Abstract
Age-related skeletal muscle loss is thought to stem from suboptimal nutrition and resistance to anabolic stimuli. Impaired microcirculatory (nutritive) blood flow may contribute to anabolic resistance by reducing delivery of amino acids to skeletal muscle. In this study, we employed contrast-enhanced ultrasound, microdialysis sampling of skeletal muscle interstitium, and stable isotope methodology, to assess hemodynamic and metabolic responses of older individuals to endurance type (walking) exercise during controlled amino acid provision. We hypothesized that older individuals would exhibit reduced microcirculatory blood flow, interstitial amino acid concentrations, and amino acid transport when compared with younger controls. We report for the first time that aging induces anabolic resistance following endurance exercise, manifested as reduced (by ∼40%) efficiency of muscle protein synthesis. Despite lower (by ∼40–45%) microcirculatory flow in the older than in the younger participants, circulating and interstitial amino acid concentrations and phenylalanine transport into skeletal muscle were all equal or higher in older individuals than in the young, comprehensively refuting our hypothesis that amino acid availability limits postexercise anabolism in older individuals. Our data point to alternative mediators of age-related anabolic resistance and importantly suggest correction of these impairments may reduce requirements for, and increase the efficacy of, dietary protein in older individuals. Durham, W. J., Casperson, S. L., Dillon, E. L., Keske, M. A., Paddon-Jones, D., Sanford, A. P., Hickner, R. C., Grady, J. J., Sheffield-Moore, M. Age-related anabolic resistance after endurance-type exercise in healthy humans.
Keywords: amino acids, blood flow, microdialysis ethanol, contrast-enhanced ultrasound, microcirculation
Most individuals experience a gradual loss of muscle mass as they age, a process termed sarcopenia. When sarcopenic losses reduce skeletal muscle mass below a critical threshold, activities of daily living are compromised and disability ensues (1). Although multiple etiologic factors have been associated with sarcopenia (1–3), the nature of sarcopenic progression is unclear. In particular, it is uncertain whether there is an early stage of resistance to anabolic stimuli that precedes frank muscle loss. Although changes in basal muscle protein metabolism have been reported to occur in some (4–8) but not all (9–13) studies of otherwise healthy adults, aberrant responses to anabolic stimuli are thought to play an important role (14). Accordingly, evidence is accumulating that skeletal muscle of older individuals exhibits resistance to anabolic stimuli such as amino acids, insulin, and resistance exercise (15, 16).
Before circulating amino acids can be used for skeletal muscle protein synthesis, they must first leave skeletal muscle capillaries, traverse the interstitial fluid, and be transported into muscle fibers. Previous studies suggest that the transit of amino acids from the blood to the interstitial space occurs primarily via diffusion and is rate-limiting for the net uptake of circulating amino acids by muscle fibers (17–19). One mediator of this uptake by skeletal muscle may be the relative distribution of blood flow between routes of optimal nutrient transfer (termed “nutritive” flow routes) and suboptimal nutrient transfer (termed “non-nutritive” routes) (20, 21). Thus, reduced nutritive flow is one potential contributor to the blunted anabolic responsiveness of older individuals to anabolic stimuli. However, there is evidence that other factors are likely involved as well. Previous studies suggest that the capacity for translation by ribosomes and their associated factors is reduced by aging (22, 23). In addition, aging has been reported to decrease skeletal muscle capillarization and increase capillary basement membrane width (24), with unknown effects on transcapillary efflux of amino acids into the interstitial fluid.
To our knowledge, no previous studies have determined whether the generalized phenomenon of age-related amino acid resistance includes the period following endurance-type (“aerobic”) exercise. Although such exercise stimulates skeletal muscle protein synthesis both acutely (25, 26) and chronically (27, 28) in the fasted state, it is not associated with hypertrophy (28), in contrast to resistance-type exercise. Divergent responses to resistance and endurance exercise may result from differences in energetic signaling responses (29–31), including intensity-dependent adaptive responses, such as mitochondrial biogenesis (32, 33). Such responses may interfere with stimulation of muscle protein synthesis by exogenous amino acids and/or increased muscle catabolism during postabsorptive conditions (27). Consistent with this notion, acute moderate-intensity (75% VO2 peak) endurance-type cycling exercise (34) blunted the anabolic response to constant amino acid administration observed in healthy young subjects at rest (35, 36). In contrast, repetitive knee extension exercise (67% maximal work rate) for 1 hour did not interfere with the anabolic response to chronic amino acid administration (37), suggesting anabolic resistance induced by endurance exercise is specific to the type of exercise performed. However, whether lower-intensity endurance exercise, which provides less energetic stress and less stimulation of mitochondrial adaptive responses, blunts amino acid-induced anabolic responses is unknown. Further, whether such blunting occurs in older subjects in response to walking, the mode of endurance exercise most commonly engaged in by older individuals, has not been studied.
Accordingly, in the current study we examined the response of muscle protein metabolism to endurance exercise in younger and older subjects during provision of amino acids. We also measured amino acid concentrations in the blood and interstitial fluid, determined phenylalanine transport rates in skeletal muscle, and utilized both contrast-enhanced ultrasound and the microdialysis ethanol technique, two independent methods for assessing skeletal muscle nutritive flow (38–46). We studied younger and older subjects who were free of disease and not taking medications in an attempt to study the effects of aging per se and reduce the potential independent effects of disease and pharmacological interventions on metabolic responses, as well as to complement investigations of older individuals who were less healthy or already exhibited markedly reduced muscle mass. We hypothesized that older subjects, as compared with the young, would be resistant to the anabolic effects of amino acids and that this resistance would be associated with reductions in postexercise nutritive blood flow, interstitial amino acid concentrations, and amino acid transport from the blood into skeletal muscle. Although we did find evidence for an age-related deficit in nutritive blood flow, our data comprehensively refute our hypothesis that impaired nutritive flow limits amino acid availability in healthy older individuals, as circulating and interstitial amino acid concentrations and phenylalanine transport into skeletal muscle were all equal or higher in older individuals than in the young. Instead, our data direct attention to metabolic and oxidative stress, altered skeletal muscle membrane integrity, and hemodynamic mechanisms apart from amino acid availability as potential mediators of age-related anabolic resistance following an acute bout of endurance-type exercise.
MATERIALS AND METHODS
Subjects
Seventeen healthy untrained men, 8 older [O; 67±1.6 (mean±se) yr] and 9 younger (Y; 29.8±1.7 yr), were studied before and after a single bout of low- to moderate-intensity endurance exercise (walking) during continuous infusion of amino acids. Informed written consent, which was approved by the Institutional Review Board of the University of Texas Medical Branch (UTMB), was obtained from all volunteers prior to any study-related procedures. Volunteers were screened at the UTMB General Clinical Research Center (GCRC) to determine study eligibility. Exclusion criteria included the following: cardiac, liver, kidney, pulmonary, autoimmune or vascular disease; hypocoagulation or hypercoagulation disorders, diabetes, cancer, obesity, anemia, infectious diseases, or an allergy to iodides. Subjects taking antihypertensive or lipid-lowering medications, anabolic steroids, or corticosteroids in the past 6 mo were excluded, as were subjects unable to discontinue anti-inflammatory or prophylactic aspirin therapy or nutritional supplement use (for 14 d prior to their study date) or those engaged in regular aerobic or resistance exercise training. All older subjects had their ankle-brachial index (ABI) determined to screen for peripheral arterial disease of the legs. Subjects were instructed to continue all regular activities of daily living and maintain their usual diet during the week preceding the study.
