Abstract
Context
Roux-en-Y gastric bypass surgery (RYGB) is currently the most effective treatment for morbid obesity and clinical studies suggest that RYGB patients change food preferences and the desire to eat.
Objective
To examine hedonic reactions to palatable foods and food choice behavior in an established rat model of Roux-en-Y gastric bypass surgery (RYGB).
Methods and Design
Male Sprague-Dawley rats and selected line obesity-prone rats that were rendered obese on a high-fat diet underwent RYGB or sham surgery and were tested for ‘liking’ and ‘wanting’ of palatable foods at different caloric densities 4 – 6 months after surgery.
Results
Compared with sham-operated (obese) and age-matched lean control rats, RYGB rats of both models exhibited more positive orofacial responses to low concentrations of sucrose but fewer to high concentrations. These changes in ‘liking’ by RYGB rats were translated into a shift of the concentration-response curve in the brief access test, with more vigorous licking of low concentrations of sucrose and corn oil, but less licking of the highest concentrations. The changes in hedonic evaluation also resulted in lower long-term preference/acceptance of high-fat diets compared with sham-operated (obese) rats. Furthermore, the reduced ‘wanting’ of a palatable reward in the incentive runway seen in sham-operated obese SD rats was fully restored after RYGB to the level found in lean control rats.
Conclusions
The results suggest that RYGB leads to a shift in hedonic evaluation, favoring low over high calorie foods and restores obesity-induced alterations in ‘liking’ and ‘wanting’. It remains to be determined whether these effects are simply due to weight loss or specific changes in gut-brain communication. Given the emerging evidence for modulation of cortico-limbic brain structures involved in reward mechanisms by gut hormones, RYGB-induced changes in the secretion of these hormones could potentially be mediating these effects.
Keywords: Bariatric surgery, liking, wanting, motivation, taste reactivity, incentive runway, brief-access, sucrose, corn oil, food preference, obesity
Introduction
Obesity, diabetes, and the metabolic syndrome are major health problems worldwide, with children increasingly among the affected. Currently used drug therapies have not proven to be very effective and often have serious negative side effects. Behavioral therapies and lifestyle changes have high failure rates, and necessary environmental changes have not yet been seriously addressed. Surgical treatment options are, therefore, increasingly considered not just to treat, but also to prevent obesity and diabetes (1, 2).
Major progress in surgical techniques has led to drastically reduced rates of mortality and complications. Thus, life expectancy and quality of life are significantly higher in gastric bypass patients compared to untreated control populations (3). This has led to a boom in bariatric surgery, with about 200,000 surgeries per year in the USA, which is limited only by the number of surgeons (4). Roux-en-Y gastric bypass (RYGB) is the most effective surgery for body weight loss and remission of T2DM. Sustained average body weight loss over 10–15 years is a remarkable 30–50%, far beyond the 5–10% achieved with drug therapies that often only last for 1–2 years, and remission of T2DM is an astounding 70–80% (3, 5, 6). Yet, the mechanisms involved in these remarkable beneficial effects are not well understood.
The major proposed candidate mechanisms for the beneficial effects of RYGB are increased secretion of the lower gut hormones PYY and GLP-1 and perhaps decreased secretion of the upper gut hormone ghrelin (7–13). However, it is highly likely that the secretion of other hormones and factors is changed in the surgically rearranged gut to achieve the changes in appetite, glucose homeostasis, lipid metabolism, and body weight (13). For each of these changed messengers from the rearranged gut, three fundamental issues need to be addressed: i) What causes the hormone or factor to be changed, ii) How is its effect communicated to the effector organ, and iii) What are its ultimate metabolic, behavioral, and other effects. Previous studies have revealed a number of answers regarding the metabolic and behavioral “phenotype” after RYGB in patients and rodent models. Among the behavioral effects, in one study, RYGB patients showed heightened acuity for sweet taste, with some patients complaining that the food was too sweet (14), but in another study there was increased acuity for bitter and sour tastes and a trend towards reduction in salt and sweet detection (15). In other human studies, preference for high-carbohydrate foods (16) and high-fat foods (16–18) was decreased after RYGB. In addition, RYGB patients were reported to lose the desire or motivation to eat (16). In a rat model of RYGB, preference for high-fat diet steadily decreased over a 5 months postsurgical period and preference for normal (low-fat) chow increased (19). Together, these observations suggest that RYGB surgery might reduce total energy intake and body weight by changing taste perception and hedonic neural processing. The consequences of RYGB, such as changes in gut hormone secretion and sensory neural communication to the brain, may not only affect homeostatic regulatory circuits in the hypothalamus and brainstem, but may also change the way the brain processes food reward. Recent neuroimaging studies increasingly associate differences in brain reward processing with obesity, although it is not yet clear whether the differences are cause or effect. Thus, some of the beneficial effects of RYGB may be achieved by reversing the effects of obesity and/or neutralizing preexisting deficits in food reward functions.
