Abstract
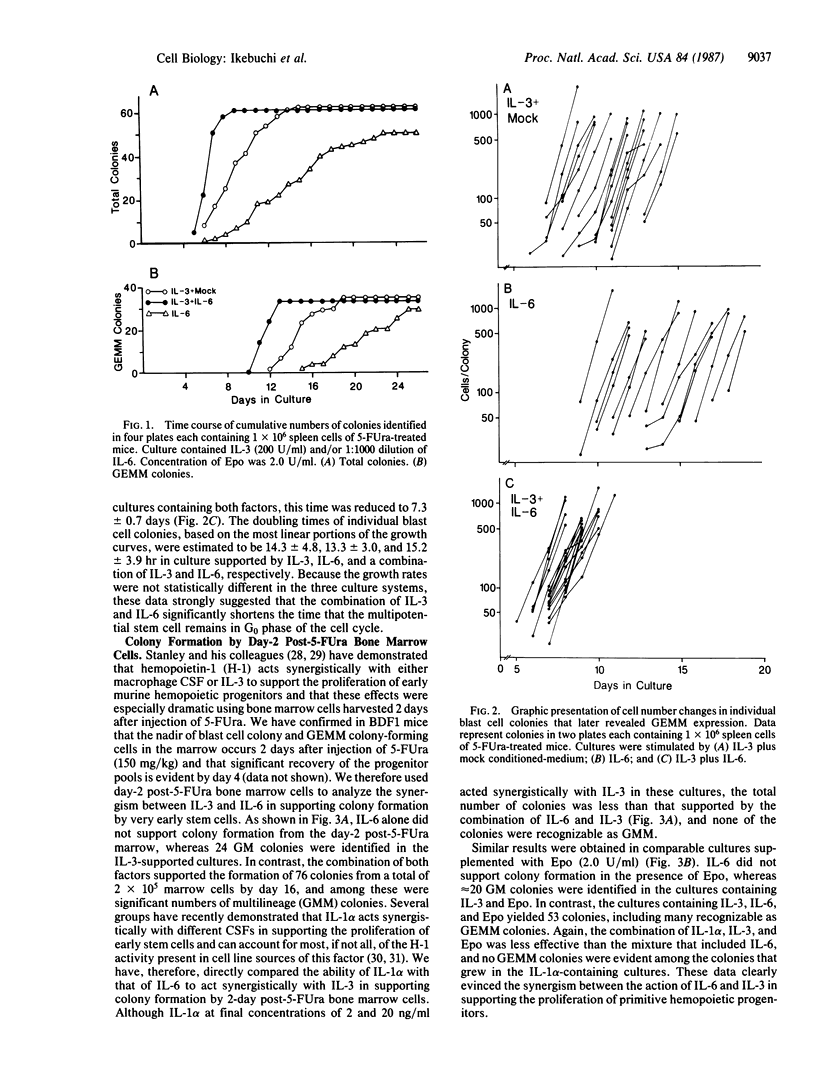
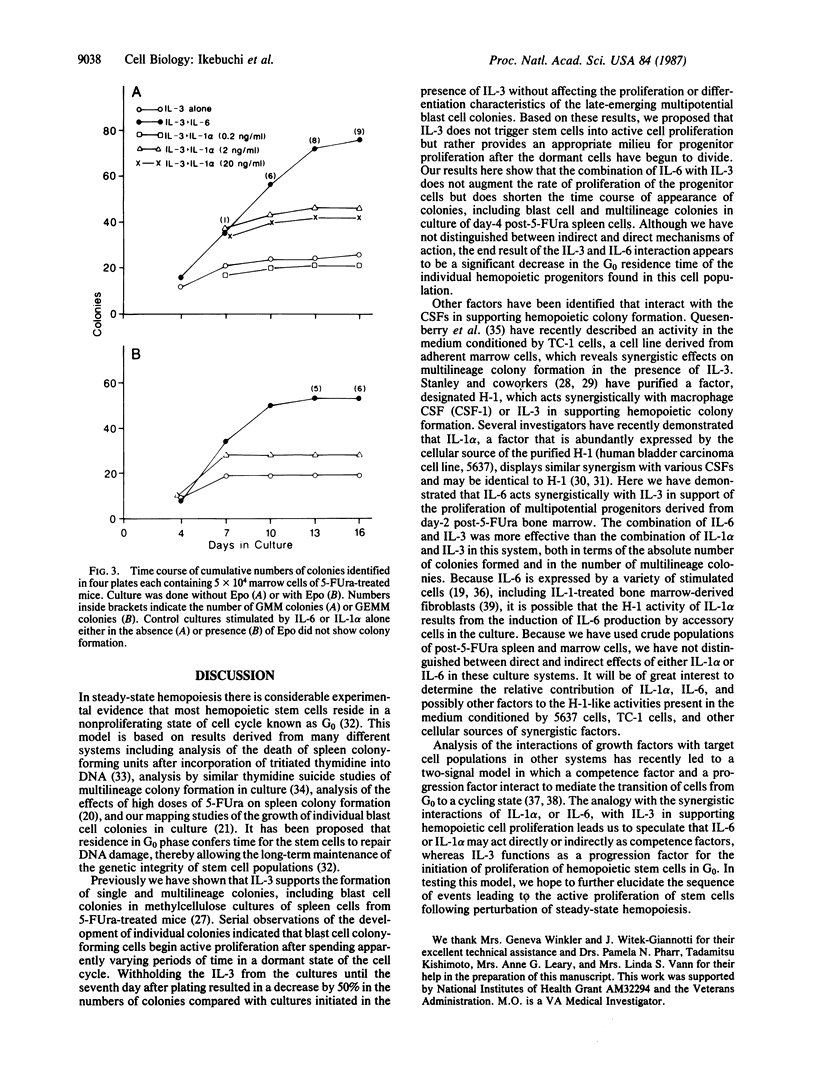
Interleukin-6 (IL-6, also known as B-cell stimulatory factor 2/interferon beta 2) was previously shown to support the proliferation of granulocyte/macrophage progenitors and indirectly support the formation of multilineage and blast cell colonies in cultures of spleen cells from normal mice. We report here that IL-3 and IL-6 act synergistically in support of the proliferation of murine multipotential progenitors in culture. The time course of total colony formation by spleen cells isolated from mice 4 days after injection of 5-fluorouracil (150 mg/kg) was significantly shortened in cultures containing both lymphokines relative to cultures supported by either of the two factors. Serial observations (mapping) of individual blast cell colonies in culture revealed that blast cell colonies emerged after random time intervals in the presence of IL-3. The average time of appearance in IL-6 alone was somewhat delayed, and in cultures containing both factors the appearance of multilineage blast cell colonies was significantly hastened relative to cultures grown in the presence of the individual lymphokines. In cultures of day-2 post-5-fluorouracil bone marrow cells, IL-6 failed to support colony formation; IL-3 alone supported the formation of a few granulocyte/macrophage colonies, but the combination of factors acted synergistically to yield multilineage and a variety of other types of colonies. In this system, IL-1 alpha also acted synergistically with IL-3, but the effect was smaller, and no multilineage colonies were seen. Together these results indicate that IL-3 and IL-6 act synergistically to support the proliferation of hemopoietic progenitors and that at least part of the effect results from a decrease in the G0 period of the individual stem cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER A. J., MCCULLOCH E. A., SIMINOVITCH L., TILL J. E. THE EFFECT OF DIFFERING DEMANDS FOR BLOOD CELL PRODUCTION ON DNA SYNTHESIS BY HEMOPOIETIC COLONY-FORMING CELLS OF MICE. Blood. 1965 Sep;26:296–308. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Zsebo K. M., Inoue H., Hines D., Boone T. C., Chazin V. R., Tsai L., Ritch T., Souza L. M. In vivo stimulation of granulopoiesis by recombinant human granulocyte colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2484–2488. doi: 10.1073/pnas.84.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue R. E., Wang E. A., Stone D. K., Kamen R., Wong G. G., Sehgal P. K., Nathan D. G., Clark S. C. Stimulation of haematopoiesis in primates by continuous infusion of recombinant human GM-CSF. 1986 Jun 26-Jul 2Nature. 321(6073):872–875. doi: 10.1038/321872a0. [DOI] [PubMed] [Google Scholar]
- Eschbach J. W., Egrie J. C., Downing M. R., Browne J. K., Adamson J. W. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987 Jan 8;316(2):73–78. doi: 10.1056/NEJM198701083160203. [DOI] [PubMed] [Google Scholar]
