Abstract
Variability in adult motor output is important for enabling animals to respond to changing external conditions. Songbirds are useful for studying variability because they alter the amount of variation in their song depending on social context. When an adult zebra finch male sings to a female (“directed”), his song is highly stereotyped, but when he sings alone (“undirected”), his song varies across renditions. Lesions of the lateral magnocellular nucleus of the anterior nidopallium (LMAN), the output nucleus of a cortical-basal ganglia circuit for song, reduce song variability to that of the stereotyped “performance” state. However, such lesions not only eliminate LMAN's synaptic input to its targets, but can also cause structural or physiological changes in connected brain regions, and thus cannot assess whether the acute activity of LMAN is important for social modulation of adult song variability. To evaluate the effects of ongoing LMAN activity, we reversibly silenced LMAN in singing zebra finches by bilateral reverse microdialysis of the GABAA receptor agonist muscimol. We found that LMAN inactivation acutely reduced undirected song variability, both across and even within syllable renditions, to the level of directed song variability in all birds examined. Song variability returned to pre-muscimol inactivation levels after drug washout. However, unlike LMAN lesions, LMAN inactivation did not eliminate social context effects on song tempo in adult birds. These results indicate that the activity of LMAN neurons acutely and actively generates social context-dependent increases in adult song variability but that social regulation of tempo is more complex.
INTRODUCTION
Elite athletes perform the same motor program time after time without apparent differences in outcome, but even champions occasionally exhibit variability that causes them to misstep or take a poor swing. Until recently, it was thought that these errors represented residual biological noise. However, it is becoming apparent that variability in a motor program may be important to allow adjustment to changing conditions—for example, aging, injury, or altered environmental inputs. Vocal production in the songbird provides a unique behavioral system to examine how the brain controls motor variability. Songbirds exhibit a well-documented form of motor learning with many parallels to human speech learning (Doupe and Kuhl 1999). A young zebra finch initially produces highly variable and noisy vocalizations, which he refines during a period of sensorimotor learning, so that by adulthood he “crystallizes” a single stereotyped song (Brainard and Doupe 2002; Brenowitz and Beecher 2005; Immelmann 1969; Konishi 2004; Price 1979; Tchernichovski et al. 2001). Once adult, when male zebra finches are singing to females (“directed” singing), they produce songs that are very consistent from rendition to rendition. However, when males sing in isolation (“undirected” singing), their songs contain subtle but biologically meaningful variability. This suggests that males switch between a state of vocal motor performance for females and a state of vocal practice when they are alone (Jarvis et al. 1998; Kao and Brainard 2006; Kao et al. 2005; Olveczky et al. 2005; Sakata et al. 2008; Sober et al. 2008; Teramitsu and White 2006). Indeed females clearly prefer listening to the less variable directed song (Woolley and Doupe 2008).
A variety of evidence supports a role for the anterior forebrain pathway (AFP), a cortical-basal ganglia circuit, not only in plasticity (Andalman and Fee 2009; Bottjer et al. 1984; Brainard and Doupe 2000; Kao et al. 2005; Nordeen and Nordeen 2010; Scharff and Nottebohm 1991; Sohrabji et al. 1990; Thompson and Johnson 2007; Williams and Mehta 1999) but also in actively modulating social context-dependent differences in vocal behavior. The AFP forms a loop between two premotor areas that are necessary for song production (Fig. 1A). The neurons of the cortex-like outflow nucleus of the AFP, the lateral magnocellular nucleus of the anterior nidopallium (LMAN), are active during singing (Hessler and Doupe 1999a; Jarvis and Nottebohm 1997) and can influence vocal motor output when stimulated during undirected song (Kao et al. 2005). When birds are engaged in undirected singing, singing-related neural activity in LMAN is greatly increased in magnitude, with more burst firing, than during directed singing (Kao et al. 2008); this likely explains the striking increases in immediate early gene expression in LMAN in the undirected context (Jarvis et al. 1998). Additionally, LMAN neural activity during undirected song is more variable in relation to song output (Hessler and Doupe 1999b; Kao et al. 2005, 2008), and complete elimination of LMAN activity by bilateral lesions causes the level of undirected song variability to decrease to the level of directed song variability (Hampton et al. 2009; Kao and Brainard 2006; Kao et al. 2005). In zebra finches, LMAN lesions also cause an attenuation of social context effects on song tempo (Kao and Brainard 2006). Taken together, these lesion data suggest that the AFP is necessary for social modulation of adult song.
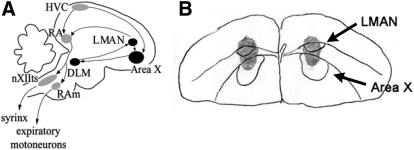
Fig. 1.
A: diagram of the major nuclei of the song system. The motor pathway is shown in gray, the anterior forebrain pathway (AFP) in black. In the motor pathway, neurons of the cortex-like nucleus HVC (abbreviation used as proper name) synapse on neurons of the robust nucleus of the arcopallium (RA), which directly and indirectly project to motor neurons that innervate the syrinx and respiratory muscles. In the AFP, HVC provides input into the striato-pallidal nucleus Area X, which projects to the medial nucleus of the dorsolateral thalamus (DLM). DLM sends excitatory projections to the lateral magnocellular nucleus of the anterior nidopallium (LMAN). LMAN projects both back to Area X, and on to RA. B: camera lucida tracing of 1 coronal section showing spread of biotinylated muscimol (gray shading). LMAN and Area X were delineated by Nissl stain (see methods).
However, the use of lesions to assess the role of a particular brain region in behavior has disadvantages. In addition to nonspecific influences of tissue damage, lesions often require substantial recovery time before behavioral effects can be measured, during which time compensatory mechanisms can develop to replace the function of the lesioned area, as has been observed for example in the primate visual system (Newsome and Wurtz 1988). These compensatory mechanisms could contribute to the examples of different behavioral outcomes from acute inactivations versus lesions of a brain region (Dias and Segraves 1999; Riquimaroux et al. 1991; Ruthazer and Stryker 1996). With respect to LMAN in particular, the destruction of LMAN not only removes its synaptic inputs to the premotor robust nucleus of the arcopallium (RA), but also eliminates LMAN's neurotrophic influences on RA (Johnson et al. 1997; Kittelberger and Mooney 2005). Moreover, damage of LMAN inputs to RA is known to elicit striking and relatively rapid structural and electrophysiological changes in the synapses from HVC (used as proper name) to RA (Kittelberger and Mooney 1999), at least in juveniles, making it difficult to assess which brain changes are responsible for the behavioral alterations seen. Thus although lesions can provide evidence for the involvement of a brain region in a behavior, the much shorter time course of inactivations can provide information about the brain region's rapid synaptic contributions to the behavior.
Our approach to investigating the acute contribution of LMAN to adult song variability was therefore to inactivate LMAN transiently in awake, singing zebra finches, using reverse microdialysis of muscimol, a GABAA receptor agonist. Indeed such acute inactivations have been used effectively in birds to examine song learning (Andalman and Fee 2009; Olveczky et al. 2005), suggesting that this technique would be useful in examining LMAN's role in social modulation of adult song. We designed the experimental setup to allow us to wash muscimol in and out without handling the bird or disrupting his singing behavior. In addition, reverse microdialysis allowed us to inactivate for long and controlled periods of time, without causing any net liquid flow into the brain, so that we could obtain the substantial amounts of both directed and undirected singing necessary for analyzing social context-dependence of song. We found that like lesions of LMAN, muscimol inactivation of LMAN completely eliminated social context-dependent song variability and even decreased a measure of within syllable variability, something that had not been seen with LMAN lesions. Moreover undirected song variability returned following drug washout, supporting the idea that LMAN firing acutely alters adult song variability. However, unlike LMAN lesions in zebra finches, LMAN inactivation did not eliminate the modulation of song tempo by social context, indicating that tempo changes are not simply caused by changes in LMAN activity.
METHODS
Subjects
Eight adult male zebra finches (Taeniopygia guttata) were used in this study, ages 80–230 days. All birds were raised in our colony with their parents and siblings in individual breeding cages until ≥60 days of age and then housed with other males in the colony. For this study we selected males whose songs contained at least one syllable with clear harmonic structure and that sang both directed and undirected song. After selection they were housed individually in sound-attenuating chambers (Acoustic Systems, Austin, TX) with a 14 h light:10 h dark photoperiod for the duration of the experiment. All procedures were performed in accordance with protocols approved by the University of California, San Francisco Institutional Animal Care and Use Committee.
Chemicals
All chemicals were from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Surgical procedures
Birds were anesthetized with a mixture of ketamine and midazolam injected intramuscularly, followed by the inhalant isoflurane for the duration of the surgery, and then were placed in a stereotaxic apparatus. Beak angle was adjusted such that the distance between the posterior bifurcation of the midsagittal sinus and the center of the ear bars was between 4.8 and 5.2 mm. In most birds, guide cannulae (CMA/7, CMA Microdialysis, Stockholm, Sweden) were implanted bilaterally, slightly rostral to and just above LMAN (relative to the posterior bifurcation of the midsagittal sinus: rostral 5.4–5.7 mm; lateral 1.7 mm; and 1.0–1.5 mm deep from the dorsal surface of the brain in most birds). In a subset of birds, cannulae were placed lateral to LMAN and angled toward the midline. Experimental results were the same in this subset of birds. Our placement of guide cannulae was designed so that microdialysis probes were positioned just anterior of LMAN to minimize damage to the nucleus. The implanted cannulae, containing dummy probes, were glued to the skull using epoxy.