The aim of the present study was to compare food reward behaviors in rats with RYGB or sham operations in two models of diet-induced obesity, outbred (SD) and genetically selected obesity-prone (OP) rats. Two distinguishable aspects of food reward behavior were assessed, hedonic evaluation (or pleasure or ‘liking’) of a food stimulus as measured by the taste reactivity test (20) and motivation (or incentive salience or ‘wanting’) to obtain food as measured by the incentive runway test (21). In a third test paradigm, brief access responding to liquid sucrose and corn oil stimuli, the combined effect of ‘liking’ and taste-guided low effort ‘wanting’ was evaluated.
Materials and Methods
Animals
Male Sprague-Dawley rats weighing 200g were purchased from Harlan Industries (Indianapolis, IN), and male obesity-prone (OP) rats from Charles River Laboratories (Wilmington, MA). Animals were housed individually in wire-mesh cages at a constant temperature of 21–23°C with a 12-h light-dark cycle (lights on 07:00, off at 19:00). To render them obese, rats were given access for 14–16 weeks to a three-choice cafeteria diet consisting of normal laboratory chow (Kcal%: carb, 58; fat, 13.5; prot, 28.5, # 5001, Purina LabDiet, Richmond IN), high-fat chow (Kcal%: carb, 20; fat, 60; prot, 20, D12492, Research Diets, New Brunswick, NJ), and chocolate-flavored liquid Ensure (Kcal%: carb, 64; fat, 21.6; prot, 14.4, Abbott Laboratories, Columbus, OH). After surgery, only Ensure was available for the first 10 days, and a 2-choice diet with regular and high-fat chow was available thereafter, except for brief periods when intake of single diets was measured.
All protocols involved in this study were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center in accordance with guidelines established by the National Institutes of Health.
Surgical procedures
At the end of the fattening period, rats were randomly assigned to either RYGB or sham surgery as described earlier (19). Briefly, a gastric pouch of approximately 20% of the total stomach volume was divided by means of a cutting stapler and care was taken not to damage the neural and vascular supply to the esophago-gastric junction and the pouch. The jejunum was transected ~40 cm from the ileocecal valve (~40 cm from the ligament of Treitz), and the distal cut end was anastomosed to the gastric pouch. The proximal cut end of the jejunum was anastomosed to the side of the lower jejunum (~ 25 cm from the ileocecal valve). This procedure resulted in a ~15 cm-long Roux limb, a 25 cm-long common limb, and a roughly 40 cm-long biliopancreatic limb. Sham-surgery consisted of the same procedure, except that the stapler was only laid over the stomach, but not fired, the divided jejunum was sewn back together, and the small stab wound in the gastric fundus was closed with sutures. An additional group of rats (lean controls) of similar age and without any surgery was fed regular chow throughout the experiment.
In some rats, chronic jugular catheters were implanted as described earlier (22) and 0.25 ml blood samples were taken during the light period in ad libitum fed rats. Plasma leptin concentrations were measured on duplicate samples using the Milliplex rat gut hormone panel with Luminex xMAP technology (Millipore, St. Charles, MO; Luminex Corp, Austin, TX).
For the first 10 days after surgery, rats were only given Ensure. After 10 days, they were slowly adapted to eating normal chow and high-fat chow provided in increasing amounts on the cage floor.