Prestudy testing
Subjects were admitted as outpatients to the GCRC 2 wk prior to conducting the metabolism study. Total body fat, leg lean mass, and leg fat mass were determined by dual-energy X-ray absorptometry (DEXA; Hologic, Inc., Natick, MA, USA). Following the DEXA, subjects were escorted to the UTMB heart station for determination of Vo2peak. Vo2peak was measured on a treadmill using expired gas analysis (SensorMedics, Yorba Linda, CA, USA) during a medically supervised progressive walk/run exercise test, as described previously (26).
Experimental protocol
The experimental protocol is outlined in Fig. 1. Subjects reported to the GCRC at noon the day before the study. Subjects were fed a standardized, meat-containing mixed meal the evening before the study and fasted from 10 PM until amino acids were initiated in the immediate preexercise (rest) period the following day. The morning of the study, polyethylene catheters were inserted into the antecubital vein of both arms for infusion of stable isotopes, amino acids, Definity microbubbles (for contrast ultrasound; Bristol-Myers Squibb, New York, NY, USA), and arterialized venous blood sampling. Polyethylene catheters were also inserted into the femoral artery and vein of one leg for arterial and venous blood sampling. Indocyanine green (ICG; Akorn, Buffalo Grove, IL) was infused into the femoral artery for measurement of leg plasma flow, as described previously (47, 48).
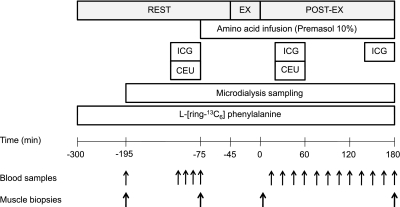
Figure 1.
Study timeline. ICG, indocyanine green sampling; EX, exercise at ∼40% VO2peak; POST-EX, postexercise; CEU, contrast-enhanced ultrasound.
Baseline blood samples were drawn ∼150 min prior to exercise (time=−195 min in Fig. 1) for the analysis of background isotopic enrichment and ICG concentration. Thereafter, blood samples were taken at t = −120, −105, −90, and −75 min for the analysis of isotopic enrichment, ICG concentration, insulin concentration, and glucose concentration. In addition, the t = −75-min sample was also analyzed for amino acid concentrations (see below). Following exercise, blood samples were obtained at 15, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, and 180 min for measurements of isotopic enrichment, ICG concentration, insulin concentration, and glucose concentration. In addition, the 60, 120, and 180 min postexercise samples were analyzed for amino acid concentrations, as described below.
A primed (2 μmol·kg−1) continuous infusion of l-[ring-13C6] phenylalanine (Phe) (0.08 μmol·kg−1·min−1) was started (∼time=−300) and continued uninterrupted until study conclusion (Fig. 1). In the final 30 min of the rest period, an infusion of amino acids (Premasol 10%; Baxter, Deerfield, IL) was started (prime=0.45 ml·kg−1; IR=1.35 ml·kg−1·h−1) and continued throughout the remainder of the study. After a 30-min stabilization period, subjects performed treadmill exercise (walking) for 45 min at ∼ 40% Vo2peak. Following exercise, subjects were immediately returned to bed for the 180-min postexercise period.
Muscle biopsies (∼100–200 mg) were taken 150 and 30 min before exercise (t = −195 and −75 min in Fig. 1) and at 10 and 180 min postexercise from the vastus lateralis muscle, ∼20 cm above the knee, as described previously (49, 50). All tissue was snap-frozen in liquid nitrogen and kept frozen at −80°C for later analysis. At the end of the study, all infusions were stopped, catheters were removed, and the subjects were fed, monitored for 2 h, and discharged home with follow-up care instructions.
Leg blood flow
Leg plasma flow was determined utilizing the ICG dye-dilution technique and converted to leg blood flow using hematocrit as described previously (47, 48).
Contrast-enhanced ultrasound
Imaging of the vastus lateralis muscle was performed in a transaxial plane ∼15–20 cm above the patella over the midportion of the muscle using a P4–2 phased array transducer interfaced with the HDI-5000 ultrasound system (Philips ATL Ultrasound, Andover, MA, USA). Power Doppler imaging was performed as described by others (51–53). In summary, an intravenous infusion (3.5 ml·min−1 for 8 min) of a suspension of perflutren microbubbles (Definity) was performed at ∼t = −85 min at rest and again at ∼50 min postexercise. Contrast-enhanced ultrasound was only performed 2 times because the FDA limits the amount of Definity that can be infused per day. A mechanical index of 1.3 was used and a compression of 80%. Once the systemic microbubble concentrations reached steady-state (∼2 min), background images were obtained at a frame rate of 1 s−1. Intermittent imaging was then performed using an internal timer at pulsing intervals (PI) ranging from 1 to 25 s, thus allowing progressively greater replenishment of the ultrasound beam elevation between destructive pulses. Depth, focus, and gain were optimized at the beginning of each study and held constant throughout. Data were recorded on an SVHS tape and digitized for analysis using an offline system. A minimum of 3 images were acquired at each PI. The background-subtracted video intensity (VI) at each PI was measured from a region of interest (ROI) within the vastus lateralis muscle. PI vs. VI data were curve fitted to the function:
where y is the video intensity at a PI of t, A is the plateau video intensity [an index of microvascular blood volume (MBV)], and β is the rate of microvascular refilling [an indicator of microvascular flow velocity (MFV)] (52). The product MBV × MFV is a measure of microvascular blood flow (MBF).
Microdialysis
Three CMA 60 microdialysis probes (30 mm, 20-kDa cutoff; CMA Microdialysis, Solna, Sweden) were inserted percutaneously into the vastus lateralis muscle of one leg with an 18-gauge needle following 1% lidocaine administration ∼20 cm above the patella. Microdialysis probes were perfused at a rate of 5.0 μl · min−1 using a CMA 102 microinfusion pump (CMA Microdialysis) with a solution consisting of Na+ (147 mEq), K+ (4 mEq), Ca2+ (2.3 mM), Cl− (156 mM), and 40 g · L−1 Dextran 70. EtOH (5 mM) was included in the microdialysis perfusion medium to monitor skeletal muscle nutritive blood flow in the area of the microdialysis probe.
Microdialysis probe recovery for phenylalanine and leucine was determined by the internal reference technique (54) by adding 0.108 μCi · ml−1 of d-[3H]phenylalanine and 0.108 μCi · ml−1 of d-[14C]leucine (Amersham Pharmacia Biotech, Piscataway, NJ, USA) to the perfusate. Probe recovery was determined by measuring disintegrations per minute (DPM) of 14C Leu and 3H Phe in the perfusate and dialysate, placing 10 μl in 15 ml of scintillation fluid and counting for 10 min on a LS 6500 multipurpose scintillation counter (Beckman Coulter, Fullerton, CA, USA). In vivo recovery was calculated using the following formula:
As described previously (55), recoveries for phenylalanine and leucine were used for lysine and alanine, respectively. For all other interstitial amino acids, the average of the phenylalanine and leucine recoveries was applied to estimate interstitial concentrations and allow relative comparisons between young and old.
Once inserted, probes were perfused for 45 min to reach equilibrium, after which dialysate samples were collected in 30-min aliquots during the preexercise and postexercise time periods in sealed microvials (Milian, Geneva, Switzerland) that were weighed before and after dialysate collection to determine dialysate volume. The microvials for each 30-min collection were immediately stored at 4°C until an aliquot was removed later in the day for ethanol analysis, with the remaining volume stored at −80°C.