Reverse microdialysis
After ≥1 wk following surgery, dummy probes were replaced with CMA/7 microdialysis probes (membrane length: 1.0 mm). Prior to implantation, probes were attached to the microdialysis apparatus and prepared with artificial cerebrospinal fluid (ACSF, in mM, 145 NaCl, 2.7 KCl, 1.0 MgCl2, 2.4 CaCl2, and 2.0 Na2HPO4, pH 7.4). The microdialysis apparatus was modified from Sasaki et al. (2006). Syringes were filled with ACSF and attached to osmotic pumps (Pump 11 Pico Plus Syringe Pumps, Harvard Apparatus, Holliston, MA) located outside of the sound-attenuating chamber. Liquid passed from the syringes through FEP tubing (CMA) attached to liquid uniswitches (1 switch for each probe; Bioanalytical Systems, West Lafayette, IN), which allowed us to switch rapidly between ACSF and drug. Outflow from one channel of each switch went to a microdialysis swivel (375/D/22QM, Instech, Plymouth Meeting, PA) and from there to the microdialysis probe. The swivel allowed the bird to turn freely in his cage without tangling the tubing. Once probes were inserted into the cannulae, ACSF was continuously perfused into the microdialysis probes at a rate of 0.125–0.5 μl/min.
On the day of muscimol inactivation, an additional set of syringes was filled with 200 μM muscimol hydrobromide and connected to the second channel of each liquid switch. We chose muscimol, rather than a sodium channel blocker, so that the drug would not affect fibers of passage traveling through LMAN. From the time we flipped the switches to send muscimol bilaterally to both probes, it took muscimol ∼20 min to reach the brain. For social context experiments, muscimol dialysis began within an hour of the bird awakening, and continued for 3–8 h. At the end of this time, switches were flipped back to ACSF.
In addition to being able to observe recovery, another advantage of pharmacological inactivation is that the experiment can be repeated in the same animal to examine the consistency of a result (Martin and Ghez 1999). In two birds, we inactivated LMAN for a second time several days after the first muscimol dialysis.
Song recording
A B3 microphone (Countryman, Menlo Park, CA) was positioned in the soundbox above the male's cage to record the bird's song. Acoustic signals were digitized and recorded by custom software. During each experiment, the bird's behavior was continuously monitored and recorded by a 1-in square zoom lens color video camera (PalmVID Video Cameras, Colorado Springs, CO).
Undirected song was defined as song produced while the male was alone in his sound-attenuating chamber. Directed song was obtained when a female zebra finch, housed in her own cage, was placed next to the male. Only songs produced when the male was facing the female were counted as directed songs. Females were presented for no more than 5 min at a time with ≥5 min between presentations. If the bird did not sing for 2 min after the female was introduced, or stopped singing for >2 min, the female was removed. We limit the time of female presentation because we have observed waning of the difference in neural activity in LMAN between directed and undirected song in a subset of males over the course of longer presentations (unpublished data). Directed and undirected songs were collected in an interleaved manner, such that the next female presentation did not occur until the male sang undirected song.
After microdialysis probe insertion, songs were recorded for 1 to 2 days prior to inactivation of LMAN. Songs from the day immediately preceding the day of muscimol inactivation were analyzed as pre-muscimol data. Songs collected the day immediately following muscimol inactivation were analyzed as post-muscimol data. Although muscimol can be washed in and out relatively rapidly by reverse microdialysis, we wanted to minimize circadian variation in song quality and drive to sing (Deregnaucourt et al. 2005; Liu and Nottebohm 2005), so for social context experiments (n = 5 birds), we collected pre-muscimol, muscimol, and post-muscimol data on consecutive days during approximately the same time range. This was typically a period of several hours from mid-morning to mid-afternoon.
However, in a subset of birds, to illustrate the rapidity of LMAN inactivation effects on song variability, we recorded and analyzed song continuously during the transition from ACSF to muscimol. In one of the five birds also used in the social context experiments, we recorded an hour of undirected song before switching to muscimol. In a sixth bird, we collected pre-muscimol data for several hours and then switched to muscimol on the same day, and analyzed undirected song from this bird continuously through the time of transition from ACSF to muscimol. We dialyzed at a rate of 0.5 μl/min for the duration of this experiment. In addition, we analyzed the time course of change in pitch variability (data kindly provided by T. Warren and M. Brainard) from two additional birds (Bengalese finches) that underwent LMAN inactivation using the same muscimol reverse microdialysis method described here.
Song analysis
Three levels of organization are typically used to classify zebra finch song. “Syllables” are basic song elements separated by silent intervals ≥5 ms in duration. Syllables of birds used in this study ranged from 30 to 210 ms in duration. “Motifs” are stereotyped sequences of syllables with a duration often <1 s. “Bouts” are one or more motifs separated by silent intervals of ≥2 s (Sossinka and Bohner 1980). At the beginning of a bout, birds often sing a series of repeated introductory elements that we did not include as part of the bird's motif nor use for syllable structure analysis.
All directed song motifs were used for analysis. An equal number of undirected song motifs, produced prior to and following female presentation, were selected for analysis; all undirected songs analyzed were produced within an hour of female-directed song. We segmented song syllables using an amplitude-based segmentation algorithm in MATLAB (MathWorks, Natick, MA) and assigned identification labels to each syllable.
To measure differences in syllable structure, we calculated the fundamental frequency (FF) of all syllables having a stable frequency component. This was the case for 2–5 syllables/bird, ∼56% of all syllables produced. For each syllable, the experimenter selected a segment of the sound waveform with a clear, constant harmonic structure (range: 16–60 ms in duration) and calculated its FF as in Kao et al. (2005). Measurements of each example syllable were screened visually by the experimenter to ensure that the correct segment of the syllable was used for calculations and that no sound artifacts (such as bird hopping or flying) obscured the syllable being measured. An average of 78 renditions of a particular syllable was used to calculate the mean and variability of FF (range: 28–155). As observed in a previous study (Kao and Brainard 2006), we did not see a consistent difference in mean FF across social contexts nor did we observe a difference in mean FF between ACSF and muscimol dialysis (data not shown). We did see a change in the variability of FF, described by the SD of FF. To compare variability across syllables, variability was described as the coefficient of variation (CV = SD/mean).
Previous studies have found the variability of FF to be the most consistent measure of song structural differences between directed and undirected singing. However, the CV of FF is a cross-rendition measure. We also examined the entropy of individual syllables as a measure of within-syllable variability, using the features of spectral entropy and spectrotemporal entropy as defined in Sakata and Brainard (2006). Spectral entropy is the entropy of the Fourier transform of the entire syllable collapsed across time, normalized such that sounds with qualities similar to pure tones have values approaching 0 and sounds similar to white noise have values approaching 1. Spectrotemporal entropy is the entropy of the distribution of power in the time-frequency representation of the syllable (here calculated using nonoverlapping 8 ms Hanning windows). These two features were calculated for the same segments of syllables with stable harmonic structure as those used to calculate FF. In addition, we calculated entropy measures for syllables without stable harmonic structure (the remaining ∼44% of all syllables produced).
We also attempted to use a local similarity score (mean accuracy) from Sound Analysis Pro 1.04 (SAP) (Tchernichovski et al. 2000), which combines measurements of FM, Wiener entropy, pitch, goodness of pitch, and AM, to estimate the variability of syllable stereotypy in songs (as in Olveczky et al. 2005; Teramitsu and White 2006). We randomly chose 10 examples of each syllable in the directed condition and performed 45 pairwise comparisons to obtain a mean accuracy score. We followed the same procedure for undirected syllables. We did not find a social context-dependent difference in the mean accuracy score even in syllables that showed significant social modulation of FF variability. This lack of measurable difference in SAP's similarity score was consistent with other adult males in our colony (Woolley and Doupe 2008) and suggests that for adult birds, this measure is less sensitive than the measurements of FF and spectral entropy. We therefore did not include SAP analysis in results. Given the relative robustness of social context-dependent changes in frequency (see results), the insensitivity of the local similarity score in our adult birds may indicate that the effects of social context on pitch are “diluted out” in this measure by other song features that are either noisier or not social context-dependent.
We analyzed three other features of zebra finch song that are modulated by social context (Cooper and Goller 2006; Kao and Brainard 2006; Sossinka and Bohner 1980): song tempo, number of motifs per bout, and number of introductory notes. To measure song tempo, we calculated the durations of each bird's most common motif ≥5 syllables in length, from onset of the first syllable to offset of the last syllable. On average, ∼70 directed and 70 undirected motifs were analyzed for each treatment day (range: 37–138). Because there can be slowing of tempo when a bird sings a very long bout (Chi and Margoliash 2001; Cooper and Goller 2006), we also calculated mean motif durations using only the first motif of each bout. This did not affect our results. Consequently we reported only the data for all motifs in a bout. To determine the number of motifs/bout, we counted all motifs in which the bird sang at least half of his canonical motif. To calculate the number of introductory notes per bout, we counted backward from the first introductory note preceding the first motif of a bout until there was ≥500 ms of silence. One of the males did not have canonical introductory notes, so only four birds were used for analysis of these notes.