Body weight, body composition, and plasma leptin levels
Body weight was monitored daily for the first month, and weekly body weight was recorded afterwards. Body composition of animals (% fat mass, fluid, and lean mass) was also measured prior to and 9–12 weeks after surgery by using a Minispec LF 90 NMR Analyzer (Bruker BioSpin Corporation, The Woodlands, TX). This method uses whole body magnetic resonance relaxometry in unanesthetized rodents with excellent linearity and reproducibility (22).
Behavioral testing and analysis
The taste reactivity test of Grill and Norgren (20) was used to quantify ‘liking’ (23). A 200 µl volume of sucrose solution was placed on the transparent floor of a cylindrical test cage, and the rat’s orofacial expressions were videotaped from below. The number of characteristic tongue protrusions was assessed by inspection of slow-motion videos (23), averaged over three consecutive bouts of ingestion and for 3 ascending concentrations of sucrose (0.01, 0.1, and 1.0 M) administered on separate days in random order. Positive hedonic orofacial responses included repeated midline tongue protrusions, lateral tongue protrusions, and paw licking. Only a few rats, and only during first exposure, demonstrated one or two aversive responses, such as headshakes, gapes, and forelimb flails.
The incentive runway was used to assess goal-oriented behavior – the motivation to obtain a food reward or ’wanting’ (21, 24). The runway consisted of start and goal boxes connected with a 158 cm long running alley and a video camera mounted above. Rats were habituated to the runway during two daily 10 min sessions. During two additional sessions, overnight food-deprived rats were enticed to eat a small food reward (Fruit Loop cereal, ~2g) in the goal box. Runway behavior was then assessed in the non food-deprived state in daily sessions of two consecutive trials over a period of 11 days. After placing the rats in the start box, the door was opened and the time to reach the goal box and consume the reward (completion time) was measured and expressed as completion speed. In addition, the time spent walking/running forward, standing still, moving backwards, and any other distractions, as well as the net running speed were assessed by replaying the video recordings in slow motion. Analysis for both tests was conducted by two independent observers that were blind to the experimental conditions.
The brief-access lick test (Davis MS-160, DiLog Instruments, Tallahassee, FL) was used to measure taste-guided reward behavior (25). Brief access [10 s] to the spout, allowing a limited number of licks for each concentration, minimizes modulation of reward behavior by postingestive learning. Rats were first adapted to the special cage and trained to lick from the spout filled with highly palatable chocolate Ensure under conditions of mild food and water deprivation. On test days, non food-deprived animals were presented with increasing concentrations of either sucrose (0, 0.005–1.5 M) or corn oil emulsion (0, 0.03–32%, in 1% Emplex emulsifier and distilled water). Each concentration was available for 10 s with 5 s intervals in two consecutive ascending series and the number of licks/10 s was averaged for each concentration.
The 2-choice food preference/acceptance test consisted of offering a choice of chow and high-fat diet for 3 consecutive days. Intake was measured each day, accounting for spillage and averaged for the 3 days.
Statistical analysis
Body composition data and plasma leptin levels were analyzed by one-way ANOVA followed by Bonferroni-adjusted multiple comparisons. Mean differences in body weight between groups across time, number of positive hedonic orofacial responses measured in the taste reactivity test, and the number of licks in the brief-access test, were analyzed by two-way repeated measures ANOVA followed by Bonferroni-adjusted multiple comparisons. Completion speed in the incentive runway was analyzed by 3-way ANOVA with treatment (RYGB, sham, and lean) as between subject factor, session and order of run in each session (1st and 2nd) as within subject factors, followed by Bonferroni-adjusted multiple comparisons. Running speed, duration and incidence of latency to get out of the start box, pauses, reversals, and energy intake and fat preference were analyzed separately by student’s t-tests. All data are expressed as mean ± SEM.