Ethanol concentration in each perfusate and dialysate sample was measured according to the method described by Hickner et al. (56, 57). Briefly, 150 μl of reagent mixture consisting of glycine-hydrazine buffer at pH 8.9 (74 μM Na4P2O7, 22 μM glycine, and 60 μmM hydrazine) and 0.54 μM NAD+ was added to a 96-well plate. Then, 2 μl of sample was added, followed by 20 μl of enzyme (1.7 mg alcohol dehydrogenase in 1 ml ddH2O). Ethanol concentrations were measured in the perfusate and dialysate solutions by fluorometric-enzymatic assay (Fluoraskan II; MTX Labs Systems, Inc., Vienna, VA, USA), with the results expressed as the ethanol outflow/inflow concentration ratio:
where Cout is the concentration of the dialysate and Cin is the concentration of the perfusate. Sample determinations from each time period (rest, 0–60 min postexercise, 61–120 min postexercise, 121–180 min postexercise) were averaged. The ethanol outflow/inflow (O/I) ratio is inversely related to local nutritive blood flow (57).
Analytical methods
Phenylalanine enrichments and concentrations in arterial and venous blood samples were determined after the addition of an internal standard, deproteinization with sulfosalicylic acid, extraction using cation exchange chromatography, and tert-butyldimethylsilyl (t-BDMS) derivatization using gas-chromatography mass-spectrometry (GCMS) in electron impact mode (GC HP 5890, MSD HP 5989; Hewlett Packard, Palo Alto, CA, USA).
Muscle samples were weighed, and the proteins were precipitated with 800 μl of 10% perchloric acid. Tissue homogenization and centrifugation were performed twice, and the supernatant was collected. The remaining pellet was then washed 3 times with 2% perchloric acid, once with 200 proof ethanol and ethyl ether, with centrifugation following each wash. The pellet was then dried overnight at 50°C. The following day, 3 ml of 6 N hydrochloric acid was added and then placed on a heating block at 110°C for 24 h to hydrolyze the proteins in the pellet. The enrichment of free tissue phenylalanine was determined on its t-BDMS derivative using GCMS (58). The enrichment of bound tissue phenylalanine was determined on its t-BDMS derivative using GCMS monitoring the ions 237 and 239 and using the standard curve approach (58).
Plasma and microdialysate amino acid concentrations were determined using a Hitachi L8800 amino acid analyzer (Hitachi, Tokyo, Japan). Plasma was precipitated to remove proteins by mixing equal volumes of 7.5% SSA in 0.02 N HCl containing internal standard with plasma sample. Samples were centrifuged for 15 min at 10,000 g. Supernatants were filtered through a 0.22-μm filter and injected on the Hitachi L8800 for analysis using standard physiological analysis methods, buffers, and reagents as provided by the manufacturer.
For analysis of AMPK and mTOR phosphorylation by immunoblotting, ∼30 mg of muscle biopsy tissue was homogenized in tissue lysis buffer [10 mM HEPES-KOH, pH 7.9; 1.5 mM MgCl26H2O; 10 mM KCl; 2 mM phenylmethylsulfonyl fluoride; 1 mM dithiothreitol; and 1 mM protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) at 1:18 w/v] followed by incubation on ice for 10 min and vortexing, and centrifugation at 16,110 g for 30 s. The supernatant was collected, and protein concentrations were determined via Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA, USA). A total of 80 μg of protein, diluted in sample buffer (NuPAGE LDS Sample Buffer; Invitrogen, Carlsbad, CA, USA) and boiled for 3 min., was loaded per lane and separated by SDS-PAGE (NuPAGE Novex 3–8% tris-acetate gels for mTOR and NuPAGE Novex Bis-Tris Gels for AMPK; Invitrogen) at a constant 150 V for 60 min. A molecular weight ladder and rodent normalization standard were also loaded on each gel. Following SDS-PAGE, proteins were transferred (100 V for 60 min) to polyvinylidene difluoride (PVDF) membranes (Hybond-P; Amersham Biosciences, Piscataway, NJ, USA) and placed in blocking buffer for 1 h. Blots were serially washed and incubated with primary antibody overnight at 4°C with constant agitation. Primary antibodies were purchased from Cell Signaling (Beverly, MA, USA): phospho-mTOR (Ser-2448; 1:1000), phospho-AMPKα (Thr172; 1:1000), total mTOR (1:1000), and total AMPKα (1:1000). The next day, the blots were rinsed and incubated with secondary antibody for 1 h at room temperature with constant agitation. Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from Amersham Biosciences (1:5000). Blots were serially washed and incubated for 5 min with enhanced chemiluminescence reagent (ECL Advanced Western blotting Detection System; Amersham Biosciences). Optical density measurements were obtained with a ChemiDoc XRS imaging system (Bio-Rad). Densitometric analysis was performed by using Quantity One 4.5.2 1-dimensional analysis software (Bio-Rad). Data are expressed as the signal from the phosphorylated kinase divided by the total protein signal for the kinase, normalized to a rodent standard that was run on each gel, in arbitrary units.
Calculations
Skeletal muscle phenylalanine transmembrane transport and intracellular kinetics were studied using established techniques, as described previously (47, 58).
To assess the efficiency of amino acid handling, we calculated the following additional characteristics: the proportion of leg delivery that was transported into the muscle (inward transport efficiency), the proportion of the intracellular rate of appearance (Ra) that was transported out of the muscle to the vein (outward transport efficiency), the proportion of intracellular Ra used for muscle protein synthesis (synthetic efficiency), the ratio of phenylalanine utilization for muscle protein synthesis to the arterial phenylalanine concentration (synthetic clearance), and the ratio of phenylalanine NB to the arterial phenylalanine concentration (anabolic clearance):
Mixed muscle fractional synthesis rate (FSR; %/h) was calculated from the incorporation of Phe into protein, using the precursor-product model:
where EP1 and EP2 are the enrichments of bound Phe in the first and second muscle biopsies of a time period, t is the time interval between biopsies (min), and EM is the mean Phe enrichment in the muscle intracellular pool (59).
Statistical analysis
The outcome variables were all continuous and approximately normally distributed. A repeated-measures general linear mixed model (GLMM) was used to analyze the treatment effects over time. Initial assessments for time × group interactions suggested no evidence of interaction in the model, and this term was dropped for all subsequent models. The models were fit in the MIXED procedure in SAS (60). To obtain meaningful (positive) values for 3-pool model fluxes calculated using both muscle and blood enrichments (e.g., FM,A), the muscle intracellular enrichment must be lower than that of the blood. In one younger and one older subject, this condition was not met during the postexercise period, and, as a result, only Fin, Fout, and NB were included in analyses for these subjects. In addition, in another older subject, the NB 60-min postexercise was negative (and >7 sd away from the mean) despite receiving an amino acid infusion; model fluxes from this subject at this time point were excluded from statistical analysis. We used a 2-sided α level of significance of 0.05 to assess statistical significance. Correlation coefficients are presented as Supplemental Data.
RESULTS
Subject characteristics
Subject characteristics are presented in Table 1. Except for age and aerobic capacity (Vo2peak), subjects were well matched, with similar heights, weights, BMI values, leg volumes, leg lean and fat mass, and body composition.
Table 1.
Characteristics of subjects
| Characteristic | Younger men (n=9) | Older men (n=8) |
|---|---|---|
| Age (yr) | 30 ± 2 | 67 ± 2 |
| Height (cm) | 170 ± 1 | 176 ± 3 |
| Weight (kg) | 78 ± 2 | 84 ± 4 |
| BMI (kg·m−2) | 27 ± 1 | 27 ± 1 |
| Body fat (%) | 22 ± 1 | 24 ± 1 |
| Total fat-free mass (kg) | 55 ± 2 | 59 ± 2 |
| Right leg | ||
| Total leg lean mass (kg) | 9.6 ± 0.3 | 9.9 ± 0.4 |
| Total leg fat mass (kg) | 3.0 ± 0.3 | 3.2 ± 0.2 |
| Total leg volume (L) | 8.8 ± 4 | 8.5 ± 3 |
| Left leg | ||
| Total leg lean mass (kg) | 9.6 ± 0.3 | 9.7 ± 0.5 |
| Total leg fat mass (kg) | 3.1 ± 0.3 | 3.2 ± 0.2 |
| Total leg volume (L) | 8.6 ± 4 | 8.4 ± 3 |
| Vo2peak (ml·kg−1·min−1) | 52 ± 4# | 41 ± 2# |
Values are expressed as means ± se.