Statistics
Statistical analyses were performed using Instat 3.0b and Prism 5.0 for Macintosh (GraphPad Software, La Jolla, CA).
We use “context” to describe the social conditions (directed or undirected song), and “treatment” to describe the conditions of the dialysate (pre-muscimol, muscimol, and post-muscimol; although the dialysate for both pre- and post-muscimol treatments is ACSF, we analyzed the data separately in case muscimol had lasting effects on the brain). To compare data across birds, we calculated “per bird” scores for each measured variable (e.g., CV of FF, motif duration, etc.) in each context for each treatment. In the two birds in which we repeated LMAN inactivation, we observed the same experimental outcomes during both inactivations (see Fig. 3). Therefore we averaged the data from both experiments for these two birds to obtain their per bird scores. We then combined the scores from all five birds in each condition and compared these means using repeated measures two-way ANOVA. If a significant interaction was observed, we conducted post hoc paired two-tailed t-tests.
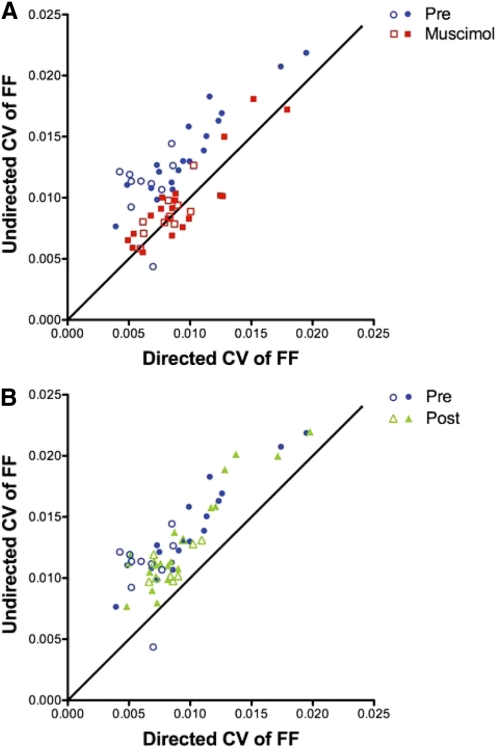
Fig. 3.
LMAN inactivation reversibly eliminates social context-dependent variability of syllable structure. Plots of variability of FF during undirected song vs. variability of FF during directed song. Each symbol represents 1 syllable recorded in both social contexts during 1 treatment condition. In 2 birds, we repeated the experiment several days after the 1st muscimol treatment. The syllables recorded in the 2nd experiment are indicated by open circles. The diagonal line represents equal variability in both social contexts. A: blue symbols represent the coefficient of variation (CV) of FF for 29 syllables recorded prior to drug. Variability was higher in the undirected context. In contrast, variability was similar in both social contexts during LMAN inactivation (red symbols: CV of FF for the same 29 syllables recorded in the muscimol condition). B: comparison of variability before muscimol to variability after muscimol washout. Green symbols representing CV of FF for the same 29 syllables post-muscimol indicate that variability of song structure was again higher in the undirected social context than in the directed.
Anatomy
Birds were deeply anesthetized with isoflurane before being transcardially perfused with 0.9% saline, followed by 3.7% formaldehyde in 0.025 M phosphate buffer (PB). Brains were postfixed for 4–24 h, then cryoprotected in 30% sucrose. Forty-micrometer coronal sections were cut on a freezing microtome. Every third section was stained with cresyl violet acetate to identify vocal control nuclei. To estimate any possible damage to LMAN due to cannula or probe implantation, we compared the area of LMAN in experimental birds to the area of LMAN in noncannulated control birds. To calculate the area of LMAN, we outlined the Nissl-stained area of the nucleus in photomicrographs imported into Image J (available at http://rsb.info.nih.gov/ij; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD) for all sections containing LMAN on each side from each bird, added the calculated areas together, and multiplied by 3. We then normalized this number by dividing by the length of the lamina pallio-subpallialis in the most caudal LMAN-containing section. The normalized area for each experimental side was divided by the mean normalized control LMAN area (calculated from 6 LMAN areas in 3 control birds) to give a ratio of experimental LMAN area: control LMAN area. The largest individual LMAN lesion observed was 27%. Overall, the ratio of experimental LMAN area: control LMAN area was 1.02 (Table 1). Thus microdialysis probes did not substantially damage LMAN in any experimental subject.
Table 1.
Normalized LMAN areas in experimental birds compared to noncannulated birds
| Normalized Area |
Ratio (Experimental/Control) |
|||
|---|---|---|---|---|
| Bird | Left | Right | Left | Right |
| Control 1 | 0.51 | 0.47 | ||
| Control 2 | 0.65 | 0.48 | ||
| Control 3 | 0.56 | 0.56 | ||
| Experiment 1 | 0.45 | 0.53 | 0.97 | 1.13 |
| Experiment 2 | 0.52 | 0.40 | 1.10 | 0.84 |
| Experiment 3 | 0.41 | 0.40 | 0.88 | 0.85 |
| Experiment 4 | 0.77 | 0.63 | 1.64 | 1.34 |
| Experiment 5 | 0.39 | 0.34 | 0.83 | 0.73 |
| Experiment 6 | 0.40 | 0.48 | 0.86 | 1.03 |
Controls are birds that did not undergo cannulae implantation. Experimental birds are the six experimental birds described in results, implanted with cannulae and probes that correctly targeted lateral magnocellular nucleus (LMAN). Values for normalized area were obtained by calculating the area of LMAN (mm2) from Nissl sections (see methods) and dividing by the length of the lamina pallio-subpallialis (mm). Ratios of normalized LMAN area for experimental birds were calculated by dividing the normalized area of each side by the mean control area. Mean control area was 0.47, mean experimental area was 0.48, and mean ratio was 1.02.
In six birds, biotinylated muscimol was reverse dialyzed into the brain for 3–6 h prior to perfusion to estimate muscimol spread. Biotinylated muscimol was made using an EZ-Link Sulfo-NHS-LC-Biotin kit (Pierce, Rockford, IL) and diluted to 200 μM in ACSF. As detailed in the preceding text, one-third of the brain sections were used for cresyl violet staining alone; the intervening two sets of sections were used to visualize spread of the biotinylated muscimol. These sets were incubated in ABC complex followed by 3′3-diaminobenzidine tetrahydrochloride (DAB) with nickel intensification (Carrillo and Doupe 2004). One set of sections stained with both DAB and cresyl violet was used for camera lucida tracing of LMAN and Area X and for an estimate of muscimol spread. A representative tracing showing DAB staining for biotinylated muscimol spread is shown in Fig. 1B.
In two of the eight birds, probe placement and staining for biotinylated muscimol suggested that probes were mistargeted such that muscimol spread was not inside LMAN but was in other areas of the brain. We refer to these birds as “sham controls” in the text.
We observed biotinylated muscimol staining in Area X in two of the six birds with probes properly targeted to LMAN. In these two birds, no more than 33% of Area X was stained on any given side, and bilaterally no more than 25% of Area X was covered by biotinylated muscimol. Area X lesions have to be extensive bilaterally to alter song or affect song plasticity (Kojima and Doupe 2007; Scharff and Nottebohm 1991; Sohrabji et al. 1990). Furthermore, in one of the sham controls, we did not observe staining for biotinylated muscimol in LMAN, but we did observe staining bilaterally over 55% of Area X. We did not observe changes in song variability during muscimol dialysis in this bird (see results). Thus it seems likely that our inactivation of virtually all of LMAN is the cause of any song changes we observed, rather than inactivation of a small part of its upstream nucleus Area X.
