation of CaM kinase II to synaptic sites in vivo. Nat Neurosci. 2003;6:217–218. doi: 10.1038/nn1011. [DOI] [PubMed] [Google Scholar]
- 19.Beattie EC, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 20.Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 21.Rose J, Jin SX, Craig AM. Heterosynaptic molecular dynamics: Locally induced propagating synaptic accumulation of CaM kinase II. Neuron. 2009;61:351–358. doi: 10.1016/j.neuron.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, et al. Interaction of calcineurin and type-A GABA receptor gamma 2 subunits produces long-term depression at CA1 inhibitory synapses. J Neurosci. 2003;23:826–836. doi: 10.1523/JNEUROSCI.23-03-00826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colbran RJ. Regulation and role of brain calcium/calmodulin-dependent protein kinase II. Neurochem Int. 1992;21:469–497. doi: 10.1016/0197-0186(92)90080-b. [DOI] [PubMed] [Google Scholar]
- 25.Bayer KU, et al. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J Neurosci. 2006;26:1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll RC, et al. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisman JE. Three Ca2+ levels affect plasticity differently: The LTP zone, the LTD zone and no man's land. J Physiol. 2001;532:285. doi: 10.1111/j.1469-7793.2001.0285f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.