P ≤ 0.05; significant difference between groups.
Leg blood flow, microvascular blood flow, and interstitial ethanol exchange
Total leg blood flow did not differ between young and older groups at any time (Fig. 2A). MBF (MBV×MFV) was significantly elevated relative to rest period at 60 min postexercise in both young and old (Fig. 2B; P=0.006 for time main effect). In addition, MBF was significantly higher in the young than in the older group (P=0.03). At 60 min postexercise, skeletal muscle microvascular blood volume was higher than at rest in both young and old (Fig. 2C; P=0.0006 for time main effect). MFV did not differ between young and old (Fig. 2D), and there was no change from baseline at 60 min postexercise. There was a marginal (P=0.06) effect of age on interstitial fluid ethanol exchange, whereas the effect of time on this variable was highly (P=0.0006) significant (Fig. 2E), with means of 0.47 ± 0.02, 0.40 ± 0.02, 0.42 ± 0.02, and 0.42 ± 0.02 for rest and 0–60, 61–120, and 121–180 min postexercise, respectively, in the repeated mixed model.
Figure 2.
Total leg blood flow and skeletal muscle nutritive flow. A) Leg blood flow did not differ between young and older groups. #P < 0.0001 for time effect. B) Microvascular blood flow was different between young and older groups and between rest and 60-post groups. *P = 0.03 for young vs. older groups; #P = 0.006 for rest vs. 60-post groups. C) Microvascular blood volume increased postexercise but did not differ between young and older individuals. #P = 0.0006. D) Microvascular flow velocity did not differ between age groups or times. E) Interstitial ethanol exchange was marginally different between age groups (P=0.06), with a significant effect of time. #P = 0.0006 for time effect.
Amino acid concentrations
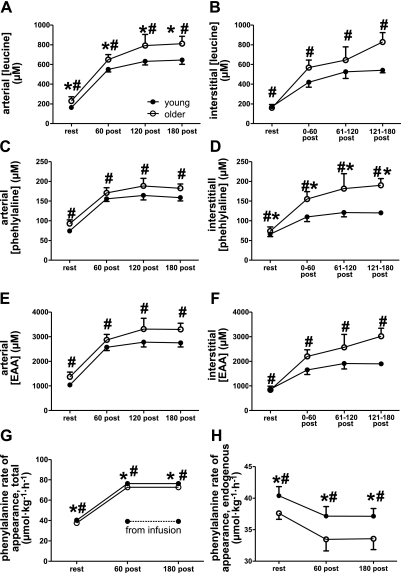
Because older individuals have elevated first-pass splanchnic extraction of oral amino acids (61), we provided amino acids via infusion in an attempt to normalize delivery of exogenous amino acids to the circulation. Arterial concentrations of several amino acids, including leucine, were marginally higher (P=0.04, 0.08, 0.08, for leucine, phenylalanine, and essential amino acids, respectively) in the older than in the younger group (Fig. 3, Supplemental Table 1). However, this was not due to an elevated overall (i.e., endogenous+exogenous) rate of appearance of amino acids in the older subjects, based on the fact that whole-body phenylalanine Ra was fairly well matched in the two groups (Fig. 3G), with values in the younger slightly (but significantly; P=0.007) higher than in the older. As expected, total whole-body phenylalanine Ra increased in response to the amino acid infusion (Fig. 3G, P<0.0001 for time main effect). Endogenous phenylalanine Ra was reduced in both groups in response to the amino acid infusion and exercise and was significantly lower in the older group (Fig. 3H; P=0.007 and P=0.02 for age and time effects, respectively).
Figure 3.
Amino acid availability. A–F) Both arterial (A, C, E) and interstitial (B, D, F) concentrations of leucine (A, B), phenylalanine (C, D), and the sum of the essential amino acids (EAA; E, F) increased with time; #P < 0.0001. Arterial leucine concentrations (A) and interstitial phenylalanine concentrations were significantly higher in older subjects; *P = 0.04. G, H) Total (endogenous+exogenous; G) and endogenous (H) rates of phenylalanine appearance in the circulation were lower in the older individuals and exhibited a significant effects of time. *P = 0.007 vs. younger group; #P<0.0001 (G), #P = 0.02 (H) for time effect. See also Results and Supplemental Tables 1 and 2 for other amino acids.
Interstitial concentrations of many amino acids were significantly correlated with plasma concentrations (Supplemental Table 3). In particular, plasma-interstitial fluid correlations for the branched chain amino acids and the sum of the essential amino acids were significant (P<0.0001) and moderately high (R2=0.65–0.76). As in the plasma, several amino acids were present at higher concentrations in the interstitium of the older individuals than in the young (Fig. 3D and Supplemental Table 2). Relative interstitial ammonia and urea levels were also significantly higher in the older group (Supplemental Table 2). We were not able to determine interstitial levels of all species measured in plasma. In particular, the summed interstitial summed essential amino acids do not include histidine, as we were not able to measure interstitial histidine levels in all subjects.
Phenylalanine kinetics
Phenylalanine concentration and enrichment data are presented in Supplemental Table 4 and demonstrate that subjects were in isotopic steady state during the postexercise period. Consistent with previous studies (9, 10), basal muscle protein metabolism was similar in the younger and older groups (Supplemental Table 5). Significant time effects were found for arterial inflow (Fin, P<0.0001,),venous outflow (Fout, P<0.0001), inward transport into muscle (FM,A, P<0.0001), arteriovenous shunting (FV,A, P<0.0001), outward transport from muscle (FV,M, P=0.003), muscle protein breakdown (FM,O, P=0.006), net balance (NB, P<0.0001), and muscle protein synthesis (FO,M, P=0.02) (Supplemental Table 5). None of these variables were found to differ significantly between the young and older subjects, although there were marginal group effects for inward transport (P=0.09) and outward transport (P=0.07).
FSR
Mixed muscle protein FSR was significantly higher postexercise than during rest (P<0.001 for time main effect), without age-related differences (0.061±0.005%·h−1 in the young subjects at rest and 0.070±0.006%·h−1 in the older subjects at rest; 0.098±0.009%·h−1 in the young subjects postexercise and 0.111±0.011%·h−1 in the older subjects postexercise).
Serum insulin concentrations
Insulin concentrations responded differently to the combined intervention of amino acid infusion and exercise in the younger and older subjects (group× time interaction P = 0.01), with older subjects exhibiting higher insulin concentrations at 60 min postexercise than the younger subjects (3.0±0.8, 3.2±1.1, and 3.6±1.1 pM at rest and at 60 and 180 min postexercise, respectively, in the young; 3.3±0.4, 7.3±1.2, 5.2±0.8 pM at rest and at 60 and 180 min postexercise, respectively, in the older subjects).