RESULTS
LMAN inactivation abolishes social context-dependent differences in variability of syllable structure
To investigate the acute influence of LMAN activity on social modulation of song variability, we measured the variability of FF while transiently inactivating LMAN by reverse microdialysis of muscimol. The variability of FF of individual song syllables is a robust measure of song structural variability. In both zebra finches and Bengalese finches, the variability of FF significantly decreases when a male sings to a female compared with when the male sings alone (Kao and Brainard 2006; Kao et al. 2005; Sakata et al. 2008). To determine if LMAN inactivation affects the social modulation of syllable structure, we measured the FF of syllables that had a constant frequency component with clear harmonic structure, before, during, and after muscimol dialysis. Representative syllables with constant frequency components of different durations, recorded from two different birds, are shown in Fig. 2, A and B. As in unmanipulated birds, during reverse microdialysis of vehicle (“pre”), the SD of FF was significantly higher for undirected song than directed song. However, during reverse microdialysis of muscimol into LMAN, the variability of FF during undirected song dropped to the level of variability during directed song, such that the difference was no longer significant (Fig. 2, A and B, “muscimol”). For example, in the syllable shown in Fig. 2A, muscimol dialysis led to a highly significant 47% decrease in the SD for undirected song (preundir vs. muscimolundir, P = 0.0006, F test for equality of variance) but a nonsignificant 9% increase in SD for directed song (predir vs. muscimoldir, P = 0.6642, F test). After washout of muscimol, the SD of FF for undirected song increased back up to pre-muscimol levels (preundir vs. postundir, P = 0.9803, F test for equality of variance), and the social context-dependent difference in the variability of FF returned. The effect of muscimol dialysis was observed consistently across syllables. In 26/29 syllables examined from five birds, undirected song was significantly more variable than directed song prior to LMAN inactivation (F test for each syllable, data not shown). In contrast, during reverse microdialysis of muscimol, only 2/29 syllables retained a significant difference in the variability of FF. The day following LMAN inactivation, 21/29 syllables were significantly more variable in the undirected context. The drop in number of syllables with directed-undirected differences in SD of FF during muscimol dialysis was not simply due to cannula or probe implantation, as the pre-muscimol data were collected with probes already in place, and the number of syllables with social context-dependent differences in FF was comparable before and after probe implantation (Table 2). These data indicate that, like LMAN lesions, acute inactivation of LMAN eliminates the social modulation of the variability of FF.
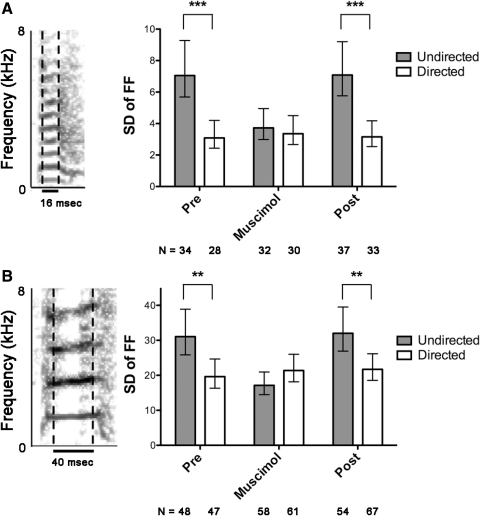
Fig. 2.
A, left: a representative spectrogram (frequency vs. time) of a song syllable sung many times during 1 experiment. Black dashed lines indicate the short portion of this syllable used for FF measurement. Right: a plot showing SDs of fundamental frequency (FF) of this syllable measured in both social contexts, before (“pre”), during (“muscimol”) and after (“post”) LMAN inactivation. Error bars indicate 95% CI of the SD. Variability of FF was much higher during directed song than undirected song in the pre-muscimol condition (P < 0.0001). During LMAN inactivation, the SD of FF during undirected song decreased dramatically, so that it equaled that of directed song (P = 0.5723). Post-muscimol, the difference in variability of FF returned (P < 0.0001). B: syllable from a different bird with a different FF and a longer duration constant-frequency portion. In the pre- and post-muscimol treatment conditions, the SD of FF was significantly higher during undirected song (P = 0.0023 and P = 0.0029, respectively). During LMAN inactivation, SD of FF was similar in both contexts (P = 0.0933). All preceding statistics are F test for equality of variance. ***P < 0.0001, **P < 0.005. Mean FF measurements (not shown) for syllable A: preundir = 637.9; predir = 634.3; muscimolundir = 631.1; muscimoldir = 631.6; postundir = 630.8; postdir = 631.9. For syllable B: preundir = 1,698.2; predir = 1,692.9; muscimolundir = 1,690.3; muscimoldir = 1,684.3; postundir = 1,695.2; postdir = 1,691.8. ANOVA followed by post hoc paired t-test did not find significant social modulation of mean FF for either syllable.
Table 2.
Proportion of syllables with significant dir-undir difference in SD of FF
| Bird | Before Surgery | After Surgery |
|---|---|---|
| Experiment 1 | n/a | Day 1–2/2 |
| Experiment 2 | Day 1–3/3 | Day 1–2/3 |
| Day 2–2/3 | ||
| Experiment 3 | Day 1–4/4 | Day 1–4/4 |
| Day 2–2/4 | ||
| Experiment 4 | Day 1–3/5 | Day 1–4/5 |
| Day 2–5/5 | Day 2–4/5 | |
| Experiment 5 | Day 1–3/5 | Day 1–5/5 |
| Day 2–5/5 | Day 2–5/5 |
SD of FF was calculated for each of the birds' harmonic stacks during directed and undirected singing as described in methods. A significant directed-undirected difference (dir-undir) in SD of fundamental frequency (FF) was defined as P < 0.05 for the F-test for equality of variance. Syllables were measured on two different days prior to surgery, and one or two different days after the implantation of cannulae and probes, during artificial cerebrospinal perfusion.
We also analyzed the variability of FF in two birds with cannulae that did not accurately target LMAN. In one bird, one of the probes was lateral to LMAN such that only ∼10% of LMAN on that side of the brain stained for biotinylated muscimol. Of the three syllables in this bird that had social-context dependent differences in variability of FF prior to drug, all three retained their differences during reverse microdialysis of muscimol. A second bird had cannulae placed such that probes were below LMAN. Biotinylated muscimol spread to ∼55% of Area X in both hemispheres but did not spread to LMAN. In this bird, five of eight syllables analyzed demonstrated social modulation of the variability of FF during ACSF dialysis, and four of these five syllables continued to be socially modulated during reverse microdialysis of muscimol. Thus the loss of syllable structure modulation by social context is specifically associated with infusion of muscimol into LMAN.
To compare changes in the variability of FF across syllables that have different mean FFs, we calculated the coefficient of variation (CV = SD/mean) of FF for all 29 syllables measured in seven experiments from the five experimental birds. Each blue symbol in Fig. 3A represents the variability in the FF of one syllable measured in the pre-muscimol condition, plotted as the CV of FF during undirected song versus the CV of FF during directed song. Before drug, all but one of the syllables lie above the diagonal, indicating that syllable structure was more variable in the undirected condition. The ratio of the variability of undirected song to the variability of directed song across the population of syllables was 1.58 ± 0.08 (P < 0.0001, Wilcoxon signed-rank test). However, when we removed LMAN activity via muscimol reverse microdialysis, syllables were evenly distributed around the diagonal (red symbols in Fig. 3A). The ratio of the variability of undirected song to the variability of directed song became ∼1 (mean ± SE: 1.06 ± 0.03; P = 0.0689, Wilcoxon signed-rank test). This attenuation of social modulation of syllable structure observed during LMAN inactivation was consistent with the effect of LMAN lesions on syllable structure variability (compare Fig. 4B in Kao and Brainard 2006). Unlike the lesion experiment, however, we were able to allow LMAN activity to return. Post-muscimol, syllables shifted back above the diagonal (1.42 ± 0.05; P < 0.0001, Wilcoxon signed-rank test), with a distribution statistically indistinguishable from that of the pre-muscimol condition (Fig. 3B), indicating that undirected song “recovered” its variability after drug washout. Therefore ongoing LMAN neural activity is necessary for the increased variability of syllable structure observed during undirected song.
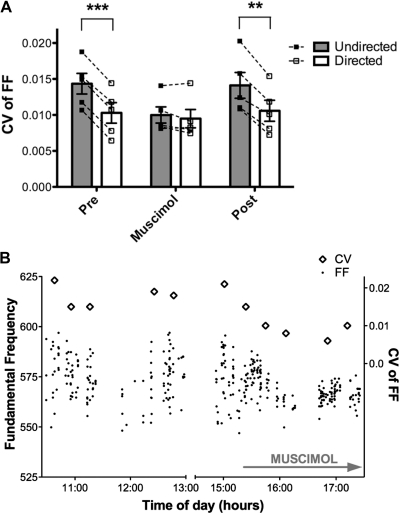
Fig. 4.
A: variability of syllable structure measured by experiment. The CV of FF for all syllables measured in each individual bird, in each condition, were averaged to give a “per bird” score (squares; dotted lines connect undirected and directed data points for each bird). Bars indicate the mean per bird score for each condition (n = 5 birds; error bars indicate ± SE). Variability of syllable structure was higher during undirected song than directed song both pre- and post-muscimol (pre: P < 0.0001; post: P = 0.0010; post hoc paired t-test). Social modulation of the variability of syllable structure was eliminated by LMAN inactivation (P = 0.4620). ***P < 0.0001, **P < 0.005. B: black dots represent the FF of 1 syllable measured during undirected song over several hours. Between 10:30 and 15:30, FF ranged over 50 Hz. However, after muscimol entered the microdialysis probes (∼15:20), the range of FF narrowed within 30 min and remained tightly distributed throughout muscimol dialysis (indicated by arrow). Diamonds indicate the CV of FF calculated at 20 min intervals. During the 2nd 20 min interval after LMAN inactivation, the CV was lower than during any previous interval that day. When data were combined across the whole treatment period, the overall CV pre-muscimol was 0.019 (n = 183), while during muscimol it was 0.011 (P < 0.0001). For comparison, the CV of directed song in this bird pre-muscimol was 0.011. The break in the x axis indicates a period during which the bird did not sing undirected song.