- Goldwasser E. Erythropoietin and the differentiation of red blood cells. Fed Proc. 1975 Dec;34(13):2285–2292. [PubMed] [Google Scholar]
- Groopman J. E., Mitsuyasu R. T., DeLeo M. J., Oette D. H., Golde D. W. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on myelopoiesis in the acquired immunodeficiency syndrome. N Engl J Med. 1987 Sep 3;317(10):593–598. doi: 10.1056/NEJM198709033171003. [DOI] [PubMed] [Google Scholar]
- Haegeman G., Content J., Volckaert G., Derynck R., Tavernier J., Fiers W. Structural analysis of the sequence coding for an inducible 26-kDa protein in human fibroblasts. Eur J Biochem. 1986 Sep 15;159(3):625–632. doi: 10.1111/j.1432-1033.1986.tb09931.x. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y., Kanakura Y., Fujita J., Takeda S., Nakano T., Tarui S., Honjo T., Kitamura Y. Interleukin 4 as an essential factor for in vitro clonal growth of murine connective tissue-type mast cells. J Exp Med. 1987 Jan 1;165(1):268–273. doi: 10.1084/jem.165.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Ogawa M. Murine hemopoietic colonies in culture containing normoblasts, macrophages, and megakaryocytes. Am J Hematol. 1978;4(1):23–34. doi: 10.1002/ajh.2830040105. [DOI] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Jubinsky P. T., Stanley E. R. Purification of hemopoietin 1: a multilineage hemopoietic growth factor. Proc Natl Acad Sci U S A. 1985 May;82(9):2764–2768. doi: 10.1073/pnas.82.9.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi T., Harada N., Severinson E., Tanabe T., Sideras P., Konishi M., Azuma C., Tominaga A., Bergstedt-Lindqvist S., Takahashi M. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature. 1986 Nov 6;324(6092):70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Koike K., Ogawa M., Ihle J. N., Miyake T., Shimizu T., Miyajima A., Yokota T., Arai K. Recombinant murine granulocyte-macrophage (GM) colony-stimulating factor supports formation of GM and multipotential blast cell colonies in culture: comparison with the effects of interleukin-3. J Cell Physiol. 1987 Jun;131(3):458–464. doi: 10.1002/jcp.1041310319. [DOI] [PubMed] [Google Scholar]
- Lajtha L. G. Stem cell concepts. Differentiation. 1979;14(1-2):23–34. doi: 10.1111/j.1432-0436.1979.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R., Burgess A. W. Direct stimulation by purified GM-CSF of the proliferation of multipotential and erythroid precursor cells. Blood. 1980 Jan;55(1):138–147. [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Mochizuki D. Y., Eisenman J. R., Conlon P. J., Larsen A. D., Tushinski R. J. Interleukin 1 regulates hematopoietic activity, a role previously ascribed to hemopoietin 1. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5267–5271. doi: 10.1073/pnas.84.15.