Sensitivity of muscle protein synthesis to amino acids
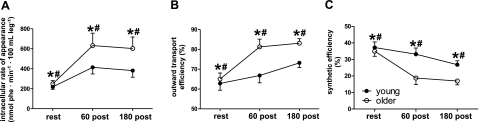
Because recent evidence suggests that older individuals exhibit resistance to anabolic stimuli, in particular, amino acids, we performed a number of calculations assessing amino acid handling and anabolic sensitivity in the young and older groups. Fractional outward transport and synthetic efficiency, two reciprocally related factors, increased and decreased, respectively, with time after exercise and were significantly different between young and old, with older subjects exhibiting greater fractional outward transport and lower synthetic efficiency (Fig. 4). We also calculated synthetic efficiency by dividing the FSR by the mean postexercise rate of phenylalanine appearance and obtained results similar to those obtained using the 3-pool model (Supplemental Fig. 1).
Figure 4.
Skeletal muscle phenylalanine trafficking. A) Intracellular rate of appearance was significantly different between young and older subjects, with a significant effect of time. *P = 0.01 vs. younger group; #P = 0.0005 for time effect. B) Outward transport efficiency was different between young and older subjects, with a significant effect of time. *P = 0.05 vs. younger group; #P = 0.0001 for time effect. C) Synthetic efficiency was higher in young than in older subjects, with a significant effect of time. *P = 0.05 vs. younger group; #P = 0.0001 for time effect.
Relationships between amino acid concentrations and muscle anabolism
Correlations between circulating and interstitial amino acid concentrations and protein synthesis, protein breakdown, and the net balance between synthesis and breakdown, using all time points (i.e., rest and 60 and 180 min postexercise) are presented in Supplemental Tables 6 and 7. For the infused amino acids, the correlations between the interstitial or plasma concentrations and protein synthesis generally suggested a weak to moderate relationship, whereas the correlations with protein breakdown suggested at most a weak relationship (Supplemental Fig. 2 and Supplemental Tables 6 and 7). In contrast, correlations between net balance and the interstitial or plasma concentrations of the infused amino acids suggested a moderate to strong association (Supplemental Fig. 2 and Supplemental Tables 6 and 7).
Energetic and anabolic signaling response (AMPK and mTOR activation)
To address the possibility that the exercise bout represented a greater metabolic stress in the skeletal muscle of the older subjects and thereby interfered with anabolic signaling responses, we assessed skeletal muscle AMPKα phosphorylation (Thr172) and mTOR (Ser-2448) phosphorylation (Supplemental Fig. 3). AMPKα phosphorylation was on average higher in the older subjects than in the young at each time point, but this difference did not reach statistical significance (P=0.12). Likewise, there were no group differences in mTOR phosphorylation; however, there was a significant effect of time (P=0.01).
Leg glucose uptake
Leg glucose uptake in the younger group was 70.9 ± 7.3, 97.9 ± 29.2, and 73.1 ± 9.5 μmol · min−1 · leg−1 at rest and at 60 and 180 min postexercise, respectively. In the older group, the leg glucose uptake was 60.4 ± 2.7, 88.2 ± 14.2, and 68.1 ± 5.6 μmol · min−1 · leg−1 at rest and at 60 and 180 min postexercise, respectively. Leg glucose uptake did not differ significantly in young and older subjects and did not exhibit a significant effect of time.
DISCUSSION
Loss of skeletal muscle mass is a common consequence of aging. Although age-related comorbidities can contribute to this loss, studies in healthy older individuals suggest that aging per se contributes to development of age-related resistance to anabolic stimuli (1, 12, 14–16). Here, we expand on this body of literature by reporting for the first time that aging induces anabolic resistance to amino acids following low-intensity endurance exercise. As expected in a group of older subjects who are healthy and nonsarcopenic, the amino acid resistance observed in this study did not manifest as marked changes in the overall balance between muscle protein synthesis and breakdown. Rather, it was a subclinical entity, evident in a reduced sensitivity of muscle protein synthesis to stimulation by amino acids.
Previous studies have determined that the stimulation of muscle protein synthesis in response to protein or amino acid administration occurs via an unidentified sensing mechanism responsive to elevated extracellular concentrations and/or increased intracellular rates of appearance of essential amino acids (15, 35, 47, 62–65). In the current study, we compared anabolic sensitivity in the younger and older groups by expressing anabolic responses relative to the intracellular rate of appearance of phenylalanine and the arterial phenylalanine concentration (Fig. 4 and Supplemental Figs. 1 and 4). These calculations uniformly demonstrated marked amino acid resistance in skeletal muscle of the older subjects. These findings are consistent with previous studies in which the response of skeletal muscle protein synthesis to amino acids was found to be blunted in the old under nonexercised conditions (14) and demonstrate that age-related anabolic resistance extends to the period following an acute bout of endurance-type exercise.
In this study, we hypothesized that reduced “nutritive” blood flow in older vs. younger subjects would be associated with reduced interstitial amino acid concentrations and inward phenylalanine transport, which would thereby limit muscle protein synthesis by limiting essential amino acid availability. In theory, impaired endothelial function could interfere with the response of skeletal muscle to elevated plasma amino acids by reducing capillary blood flow and thus limiting transcapillary amino acid exchange with the interstitial fluid. Our results comprehensively refute our hypothesis. Despite evidence of reduced nutritive blood flow by two independent measures, the older individuals had equal or greater amino acid availability than the young, based on circulating and interstitial amino acid concentrations and intracellular appearance rates of phenylalanine. Thus, age-related anabolic resistance to amino acids is apparently not mediated via reduced amino acid availability secondary to impaired hemodynamics. However, we cannot rule out a role for impaired microvascular flow in anabolic resistance, but by some mechanism other than amino acid availability [e.g., reduced hormone/nutrient delivery (66) and/or reduced removal of cellular “waste” products such as ammonia (Supplemental Table 2)], as both capillary recruitment and microvascular blood flow were positively correlated with net balance.
The markedly higher interstitial ammonia levels (Supplemental Table 2) in skeletal muscle of the older subjects were unexpected. Skeletal muscle ammonia production can occur by several mechanisms involving deamination of AMP or amino acids (67–69), although the relative importance of these are still not certain, with the exception of very intense exercise, during which AMP deamination is generally agreed to be the most important pathway for skeletal muscle ammoniagenesis. Recent human and animal studies suggest that the susceptibility of older skeletal muscle to metabolic stress is increased, on the basis of activation of the energy sensor AMPK (10, 70). Such findings are consistent with the notion that mitochondrial ATP-generating capacity is reduced with aging (71). It is conceivable that this greater metabolic stress in older skeletal muscle results in significant ammonia production from AMP deamination. In the current study, skeletal muscle AMPK phosphorylation was, on average, higher in the older than in the younger subjects, but this difference did not reach statistical significance. The finding that arterial glutamine levels were significantly higher in the old than in the young, whereas interstitial concentrations were not different between the two groups, could also indicate that activity of glutaminase, which catalyzes conversion of glutamine to ammonia and glutamate, is higher in skeletal muscles of older vs. younger individuals. However, the facts that glutamate, aspartate, and alanine were given in the amino acid infusion and no tracers were infused for the purpose of studying amino acid deamination prevent any strong conclusions regarding which, if any, amino acid deamination pathways were differentially stimulated in older and younger skeletal muscle.
Circulating levels of 3-methylhistidine, an accepted marker of myofibrillar protein breakdown (72), were significantly higher in the old than in the young (Supplemental Table 1). However, the fact that neither skeletal muscle protein breakdown nor whole body phenylalanine Ra were greater in the old subjects is inconsistent with greater release of 3-methylhistidine from protein breakdown of skeletal muscle or other tissues. An alternative explanation is that skeletal muscle sarcolemmal integrity is compromised with aging, possibly due to chronic suboptimal nutrition (73, 74), with the result that exchange between muscle fibers and the interstitial fluid is increased, as suggested previously (75). The significantly greater fractional outward transport from skeletal muscle in the older subjects is consistent with such an interpretation, as our model of phenylalanine kinetics does not distinguish between transporter-mediated exchange and amino acid exchange occurring through a compromised sarcolemma. Notably, a metabolomic investigation found that 3-methylhistidine is a sensitive marker of oxidative stress (76), suggesting that age-related oxidative stress may have contributed to reduced sarcolemmal integrity in the older subjects. Likewise, cystine, the oxidized form of cysteine, was also higher in both interstitial fluid and plasma of the older subjects than in the young, confirming previous reports of higher circulating cystine levels in the elderly (77–80) and consistent with the development of redox stress with aging (81–83).