We also examined the data grouped by bird (Fig. 4A; n = 5 birds, 2–5 syllables measured in each bird; see methods). We observed a significant interaction between treatment and context (P < 0.0001; F = 32.88); that is, social context-dependent modulation of the variability of FF was different across treatment groups. Post hoc paired t-test revealed a significantly higher mean CV of FF across experiments in the undirected condition than in the directed condition during dialysis of ACSF. This was true both pre- (mean CV ± SE: undir = 0.0145 ± 0.0014; dir = 0.0106 ± 0.0013; P < 0.0001) and post-muscimol (undir = 0.0140 ± 0.0018; dir = 0.0103 ± 0.0016; P = 0.0010). However, during LMAN inactivation, there was no social context modulation of the variability of FF across experiments (undir = 0.0099 ± 0.0012; dir = 0.0095 ± 0.0013, P = 0.4620). This was due to a 32% decrease in undirected song variability during muscimol dialysis (preundir vs. muscimolundir, P = 0.0361). In contrast, directed song variability of FF only decreased 10% during muscimol dialysis (predir vs. muscimoldir, P = 0.3110). Consequently, analysis on a per bird basis supports the analysis on a per syllable basis. These measurements indicate that the loss of social modulation of variability of FF was highly consistent across experiments in birds with different songs.
In the preceding birds, we analyzed song for social context-dependent changes in variability of FF beginning 1–1.5 h after muscimol reached the brain, based on data from muscimol injections into LMAN in which decreased syllable variability was observed in juvenile birds ∼50 min after TTX or muscimol injections (Olveczky et al. 2005). To verify the rapidity with which LMAN inactivation could reduce the variability of FF in our reverse microdialysis system, we continuously recorded undirected song in a subset of our birds before and during the switch from ACSF to muscimol, over the course of a few hours in 1 day (Fig. 4B). We observed a sharp decrease in variability of FF within 20–40 min of muscimol reaching the dialysis probes. We calculated the CV of FF for all 20 min intervals in which there were ≥20 motifs of song produced. For the example shown, the CV of FF was 0.018 and 0.021 in the final two intervals prior to LMAN inactivation. In the first 20 min interval after muscimol reached the probes, the CV of FF had decreased to 0.015. In the next 20 min, the CV dropped further, to 0.010, and remained at or below this level for the duration of muscimol dialysis. We compared the variability between intervals using the F test for equality of variance (Table 3) and found that of five syllables measured from two birds, the variability of FF significantly decreased for 2/5 syllables within the first 20 min after muscimol reached the brain and 3/5 syllables within 40 min. Additionally, we analyzed data from similar muscimol inactivations of LMAN performed in Bengalese finches and found that 3/4 syllables measured from two birds showed significantly decreased variability of FF within a window 20–40 min after inactivation (T. Warren and M. S. Brainard, unpublished data). We conclude that reverse microdialysis of muscimol quickly (within minutes or tens of minutes) inactivates LMAN, thereby causing a rapid decrease in variability of undirected song.
Table 3.
P values for comparisons of SD of FF in 20-min intervals before and after switch to muscimol
| Pre1 vs. Pre2 | Pre2 vs. Mus1 | Pre2 vs. Mus2 | |
|---|---|---|---|
| Experiment 1 | |||
| Syllable 1 | 0.4685 | 0.6930 | 0.0045 |
| Syllable 2 | 0.9231 | 0.3499 | 0.0312 |
| Experiment 6 | |||
| *Syllable 1 | 0.3426 | 0.0303 | 0.0001 |
| Syllable 2 | 0.7420 | 0.0030 | 0.0269 |
| Syllable 3 | 0.8554 | 0.2119 | 0.0274 |
The SD of FF was calculated in 20-min intervals for 5 syllables from 2 experimental birds (experiments 1 and 6). The SDs for each syllable were then compared for the two time windows immediately preceding the switch to muscimol (Pre1 vs. Pre2), and for the window immediately preceding the switch to muscimol (Pre2) vs. the first 20-min window after muscimol reaches the brain (Mus1) and the second muscimol window (Mus2). Bold indicates a significant difference between two intervals.
, the syllable shown in Fig. 4B.
LMAN inactivation abolishes social context-dependent differences in spectral entropy
Although the variability of FF is a robust measure of social context-dependent behavioral variability, it must be calculated across renditions of song. We were also interested in measures of variability that could be calculated for single renditions of a syllable, as any socially modulated changes in such measures would of necessity reflect effects of LMAN activity on a millisecond time scale (during the course of a single syllable); we then also could ask whether these measures of variability were affected by LMAN inactivation. Therefore we calculated the spectral entropy of syllables produced during directed and undirected song (Sakata and Brainard 2006). Spectral entropy describes the “noisiness” of a particular syllable normalized so that a pure tone would have a value of 0 and white noise a value of 1. Initially we examined only syllables with a constant frequency component and clear harmonic structure as we did for FF. Consistent with social context effects on a moment-by-moment time scale, the spectral entropy of these syllables was higher when males sang alone compared with when they sang to a female (Fig. 5A; blue circles) so that the ratio of spectral entropy of undirected song: directed song across the population of syllables was significantly >1 (P < 0.0326, Wilcoxon signed-rank test; n = 29). When examined individually, 21/29 syllables had lower spectral entropy during directed song, and the difference reached significance in 8/29 syllables. This is the first report of social modulation of spectral entropy.
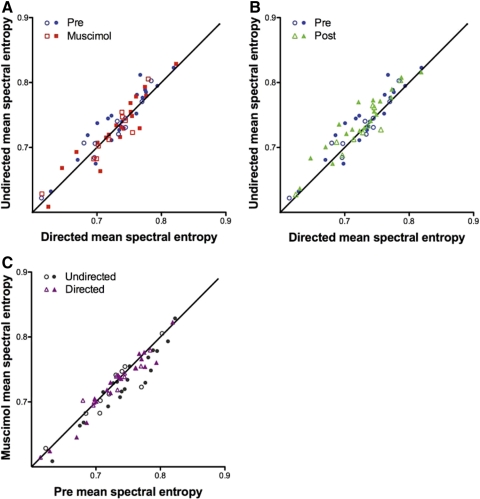
Fig. 5.
LMAN inactivation reversibly eliminates social context-dependent spectral entropy. A and B: plots of mean spectral entropy during undirected song vs. mean spectral entropy during directed song. Each symbol represents 1 syllable recorded in both social contexts during 1 treatment condition. In 2 birds, we repeated the experiment several days after the 1st muscimol treatment. The syllables recorded in the 2nd experiment are indicated by open circles. The diagonal line represents equal spectral entropy in both social contexts. A: blue symbols represent mean spectral entropy for 29 syllables recorded prior to muscimol. Spectral entropy was higher in the undirected context. In contrast, entropy was similar in both social contexts during LMAN inactivation (red symbols: mean spectral entropy for the same 29 syllables, recorded during muscimol). B: comparison of spectral entropy before muscimol to spectral entropy after muscimol washout. Green symbols representing spectral entropy for the same 29 syllables post-muscimol indicate that entropy of syllable structure was again higher in the undirected social context than in the directed. C: plot of mean spectral entropy during muscimol vs. mean spectral entropy pre-muscimol. During directed song, syllables have approximately the same spectral entropy with and without drug, as shown by the even distribution of purple symbols along the diagonal (P = 0.1689, Wilcoxon signed-rank test, n = 29). However, during undirected song, spectral entropy is lower in syllables produced during LMAN inactivation (gray symbols), and the ratio of muscimol: pre-muscimol spectral entropy is significantly <1 (P = 0.0010).
Moreover reverse microdialysis of muscimol eliminated the difference in spectral entropy between directed and undirected song (Fig. 5A; red squares; P = 0.9830). This was due to a noticeable decrease in the spectral entropy of undirected song during muscimol treatment (Fig. 5C), while the spectral entropy of syllables produced during directed song remained constant during LMAN inactivation. Following muscimol washout, the social-context dependent difference in spectral entropy returned (Fig. 5B, green triangles), such that the ratio of undirected: directed spectral entropy was >1 (P = 0.0010).
Spectral entropy can also be calculated for syllables that are not harmonic stacks. However, we did not find a social context-dependent difference in such nonharmonic syllables (P = 0.8596, Wilcoxon signed-rank test, n = 20). Additionally, we did not see a social context-dependent difference, nor a muscimol-dependent decrease, in the spectrotemporal entropy of syllables of either type (data not shown). The difference in results between spectral entropy and spectrotemporal entropy may be explained by the difference in time scales over which the spectral power is calculated for these two measures (see discussion).
LMAN inactivation does not abolish social context-dependent differences in song tempo or other higher-level features of song
The social context in which a bird sings can affect several characteristics of song at the motif or bout level, including higher-level, supra-syllabic features of song such as the number of introductory notes per bout of song, the number of motifs per bout of song, and the song tempo (Cooper and Goller 2006; Kao and Brainard 2006; Sakata et al. 2008; Sossinka and Bohner 1980). We examined these three features of song during LMAN inactivation.