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. A., Warren D. J. Synergy of interleukin 1 and granulocyte colony-stimulating factor: in vivo stimulation of stem-cell recovery and hematopoietic regeneration following 5-fluorouracil treatment of mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7134–7138. doi: 10.1073/pnas.84.20.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Clonal origin of murine hemopoietic colonies with apparent restriction to granuclocyte-macrophage-megakaryocyte (GMM) differentiation. J Cell Physiol. 1982 Jun;111(3):239–246. doi: 10.1002/jcp.1041110304. [DOI] [PubMed] [Google Scholar]
- Nakahata T., Ogawa M. Identification in culture of a class of hemopoietic colony-forming units with extensive capability to self-renew and generate multipotential hemopoietic colonies. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3843–3847. doi: 10.1073/pnas.79.12.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Quesenberry P., Song Z. X., McGrath E., McNiece I., Shadduck R., Waheed A., Baber G., Kleeman E., Kaiser D. Multilineage synergistic activity produced by a murine adherent marrow cell line. Blood. 1987 Mar;69(3):827–835. [PubMed] [Google Scholar]
- Sanderson C. J., O'Garra A., Warren D. J., Klaus G. G. Eosinophil differentiation factor also has B-cell growth factor activity: proposed name interleukin 4. Proc Natl Acad Sci U S A. 1986 Jan;83(2):437–440. doi: 10.1073/pnas.83.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Bartocci A., Patinkin D., Rosendaal M., Bradley T. R. Regulation of very primitive, multipotent, hemopoietic cells by hemopoietin-1. Cell. 1986 Jun 6;45(5):667–674. doi: 10.1016/0092-8674(86)90781-6. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M., Ihle J. N. Permissive role of interleukin 3 (IL-3) in proliferation and differentiation of multipotential hemopoietic progenitors in culture. J Cell Physiol. 1985 Aug;124(2):182–190. doi: 10.1002/jcp.1041240203. [DOI] [PubMed] [Google Scholar]
- Suda T., Suda J., Ogawa M. Proliferative kinetics and differentiation of murine blast cell colonies in culture: evidence for variable G0 periods and constant doubling rates of early pluripotent hemopoietic progenitors. J Cell Physiol. 1983 Dec;117(3):308–318. doi: 10.1002/jcp.1041170305. [DOI] [PubMed] [Google Scholar]
- Welte K., Bonilla M. A., Gillio A. P., Boone T. C., Potter G. K., Gabrilove J. L., Moore M. A., O'Reilly R. J., Souza L. M. Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J Exp Med. 1987 Apr 1;165(4):941–948. doi: 10.1084/jem.165.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton W. Hormonal regulation of discrete portions of the cell cycle: commitment to DNA synthesis is commitment to cellular division. J Cell Physiol. 1983 Dec;117(3):423–429. doi: 10.1002/jcp.1041170318. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Ruggieri R., Korn J. H., Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986 Oct;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