By design, we studied healthy older subjects who do not take prescription medications, are glucose tolerant, and do not participate in a regular exercise program. This allowed us to study the effects of aging per se in the absence of comorbidities or pharmacological effects. However, our results, therefore, may underestimate the extent of impairment in anabolic responsiveness in older individuals who are not as healthy. Likewise, by selecting glucose-tolerant elderly subjects, we have minimized the likelihood of observing impairments in leg glucose uptake. Future studies will be important to determine the degree of impairment in less healthy populations.
Regardless of how it occurs, the existence of age-related anabolic resistance to amino acids, as found in this study, as well as in several previous ones, suggests two general, nonexclusive approaches for treating or preventing sarcopenia: increase ingestion of amino acids and/or protein by older individuals; and increase the anabolic sensitivity of older skeletal muscle to amino acids. The first approach may be viable in principle, as rates of skeletal muscle protein synthesis in older subjects have equaled those of younger subjects in some (84, 85) (as well as the present study), but not all (14), studies when large enough amounts of protein or amino acids were given. However, the diminished anabolic responsiveness of older individuals to amino acids, as well as the uncertain effects of long-term high protein/amino acid diets on deleterious signaling in tissues other than skeletal muscle (86, 87), implies that increasing anabolic sensitivity is the preferable approach. The present study suggests that interventions to reduce metabolic and redox stress and maintain sarcolemmal integrity are worthy of investigation in this regard.
Supplementary Material
Acknowledgments
The authors thank the study volunteers, General Clinical Research Center (GCRC) nurses, and the Sealy Center on Aging recruitment coordinators, Sue Minello and Roxana Hirst. The authors thank Chuan Hong for assistance with the statistical analysis. The authors thank the Biomolecular Resource Facility and the University of Texas Medical Branch (UTMB) Protein Chemistry Lab for assistance with plasma and interstitial amino acid analysis.
This research was supported by National Institutes of Health/National Institute on Aging grants R01 AG21539 (M.S.-M.) and R21 AG19209 (R.H.) and Claude D. Pepper Older Americans Independence Center grant P30 AG024832 (J.G.). The authors thank Bristol-Myers Squibb (New York, NY, USA) for providing the Definity contrast agent for this study. Studies were conducted at the GCRC, UTMB at Galveston, funded by grant M01 RR 00073 from the National Center for Research Resources, National Institutes of Health, U.S. Public Health Service.
REFERENCES
- 1.Paddon-Jones D., Short K. R., Campbell W. W., Volpi E., Wolfe R. R. (2008) Role of dietary protein in the sarcopenia of aging. Am. J. Clin. Nutr. 87, 1562S–1566S [DOI] [PubMed] [Google Scholar]
- 2.Alway S. E., Siu P. M. (2008) Nuclear apoptosis contributes to sarcopenia. Exerc. Sport. Sci. Rev. 36, 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston A. P., De Lisio M., Parise G. (2008) Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl. Physiol. Nutr. Metab. 33, 191–199 [DOI] [PubMed] [Google Scholar]
- 4.Balagopal P., Rooyackers O. E., Adey D. B., Ades P. A., Nair K. S. (1997) Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am. J. Physiol. Endocrinol. Metab. 273, E790–E800 [DOI] [PubMed] [Google Scholar]
- 5.Yarasheski K. E., Pak-Loduca J., Hasten D. L., Obert K. A., Brown M. B., Sinacore D. R. (1999) Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men ≥76 yr old. Am. J. Physiol. Endocrinol. Metab. 277, E118–E125 [DOI] [PubMed] [Google Scholar]
- 6.Welle S., Thornton C., Statt M. (1995) Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am. J. Physiol. Endrocrinol. Metab. 268, E422–E427 [DOI] [PubMed] [Google Scholar]
- 7.Hasten D. L., Pak-Loduca J., Obert K. A., Yarasheski K. E. (2000) Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am. J. Physiol. Endocrinol. Metab. 278, E620–E626 [DOI] [PubMed] [Google Scholar]
- 8.Yarasheski K. E., Zachwieja J. J., Bier D. M. (1993) Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am. J. Physiol. Endrocrinol. Metab. 265, E210–E214 [DOI] [PubMed] [Google Scholar]
- 9.Volpi E., Sheffield-Moore M., Rasmussen B. B., Wolfe R. R. (2001) Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286, 1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond M. J., Dreyer H. C., Pennings B., Fry C. S., Dhanani S., Dillon E. L., Sheffield-Moore M., Volpi E., Rasmussen B. B. (2008) Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J. Appl. Physiol. 104, 1452–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita S., Rasmussen B. B., Cadenas J. G., Drummond M. J., Glynn E. L., Sattler F. R., Volpi E. (2007) Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 56, 1615–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen B. B., Fujita S., Wolfe R. R., Mittendorfer B., Roy M., Rowe V. L., Volpi E. (2006) Insulin resistance of muscle protein metabolism in aging. FASEB J. 20, 768–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpi E., Mittendorfer B., Rasmussen B. B., Wolfe R. R. (2000) The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 85, 4481–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuthbertson D., Smith K., Babraj J., Leese G., Waddell T., Atherton P., Wackerhage H., Taylor P. M., Rennie M. J. (2005) Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 19, 422–424 [DOI] [PubMed] [Google Scholar]
- 15.Kumar V., Atherton P., Smith K., Rennie M. J. (2009) Human muscle protein synthesis and breakdown during and after exercise. J. Appl. Physiol. 106, 2026–2039 [DOI] [PubMed] [Google Scholar]
- 16.Rennie M. J. (2009) Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl. Physiol. Nutr. Metab. 34, 377–381 [DOI] [PubMed] [Google Scholar]
- 17.Gore D. C., Wolfe R. R., Chinkes D. L. (2007) Quantification of amino acid transport through interstitial fluid: assessment of four-compartment modeling for muscle protein kinetics. Am. J. Physiol. Endocrinol. Metab. 292, E319–E323 [DOI] [PubMed] [Google Scholar]
- 18.Miller S., Chinkes D., MacLean D. A., Gore D., Wolfe R. R. (2004) In vivo muscle amino acid transport involves two distinct processes. Am. J. Physiol. Endocrinol. Metab. 287, E136–E141 [DOI] [PubMed] [Google Scholar]
- 19.Mehta D., Malik A. B. (2006) Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 86, 279–367 [DOI] [PubMed] [Google Scholar]
- 20.Clark M. G. (2008) Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am. J. Physiol. Endocrinol. Metab. 295, E732–E750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark M. G., Rattigan S., Barrett E. J. (2006) Nutritive blood flow as an essential element supporting muscle anabolism. Curr. Opin. Clin. Nutr. Metab. Care. 9, 185–189 [DOI] [PubMed] [Google Scholar]