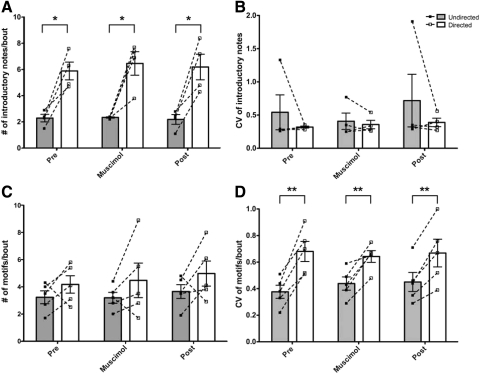
As previously reported (Cooper and Goller 2006; Kao and Brainard 2006; Sakata et al. 2008; Sossinka and Bohner 1980), the number of introductory notes produced per bout of song was robustly modulated by social context in our experiments (Fig. 6A). Prior to LMAN inactivation, birds produced on average twice as many introductory notes per bout during directed song as undirected song (mean ± SE: dir = 5.88 ± 0.68; undir = 2.27 ± 0.29; P = 0.0146; n = 4). This was also true during LMAN inactivation (dir = 6.45 ± 0.90; undir = 2.33 ± 0.05; P = 0.0191) and following washout of muscimol (dir = 6.18 ± 0.98; undir = 2.18 ± 0.37; P = 0.0193). When we compared the data across all conditions by a repeated measures two-way ANOVA, there was no significant interaction between treatment and context (P = 0.8971; F = 0.11); that is, social context-dependent modulation of introductory note number was consistent before, during, and after LMAN inactivation. Because social context could also affect the variance rather than the mean features of song, as it does for FF, we examined the variability of the number of introductory notes per bout of song (Fig. 6B). Across birds, there was no effect of social context on the CV of introductory notes per bout prior to muscimol treatment (dir = 0.32 ± 0.01; undir = 0.54 ± 0.26; P = 0.4438; n = 4), or during muscimol treatment (dir = 0.36 ± 0.06; undir = 0.41 ± 0.12; P = 0.5305). In summary, birds produced more introductory notes per bout during directed song, but the variability in the number of introductory notes per bout was unaffected by social context. LMAN inactivation did not affect either aspect of the production of introductory notes.
Fig. 6.
LMAN inactivation did not affect the social modulation of several supra-syllabic features of song. A: number of introductory notes per bout. Solid squares indicate the number of introductory notes per bout during undirected song for 1 bird, and open squares indicate the number during directed song. For A–D, dotted lines connect the data points for each bird, and bars indicate the mean group number for each condition (±SE). The number of introductory notes produced per bout was always higher during directed song even during muscimol treatment. B: CV of introductory notes per bout. In 3 of 4 birds, there was no difference in CV between directed and undirected song, and across birds there was no effect of LMAN inactivation on the variability in the number of introductory notes produced per bout of song (P = 0.7327, repeated measures 2-way ANOVA). C: number of motifs per bout. Solid squares indicate the number of motifs per bout during undirected song for 1 bird, and open squares indicate the number during directed song. Four of 5 birds sang more motifs per bout during directed song than during undirected song. One bird sang more motifs per bout in the undirected context. Neither pattern changed during LMAN inactivation. D: CV of the number of motifs per bout. The number of motifs produced per bout of song was more variable during directed song, and this remained true during muscimol treatment. **P < 0.01, *P < 0.05.
In addition to singing more introductory notes during directed song, zebra finches often sing more motifs per bout of song during directed song (Kao and Brainard 2006; Sossinka and Bohner 1980). This proved true in four of our five birds (Fig. 6C). When data from all five birds were combined, males sang approximately one more motif per bout in the directed context than when singing alone prior to LMAN inactivation (dir = 4.18 ± 0.64; undir = 3.24 ± 0.47). This trend continued during LMAN inactivation and following muscimol washout (muscimol: dir = 4.48 ± 0.127; undir = 3.2 ± 0.40; post: dir = 4.98 ± 0.92; undir 3.66 ± 0.51). We did not find a significant interaction between treatment and context (P = 0.9465; F = 0.06, repeated measures 2-way ANOVA), indicating that LMAN inactivation did not affect social modulation of number of motifs per bout.
We also found that the variability in the number of motifs produced per bout of song was affected by social context (Fig. 6D): prior to LMAN inactivation, the CV of motifs per bout was significantly higher during directed song than undirected song (dir = 0.68 ± 0.08; undir = 0.38 ± 0.05; P = 0.0032). However, this variability in the number of motifs per bout was unaffected by LMAN inactivation (P = 0.2381; F = 1.621, repeated measures 2-way ANOVA, n = 5).
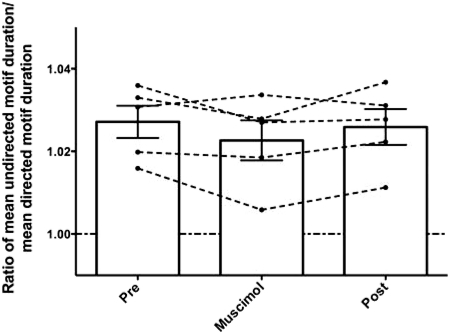
Finches generally sing at a slower rate when they sing alone than when they sing to females (Cooper and Goller 2006; Sakata et al. 2008; Sossinka and Bohner 1980). In zebra finches, it has been reported that the social modulation of song tempo is eliminated following LMAN lesions (Kao and Brainard 2006). On the day preceding LMAN inactivation, we found that song tempo was significantly slower when our birds were singing alone compared with when they were singing to females (2.7 ± 0.4% slower; range: 1.6–3.6%). This was a significant effect of social context on tempo (ratio of undirected:directed song tempo significantly different from 1; P = 0.0313, 1-tailed Wilcoxon signed-rank test). Surprisingly, unlike LMAN lesions in zebra finches, short-term LMAN inactivation did not speed up undirected song to the level of directed song (Fig. 7). During LMAN inactivation, song tempo remained significantly slower during undirected song (2.3 ± 0.5% slower; P = 0.0313, 1-tailed Wilcoxon signed-rank test). Taken together, the results indicate that LMAN activity is not necessary for socially driven modulation of higher-level features of song, including song tempo, and the number and variance of introductory notes and motifs produced per bout of song.
Fig. 7.
LMAN inactivation did not affect song tempo. ●, the ratio of mean undirected motif duration to the mean directed motif duration for 1 bird; …, connecting data points for the same bird during different treatments. A data point above 1 indicates that undirected song tempo is slower than directed song tempo. Bars indicate group means; error bars indicate SE. There are no differences in the means for each treatment (n = 5 birds; P = 0.2297, repeated measures ANOVA).
DISCUSSION
By transiently silencing LMAN in awake, behaving birds, we demonstrated that ongoing neural activity in LMAN is necessary for social modulation of the variability of acoustic structure in zebra finch song. We observed that during reverse microdialysis of muscimol into LMAN the variability of syllable structure of undirected song rapidly dropped to the level of variability of directed song. Undirected song variability recovered following muscimol washout. In contrast, neural activity in LMAN was not necessary for social context-dependent changes in bout structure or in song tempo.
The loss of social modulation of the variability of acoustic structure seen here reproduces, on a short time scale, the effect of bilateral LMAN lesions (Hampton et al. 2009; Kao and Brainard 2006; Kao et al. 2005). Both lesions and inactivations eliminate glutamatergic transmission from LMAN to its downstream target nucleus, RA. With lesions, however, it is not clear if factors besides loss of synaptic input to RA cause the attenuation of variability. Other possible mechanisms by which lesions could affect vocal motor control include general damage from the lesion, loss of neurotrophic factors produced by LMAN, or plasticity induced by the lesion. In very young birds (20 days of age), LMAN lesions cause neuronal apoptosis in the premotor nucleus RA (Akutagawa and Konishi 1994; Johnson et al. 1997), which can be prevented by injections of neurotrophins into RA (Johnson et al. 1997). In slightly older birds (40–50 days of age), loss of LMAN does not cause cell death but elicits rapid (4–7 days) changes in RA dendritic morphology and soma size and strengthens synaptic connections from HVC to RA (Kittelberger and Mooney 1999). In our experiments, we observed the return of social context-dependent variability, both of FF across syllable renditions, and of frequency structure within individual syllables, after muscimol washout. Thus the loss of variability during muscimol dialysis could not be due to permanent damage to the brain or structural modifications in RA. If neurotrophin release in RA is strictly activity-dependent, it would also have been abolished during our inactivation of LMAN projection neurons. It therefore remains possible that acute effects of decreased neurotrophin release play a role in the loss of variability that we observed (Kittelberger and Mooney 2005). However, some neurotrophins, including BDNF, can still be secreted in the absence of neural activity (Aicardi et al. 2004). Moreover, because we observed substantial effects on song within 20–40 min of LMAN inactivation, we are confident that long term plasticity in RA in response to a loss of input from LMAN was not responsible for decreasing the variability of undirected song.
We report here for the first time that spectral entropy can also be used to differentiate undirected song from female-directed song. The frequency structure of individual syllables is, on average, less entropic when males sing to females than when males sing alone. Additionally, LMAN inactivation decreases the spectral entropy of undirected song to the level of directed song. This provides evidence that LMAN activity is not only necessary for song variability between renditions of song but also for variability of vocal production within a single syllable. A decrease in spectral entropy was not observed following LMAN lesions in Bengalese finches (Hampton et al. 2009). This contrast in results may be an example of different outcomes between lesion and inactivation experiments. Alternatively, spectral entropy may be less influenced by LMAN in Bengalese finches, or a possible result could have been masked, if all types of syllables were analyzed together. In zebra finches, we only observed an effect in harmonic stacks, consistent with the fine-grained nature of LMAN-driven changes, which are most easily detected in syllables of simple frequency structure. Visual inspection of “noisier,” more complex syllables suggested that tonal components embedded within the noise of those syllables could also become less variable during directed song; however, such changes were not detected by our measure of spectral entropy. Our measure may obscure changes in more complex syllables because it is in effect calculating a mean entropy across many types of sounds within the syllable, each with different baseline entropies.
In contrast to undirected song, the spectral entropy of directed song remained constant during LMAN inactivation, as did the variability of fundamental frequency across trials. These data suggest that there is either some limit to the amount of control the bird has over variability in his vocal production or that there are other sources of variability outside of LMAN.
Interestingly, we did not observe social context modulation of spectrotemporal entropy nor a change in spectrotemporal entropy during LMAN inactivation. In contrast to spectral entropy, in which entropy of the power in each frequency is calculated for the whole syllable collapsed across time, spectrotemporal entropy is the entropy of the distribution of power calculated for each time point of the time-frequency representation of the syllable. If a frequency band became significantly more noisy (i.e., randomly varying) or had a broader frequency during undirected song, we would expect to see a difference in spectrotemporal entropy as well as spectral entropy. However, if syllables became consistently more “wavery”—that is, if they showed slower, smoother variation in frequency than that seen with noise, on a time scale closer to or longer than the spectrographic time window used to measure spectrotemporal entropy—the distribution of the measurements of power within many such small time-frequency windows would not necessarily show an increase in entropy. In contrast, measurements of power in frequencies across the whole syllable still would. Thus it seems possible, based on the difference in results between spectral entropy and spectrotemporal entropy, that LMAN contributes to the ongoing waveriness of syllable structure during undirected song.
Inactivation of LMAN did not affect social modulation of the number of introductory notes or the number of motifs per bout. This finding was consistent with the results from LMAN lesions in which changes in song sequence and higher-order features due to social context remained after lesion (Hampton et al. 2009; Kao and Brainard 2006). The negative findings in lesion experiments, however, could have reflected compensation for the loss of LMAN in the days following lesions. We also found that the variability of the number of introductory notes and number of motifs per bout were not affected by LMAN inactivation. Consequently, our results from acute inactivations provide important additional evidence that other brain regions modulate aspects of courtship song. Changes in higher order levels of song structure are likely to be regulated upstream in the premotor pathway, possibly by HVC or its inputs (Foster and Bottjer 2001; Horita et al. 2008; Thompson and Johnson 2007; Vu et al. 1994). Neuromodulatory input directly into HVC, RA, or elsewhere may signal the presence of a female and thus cause changes in overall bout structure (reviewed in Ball et al. 2003; Hara et al. 2007; Heimovics et al. 2009; Riters and Alger 2004; Yanagihara and Hessler 2006).
Strikingly, however, a prominent effect of LMAN lesions was not reproduced in our experiments. Social modulation of song tempo was not dependent on ongoing LMAN activity. This result contrasts with a previous lesion experiment, which found that four of five zebra finches no longer had social context-dependent differences in song tempo 1 wk after LMAN lesions (Kao and Brainard 2006). In the fifth LMAN-lesioned bird, undirected song became significantly faster than directed song (Kao and Brainard 2006). This might be an early emergence of a known long-term effect of LMAN lesions, which is a gradual acceleration of undirected song, well beyond the normal range of tempo variability, that occurs over days to weeks after lesions (Brainard and Doupe 2001; Williams and Mehta 1999). These studies strongly suggested a contribution of LMAN to temporal aspects of song regulation. As in our inactivation experiment, however, a lesion study in the Bengalese finch found no change in song tempo, and no loss of socially modulated differences in song tempo, 2 wk after LMAN lesions (Hampton et al. 2009). Song tempo and context-dependent changes in tempo may be under more complex regulation by many brain regions as well as by long-term trophic control. For example, HVC exhibits rhythmic, patterned activity (Hahnloser et al. 2002; Solis and Perkel 2005), precisely time-locked to song in the awake bird, and cooling of HVC slows song tempo, consistent with an important role for this nucleus in the commands for song timing (Long and Fee 2008). However, because HVC is part of numerous feedback loops, with direct and indirect connections from RA and brain stem nuclei, these structures may also affect song timing (Ashmore et al. 2005, 2008; Roberts et al. 2008; Striedter and Vu 1998). An example of the complexity of tempo is that syllable durations may be controlled by different timing mechanisms than intersyllable interval durations (Cooper and Goller 2006; Glaze and Troyer 2006); for example, expiratory events (associated with syllable production) vary in duration between directed and undirected song, whereas inspiratory events (often associated with silent intervals) do not.
Models of motor and reinforcement learning have long stressed the importance of variation for learning (e.g., Sutton and Barto 1998). Studies in songbirds have been important in suggesting a possible neural source for such variability: lesion and inactivation studies of the AFP show that this specialized cortical-basal ganglia circuit can actively provide motor variability critical for learning (Andalman and Fee 2009; Bottjer et al. 1984; Kao et al. 2005, 2008; Olveczky et al. 2005; Scharff and Nottebohm 1991). In adult birds, AFP-dependent motor variability is much lower than in young birds learning song for the first time but can still be used to enable adult song plasticity (Andalman and Fee 2009; Tumer and Brainard 2007). Moreover, adult birds can alter the degree of neural and vocal variability: LMAN neural activity decreases, loses its prominent burst firing, and becomes more precisely timed with respect to the song motif when birds are “performing” for a female, and at the same time, the song becomes much more stereotyped (Hampton et al. 2009; Hessler and Doupe 1999b; Kao and Brainard 2006; Kao et al. 2005, 2008). Although it remains unclear whether neural variability arises within LMAN itself, or is transmitted to LMAN from other brain areas, the data presented here, using acute LMAN inactivation, indicate that LMAN firing during undirected song is required for context-sensitive song variability.
Notably, the variability of song with LMAN inactivated is not different from that during directed singing. Because LMAN is not completely silenced during directed singing in the unmanipulated bird, more subtle manipulations of activity in LMAN and elsewhere have the potential to reveal which neural signals in the cortical-basal ganglia circuit are important for modulating the variability in song and how the brain generates these. This in turn should give us insights into the neural signals necessary for song learning and plasticity and their regulation by social cues.
GRANTS
This work was supported by National Institute of Mental Health Grants MH-78824 and MH-55987 to A. J. Doupe and F32 MH-76467 to L. Stepanek, and grants from National Alliance for Research on Schizophrenia and Depression, the National Parkinson Foundation, and the Sandler Family Supporting Fund to A. J. Doupe.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank A. Arteseros and G. Carrillo for technical assistance, T. Warren for Bengalese finch data, and M. Brainard, M. Kao, J. Sakata, and O. Tchernichovski for thoughtful comments on this manuscript.