- 22.Lewis S. E., Kelly F. J., Goldspink D. F. (1984) Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem. J. 217, 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pluskal M. G., Moreyra M., Burini R. C., Young V. R. (1984) Protein synthesis studies in skeletal muscle of aging rats. I. Alterations in nitrogen composition and protein synthesis using a crude polyribosome and pH 5 enzyme system. J. Gerontol. 39, 385–391 [DOI] [PubMed] [Google Scholar]
- 24.Williamson J. R., Hoffmann P. L., Kohrt W. M., Spina R. J., Coggan A. R., Holloszy O. (1996) Endurance exercise training decreases capillary basement membrane width in older nondiabetic and diabetic adults. J. Appl. Physiol. 80, 747–753 [DOI] [PubMed] [Google Scholar]
- 25.Carraro F., Stuart C. A., Hartl W. H., Rosenblatt J., Wolfe R. R. (1990) Effect of exercise and recovery on muscle protein synthesis in human subjects. Am. J. Physiol. Endrocrinol Metab. 259, E470–E476 [DOI] [PubMed] [Google Scholar]
- 26.Sheffield-Moore M., Yeckel C. W., Volpi E., Wolf S. E., Morio B., Chinkes D. L., Paddon-Jones D., Wolfe R. R. (2004) Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am. J. Physiol. Endocrinol. Metab. 287, E513–E522 [DOI] [PubMed] [Google Scholar]
- 27.Pikosky M. A., Gaine P. C., Martin W. F., Grabarz K. C., Ferrando A. A., Wolfe R. R., Rodriguez N. R. (2006) Aerobic exercise training increases skeletal muscle protein turnover in healthy adults at rest. J. Nutr. 136, 379–383 [DOI] [PubMed] [Google Scholar]
- 28.Short K. R., Vittone J. L., Bigelow M. L., Proctor D. N., Nair K. S. (2004) Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab. 286, E92–E101 [DOI] [PubMed] [Google Scholar]
- 29.Bodine S. C. (2006) mTOR signaling and the molecular adaptation to resistance exercise. Med. Sci. Sports. Exerc. 38, 1950–1957 [DOI] [PubMed] [Google Scholar]
- 30.Atherton P. J., Babraj J., Smith K., Singh J., Rennie M. J., Wackerhage H. (2005) Selective activation of AMPK-PGC-1alpha or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 19, 786–788 [DOI] [PubMed] [Google Scholar]
- 31.Camera D. M., Edge J., Short M. J., Hawley J. A., Coffey V. G. (2010) Early time-course of Akt phosphorylation following endurance and resistance exercise. [E-pub ahead of print]Med. Sci. Sports Exerc. PMID: 20195183 [DOI] [PubMed] [Google Scholar]
- 32.Holloszy J. O. (1967) Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 242, 2278–2282 [PubMed] [Google Scholar]
- 33.Hood D. A. (2009) Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl. Physiol. Nutr. Metab. 34, 465–472 [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson S. B., Phillips S. M., Atherton P. J., Patel R., Yarasheski K. E., Tarnopolsky M. A., Rennie M. J. (2008) Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J. Physiol. 586, 3701–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohe J., Low A., Wolfe R. R., Rennie M. J. (2003) Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J. Physiol. 552, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohe J., Low J. F., Wolfe R. R., Rennie M. J. (2001) Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J. Physiol. 532, 575–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller B. F., Olesen J. L., Hansen M., Dossing S., Crameri R. M., Welling R. J., Langberg H., Flyvbjerg A., Kjaer M., Babraj J. A., Smith K., Rennie M. J. (2005) Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J. Physiol. 567, 1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincent M. A., Clerk L. H., Lindner J. R., Klibanov A. L., Clark M. G., Rattigan S., Barrett E. J. (2004) Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53, 1418–1423 [DOI] [PubMed] [Google Scholar]
- 39.Adams F., Jordan J., Schaller K., Luft F. C., Boschmann M. (2005) Blood flow in subcutaneous adipose tissue depends on skin-fold thickness. Horm. Metab. Res. 37, 68–73 [DOI] [PubMed] [Google Scholar]
- 40.Hickner R. C., Fisher J. S., Ehsani A. A., Kohrt W. M. (1997) Role of nitric oxide in skeletal muscle blood flow at rest and during dynamic exercise in humans. Am. J. Physiol. Heart Circ. Physiol. 273, H405–H410 [DOI] [PubMed] [Google Scholar]
- 41.Hickner R. C., Kemeny G., McIver K., Harrison K., Hostetler M. E. (2003) Lower skeletal muscle nutritive blood flow in older women is related to eNOS protein content. J. Gerontol. A Biol. Sci. Med. Sci. 58, 20–25 [DOI] [PubMed] [Google Scholar]
- 42.McIver K. L., Evans C., Kraus R. M., Ispas L., Sciotti V. M., Hickner R. C. (2006) NO-mediated alterations in skeletal muscle nutritive blood flow and lactate metabolism in fibromyalgia. Pain 120, 161–169 [DOI] [PubMed] [Google Scholar]
- 43.Newman J. M., Di Maria C. A., Rattigan S., Clark M. G. (2001) Nutritive blood flow affects microdialysis O/I ratio for [(14)C]ethanol and 3H2O in perfused rat hindlimb. Am. J. Physiol. Heart. Circ. Physiol. 281, H2731–H2737 [DOI] [PubMed] [Google Scholar]
- 44.Roberts J. L., Newman J. M., Warner R., Rattigan S., Clark M. G. (2005) Axially symmetric semi-infinite domain models of microdialysis and their application to the determination of nutritive flow in rat muscle. J. Physiol. 563, 213–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiffelers S. L., Akkermans J. A., Saris W. H., Blaak E. E. (2003) Lipolytic and nutritive blood flow response to beta-adrenoceptor stimulation in situ in subcutaneous abdominal adipose tissue in obese men. Int. J. Obes. Relat. Metab. Disord. 27, 227–231 [DOI] [PubMed] [Google Scholar]
- 46.Van Pelt R. E., Gozansky W. S., Hickner R. C., Schwartz R. S., Kohrt W. M. (2006) Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity (Silver Spring) 14, 2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biolo G., Fleming R. Y., Maggi S. P., Wolfe R. R. (1995) Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am. J. Physiol. Endrocrinol. Metab. 268, E75–E84 [DOI] [PubMed] [Google Scholar]
- 48.Jorfeldt L., Wahren J. (1971) Leg blood flow during exercise in man. Clin. Sci. 41, 459–473 [DOI] [PubMed] [Google Scholar]
- 49.Bergstrom J., Furst P., Noree L. O., Vinnars E. (1974) Intracellular free amino acid concentration in human muscle tissue. J. Appl. Physiol. 36, 693–697 [DOI] [PubMed] [Google Scholar]
- 50.Sheffield-Moore M., Urban R. J., Wolf S. E., Jiang J., Catlin D. H., Herndon D. N., Wolfe R. R., Ferrando A. A. (1999) Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J. Clin. Endocrinol. Metab. 84, 2705–2711 [DOI] [PubMed] [Google Scholar]
- 51.Vincent M. A., Clerk L. H., Lindner J. R., Price W. J., Jahn L. A., Leong-Poi H., Barrett E. J. (2006) Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am. J. Physiol. Endocrinol. Metab. 290, E1191–E1197 [DOI] [PubMed] [Google Scholar]
- 52.Wei K., Jayaweera A. R., Firoozan S., Linka A., Skyba D. M., Kaul S. (1998) Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97, 473–483 [DOI] [PubMed] [Google Scholar]
- 53.Clerk L. H., Vincent M. A., Jahn L. A., Liu Z., Lindner J. R., Barrett E. J. (2006) Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55, 1436–1442 [DOI] [PubMed] [Google Scholar]
- 54.Scheller D., Kolb J. (1991) The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J. Neurosci. Methods 40, 31–38 [DOI] [PubMed] [Google Scholar]