REFERENCES
- Aicardi et al., 2004.Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci USA 101: 15788–15792, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akutagawa and Konishi, 1994.Akutagawa E, Konishi M. Two separate areas of the brain differentially guide the development of a song control nucleus in the zebra finch. Proc Natl Acad Sci USA 91: 12413–12417, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalman and Fee, 2009.Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc Natl Acad Sci USA 106: 12518–12523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore et al., 2008.Ashmore RC, Renk JA, Schmidt MF. Bottom-up activation of the vocal motor forebrain by the respiratory brain stem. J Neurosci 28: 2613–2623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore et al., 2005.Ashmore RC, Wild JM, Schmidt MF. Brain stem and forebrain contributions to the generation of learned motor behaviors for song. J Neurosci 25: 8543–8554, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball et al., 2003.Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. The activation of birdsong by testosterone: multiple sites of action and role of ascending catecholamine projections. Ann NY Acad Sci 1007: 211–231, 2003 [DOI] [PubMed] [Google Scholar]
- Bottjer et al., 1984.Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224: 901–903, 1984 [DOI] [PubMed] [Google Scholar]
- Brainard and Doupe, 2000.Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature 404: 762–766, 2000 [DOI] [PubMed] [Google Scholar]
- Brainard and Doupe, 2001.Brainard MS, Doupe AJ. Postlearning consolidation of birdsong: stabilizing effects of age and anterior forebrain lesions. J Neurosci 21: 2501–2517, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard and Doupe, 2002.Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature 417: 351–358, 2002 [DOI] [PubMed] [Google Scholar]
- Brenowitz and Beecher, 2005.Brenowitz EA, Beecher MD. Song learning in birds: diversity and plasticity, opportunities and challenges. Trends Neurosci 28: 127–132, 2005 [DOI] [PubMed] [Google Scholar]
- Carrillo and Doupe, 2004.Carrillo GD, Doupe AJ. Is the songbird Area X striatal, pallidal, or both? An anatomical study. J Comp Neurol 473: 415–437, 2004 [DOI] [PubMed] [Google Scholar]
- Chi and Margoliash, 2001.Chi Z, Margoliash D. Temporal precision and temporal drift in brain and behavior of zebra finch song. Neuron 32: 899–910, 2001 [DOI] [PubMed] [Google Scholar]
- Cooper and Goller, 2006.Cooper BG, Goller F. Physiological insights into the social-context-dependent changes in the rhythm of the song motor program. J Neurophysiol 95: 3798–3809, 2006 [DOI] [PubMed] [Google Scholar]
- Deregnaucourt et al., 2005.Deregnaucourt S, Mitra PP, Feher O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature 433: 710–716, 2005 [DOI] [PubMed] [Google Scholar]
- Dias and Segraves, 1999.Dias EC, Segraves MA. Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J Neurophysiol 81: 2191–2214, 1999 [DOI] [PubMed] [Google Scholar]
- Doupe and Kuhl, 1999.Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci 22: 567–631, 1999 [DOI] [PubMed] [Google Scholar]
- Foster and Bottjer, 2001.Foster EF, Bottjer SW. Lesions of a telencephalic nucleus in male zebra finches: influences on vocal behavior in juveniles and adults. J Neurobiol 46: 142–165, 2001 [DOI] [PubMed] [Google Scholar]
- Glaze and Troyer, 2006.Glaze CM, Troyer TW. Temporal structure in zebra finch song: implications for motor coding. J Neurosci 26: 991–1005, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser et al., 2002.Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419: 65–70, 2002 [DOI] [PubMed] [Google Scholar]
- Hampton et al., 2009.Hampton CM, Sakata JT, Brainard MS. An avian basal ganglia-forebrain circuit contributes differentially to syllable versus sequence variability of adult Bengalese finch song. J Neurophysiol 101: 3235–3245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara et al., 2007.Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci 25: 3406–3416, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics et al., 2009.Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience 159: 962–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler and Doupe, 1999a.Hessler NA, Doupe AJ. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J Neurosci 19: 10461–10481, 1999a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler and Doupe, 1999b.Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci 2: 209–211, 1999b [DOI] [PubMed] [Google Scholar]
- Horita et al., 2008.Horita H, Wada K, Jarvis ED. Early onset of deafening-induced song deterioration and differential requirements of the pallial-basal ganglia vocal pathway. Eur J Neurosci 28: 2519–2532, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immelmann, 1969.Immelmann K. Song development in zebra finch and other Estrildid finches. In: Bird Vocalisations, edited by Hinde RA. London: Cambridge University Press, 1969, p. 61–74 [Google Scholar]
- Jarvis and Nottebohm, 1997.Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci USA 94: 4097–4102, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis et al., 1998.Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron 21: 775–788, 1998 [DOI] [PubMed] [Google Scholar]
- Johnson et al., 1997.Johnson F, Hohmann SE, DiStefano PS, Bottjer SW. Neurotrophins suppress apoptosis induced by deafferentation of an avian motor-cortical region. J Neurosci 17: 2101–2111, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao and Brainard, 2006.Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol 96: 1441–1455, 2006 [DOI] [PubMed] [Google Scholar]
- Kao et al., 2005.Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature 433: 638–643, 2005 [DOI] [PubMed] [Google Scholar]
- Kao et al., 2008.Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci 28: 13232–13247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelberger and Mooney, 1999.Kittelberger JM, Mooney R. Lesions of an avian forebrain nucleus that disrupt song development alter synaptic connectivity and transmission in the vocal premotor pathway. J Neurosci 19: 9385–9398, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelberger and Mooney, 2005.Kittelberger JM, Mooney R. Acute injections of brain-derived neurotrophic factor in a vocal premotor nucleus reversibly disrupt adult birdsong stability and trigger syllable deletion. J Neurobiol 62: 406–424, 2005 [DOI] [PubMed] [Google Scholar]
- Kojima and Doupe, 2007.Kojima S, Doupe A. Lesions of basal ganglia nucleus Area X prevent song deterioration after deafening in adult zebra finches (Abstract). In: Society for Neuroscience Annual Meeting. San Diego, CA: Society for Neuroscience, 2007 [Google Scholar]
- Konishi, 2004.Konishi M. The role of auditory feedback in birdsong. Ann NY Acad Sci 1016: 463–475, 2004 [DOI] [PubMed] [Google Scholar]
- Liu and Nottebohm, 2005.Liu WC, Nottebohm F. Variable rate of singing and variable song duration are associated with high immediate early gene expression in two anterior forebrain song nuclei. Proc Natl Acad Sci USA 102: 10724–10729, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long and Fee, 2008.Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature 456: 189–194, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin and Ghez, 1999.Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods 86: 145–159, 1999 [DOI] [PubMed] [Google Scholar]
- Newsome and Wurtz, 1988.Newsome WT, Wurtz RH. Probing visual cortical function with discrete chemical lesions. Trends Neurosci 11: 394–400, 1988 [DOI] [PubMed] [Google Scholar]
- Nordeen and Nordeen, 2010.Nordeen KW, Nordeen EJ. Deafening-induced vocal deterioration in adult songbirds is reversed by disrupting a basal ganglia-forebrain circuit. J Neurosci 30: 7392–7400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olveczky et al., 2005.Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol 3: e153, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, 1979.Price PH. Developmental determinants of structure in zebra finch song. J Comp Physiol Psychol 93: 268–277, 1979 [Google Scholar]
- Riquimaroux et al., 1991.Riquimaroux H, Gaioni SJ, Suga N. Cortical computational maps control auditory perception. Science 251: 565–568, 1991 [DOI] [PubMed] [Google Scholar]
- Riters and Alger, 2004.Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res 316: 35–44, 2004 [DOI] [PubMed] [Google Scholar]
- Roberts et al., 2008.Roberts TF, Klein ME, Kubke MF, Wild JM, Mooney R. Telencephalic neurons monosynaptically link brain stem and forebrain premotor networks necessary for song. J Neurosci 28: 3479–3489, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer and Stryker, 1996.Ruthazer ES, Stryker MP. The role of activity in the development of long-range horizontal connections in area 17 of the ferret. J Neurosci 16: 7253–7269, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata et al., 2008.Sakata JT, Hampton CM, Brainard MS. Social modulation of sequence and syllable variability in adult birdsong. J Neurophysiol 99: 1700–1711, 2008 [DOI] [PubMed] [Google Scholar]
- Sakata and Brainard, 2006.Sakata JT, Brainard MS. Real-time contributions of auditory feedback to avian vocal motor control. J Neurosci 26: 9619–9628, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki et al., 2006.Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci 26: 9010–9014, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff and Nottebohm, 1991.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci 11: 2896–2913, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober et al., 2008.Sober SJ, Wohlgemuth MJ, Brainard MS. Central contributions to acoustic variation in birdsong. J Neurosci 28: 10370–10379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji et al., 1990.Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol 53: 51–63, 1990 [DOI] [PubMed] [Google Scholar]
- Solis and Perkel, 2005.Solis MM, Perkel DJ. Rhythmic activity in a forebrain vocal control nucleus in vitro. J Neurosci 25: 2811–2822, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossinka and Bohner, 1980.Sossinka R, Bohner J. Song types in the zebra finch (Poephila guttata castanotis). Z Tierpsychol 53: 123–132, 1980 [Google Scholar]
- Striedter and Vu, 1998.Striedter GF, Vu ET. Bilateral feedback projections to the forebrain in the premotor network for singing in zebra finches. J Neurobiol 34: 27–40, 1998 [PubMed] [Google Scholar]
- Sutton and Barto, 1998.Sutton RS, Barto AG. Reinforcement learning: an introduction. IEEE Trans Neural Netw 9: 1054, 1998 [Google Scholar]
- Tchernichovski et al., 2000.Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Procedure for an automated measurement of song similarity. Anim Behav 59: 1167–1176, 2000 [DOI] [PubMed] [Google Scholar]
- Tchernichovski et al., 2001.Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science 291: 2564–2569, 2001 [DOI] [PubMed] [Google Scholar]
- Teramitsu and White, 2006.Teramitsu I, White SA. FoxP2 regulation during undirected singing in adult songbirds. J Neurosci 26: 7390–7394, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson and Johnson, 2007.Thompson JA, Johnson F. HVC microlesions do not destabilize the vocal patterns of adult male zebra finches with prior ablation of LMAN. Dev Neurobiol 67: 205–218, 2007 [DOI] [PubMed] [Google Scholar]
- Tumer and Brainard, 2007.Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of “crystallized” adult birdsong. Nature 450: 1240–1244, 2007 [DOI] [PubMed] [Google Scholar]
- Vu et al., 1994.Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci 14: 6924–6934, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams and Mehta, 1999.Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol 39: 14–28, 1999 [PubMed] [Google Scholar]
- Woolley and Doupe, 2008.Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol 6: e62, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara and Hessler, 2006.Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur J Neurosci 24: 3619–3627, 2006 [DOI] [PubMed] [Google Scholar]