- 55.Borsheim E., Kobayashi H., Traber D. L., Wolfe R. R. (2006) Compartmental distribution of amino acids during hemodialysis-induced hypoaminoacidemia. Am. J. Physiol. Endocrinol. Metab. 290, E643–E652 [DOI] [PubMed] [Google Scholar]
- 56.Hickner R. C., Ekelund U., Mellander S., Ungerstedt U., Henriksson J. (1995) Muscle blood flow in cats: comparison of microdialysis ethanol technique with direct measurement. J. Appl. Physiol. 79, 638–647 [DOI] [PubMed] [Google Scholar]
- 57.Hickner R. C., Rosdahl H., Borg I., Ungerstedt U., Jorfeldt L., Henriksson J. (1992) The ethanol technique of monitoring local blood flow changes in rat skeletal muscle: implications for microdialysis. Acta Physiol. Scand. 146, 87–97 [DOI] [PubMed] [Google Scholar]
- 58.Wolfe R. R. (1992) Radioactive and Stable Isotope Tracers in Biomedicine. Principles and Practice of Kinetic Analysis, Wiley-Liss, New York [Google Scholar]
- 59.Baumann P. Q., Stirewalt W. S., O'Rourke B. D., Howard D., Nair K. S. (1994) Precursor pools of protein synthesis: a stable isotope study in a swine model. Am. J. Physiol. Endocrinol. Metab. 267, E203–E209 [DOI] [PubMed] [Google Scholar]
- 60.SAS Institute (2004) SAS/STAT 9.1 User's Guide, SAS Institute Inc., Cary, NC, USA [Google Scholar]
- 61.Volpi E., Mittendorfer B., Wolf S. E., Wolfe R. R. (1999) Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am. J. Physiol. Endocrinol. Metab. 277, E513–520 [DOI] [PubMed] [Google Scholar]
- 62.Tipton K. D., Ferrando A. A. (2008) Improving muscle mass: response of muscle metabolism to exercise, nutrition and anabolic agents. Essays Biochem. 44, 85–98 [DOI] [PubMed] [Google Scholar]
- 63.Drummond M. J., Dreyer H. C., Fry C. S., Glynn E. L., Rasmussen B. B. (2009) Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J. Appl. Physiol. 106, 1374–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyazaki M., Esser K. A. (2009) Cellular mechanisms regulating protein synthesis and skeletal muscle hypertrophy in animals. J. Appl. Physiol. 106, 1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vary T. C., Lynch C. J. (2007) Nutrient signaling components controlling protein synthesis in striated muscle. J. Nutr. 137, 1835–1843 [DOI] [PubMed] [Google Scholar]
- 66.Bergman R. N. (1997) New concepts in extracellular signaling for insulin action: the single gateway hypothesis. Recent. Prog. Horm. Res. 52, 359–385; discussion 385–357 [PubMed] [Google Scholar]
- 67.van Hall G., van der Vusse G. J., Soderlund K., Wagenmakers A. J. (1995) Deamination of amino acids as a source for ammonia production in human skeletal muscle during prolonged exercise. J. Physiol. 489, 251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hellsten Y., Richter E. A., Kiens B., Bangsbo J. (1999) AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. J. Physiol. 520, 909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graham T. E., MacLean D. A. (1998) Ammonia and amino acid metabolism in skeletal muscle: human, rodent and canine models. Med. Sci. Sports Exerc. 30, 34–46 [DOI] [PubMed] [Google Scholar]
- 70.Thomson D. M., Brown J. D., Fillmore N., Ellsworth S. K., Jacobs D. L., Winder W. W., Fick C. A., Gordon S. E. (2009) AMP-activated protein kinase response to contractions and treatment with the AMPK activator AICAR in young adult and old skeletal muscle. J. Physiol. 587, 2077–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Short K. R., Bigelow M. L., Kahl J., Singh R., Coenen-Schimke J., Raghavakaimal S., Nair K. S. (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U. S. A. 102, 5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young V. R., Munro H. N. (1978) Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed. Proc. 37, 2291–2300 [PubMed] [Google Scholar]
- 73.Fischer L. M., daCosta K. A., Kwock L., Stewart P. W., Lu T. S., Stabler S. P., Allen R. H., Zeisel S. H. (2007) Sex and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 85, 1275–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeisel S. H. (2006) Choline: critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 26, 229–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McBride T. (2000) Increased depolarization, prolonged recovery and reduced adaptation of the resting membrane potential in aged rat skeletal muscles following eccentric contractions. Mech. Ageing Dev. 115, 127–138 [DOI] [PubMed] [Google Scholar]
- 76.Lee R., West D., Phillips S. M., Britz-McKibbin P. (2010) Differential metabolomics for quantitative assessment of oxidative stress with strenuous exercise and nutritional intervention: thiol-specific regulation of cellular metabolism with N-acetyl-l-cysteine pretreatment. Anal. Chem. 82, 2959–2968 [DOI] [PubMed] [Google Scholar]
- 77.Hack V., Breitkreutz R., Kinscherf R., Rohrer H., Bartsch P., Taut F., Benner A., Droge W. (1998) The redox state as a correlate of senescence and wasting and as a target for therapeutic intervention. Blood 92, 59–67 [PubMed] [Google Scholar]
- 78.Hack V., Schmid D., Breitkreutz R., Stahl-Henning C., Drings P., Kinscherf R., Taut F., Holm E., Droge W. (1997) Cystine levels, cystine flux, and protein catabolism in cancer cachexia, HIV/SIV infection, and senescence. FASEB J. 11, 84–92 [DOI] [PubMed] [Google Scholar]
- 79.Hildebrandt W., Kinscherf R., Hauer K., Holm E., Droge W. (2002) Plasma cystine concentration and redox state in aging and physical exercise. Mech. Ageing Dev. 123, 1269–1281 [DOI] [PubMed] [Google Scholar]
- 80.Jones D. P., Mody V. C., Jr., Carlson J. L., Lynn M. J., Sternberg P., Jr. (2002) Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic. Biol. Med. 33, 1290–1300 [DOI] [PubMed] [Google Scholar]
- 81.Droge W. (2002) Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 [DOI] [PubMed] [Google Scholar]
- 82.Jackson M. J. (2008) Redox regulation of skeletal muscle. IUBMB Life 60, 497–501 [DOI] [PubMed] [Google Scholar]
- 83.Reid M. B., Durham W. J. (2002) Generation of reactive oxygen and nitrogen species in contracting skeletal muscle: potential impact on aging. Ann. N. Y. Acad. Sci. 959, 108–116 [DOI] [PubMed] [Google Scholar]
- 84.Symons T. B., Schutzler S. E., Cocke T. L., Chinkes D. L., Wolfe R. R., Paddon-Jones D. (2007) Aging does not impair the anabolic response to a protein-rich meal. Am. J. Clin. Nutr. 86, 451–456 [DOI] [PubMed] [Google Scholar]
- 85.Symons T. B., Sheffield-Moore M., Wolfe R. R., Paddon-Jones D. (2009) A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J. Am. Diet. Assoc. 109, 1582–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harrison D. E., Strong R., Sharp Z. D., Nelson J. F., Astle C. M., Flurkey K., Nadon N. L., Wilkinson J. E., Frenkel K., Carter C. S., Pahor M., Javors M. A., Fernandez E., Miller R. A. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Selman C., Tullet J. M., Wieser D., Irvine E., Lingard S. J., Choudhury A. I., Claret M., Al-Qassab H., Carmignac D., Ramadani F., Woods A., Robinson I. C., Schuster E., Batterham R. L., Kozma S. C., Thomas G., Carling D., Okkenhaug K., Thornton J. M., Partridge L., Gems D., Withers D. J. (2009) Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.