Results
Effect of RYGB on body weight and body composition
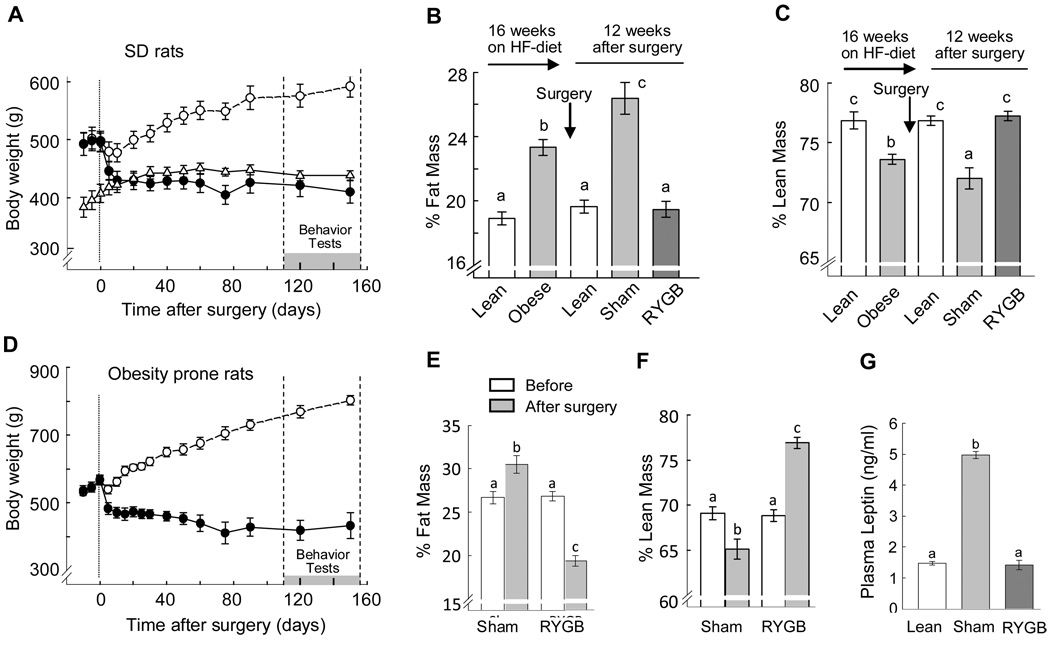
In both outbred Sprague-Dawley rats and genetically selected obesity-prone rats, RYGB produced loss of body weight and fat mass compared to sham-operated controls (Fig. 1A,B). SD rats undergoing RYGB started at about 500g and rapidly lost ~80 g (16%) of body weight within the first 10 days after surgery and stabilized at this level over the next 5 months. Sham-operated rats continued to gain weight and fat mass, so that they were about 170g heavier during the period of the behavioral tests. Five months after RYGB, body weight, percent fat and lean mass, as well as plasma leptin levels were not significantly different from non-operated, age-matched control rats fed chow throughout the experiment (Fig. 1B,C,G).
Fig. 1.
Body weight, body composition, and leptin levels after RYGB and sham surgery. A–C, G: Body weight (A), adiposity (B), percent lean mass (C), and plasma leptin levels (G), of outbred Sprague-Dawley rats with either Roux-en-Y gastric bypass surgery (RYGB, filled circles; n = 11) or sham surgery (sham, open circles; n = 11), and non-operated, chow-fed, lean controls (lean, open triangles; n = 10). Body weight in RYGB rats was significantly reduced at all time points after surgery compared with sham-operated controls, but was not significantly different from lean controls. D–F: Body weight (D), adiposity (E), and percent lean mass (F) of genetically select obesity-prone (CD–OP) rats with RYGB (n = 6) or sham-surgery (n = 5). Bars that do not share the same letter are significantly (P < 0.05) different from each other (based on ANOVA and Bonferroni adjusted multiple comparisons).
Obesity-prone rats started at 580 g and lost about 140g over 5 months, while their sham-operated counterparts continued to gain additional weight and fat mass, so that they were about 360 g heavier at the time of behavioral testing (Fig. 1D). Percent fat and lean mass of obesity prone rats with RYGB was similar to both lean SD rats and SD rats after RYGB (Fig. 1E,F).
RYGB increases ‘liking’ of low concentrations but decreases ‘liking’ of high concentrations of sucrose
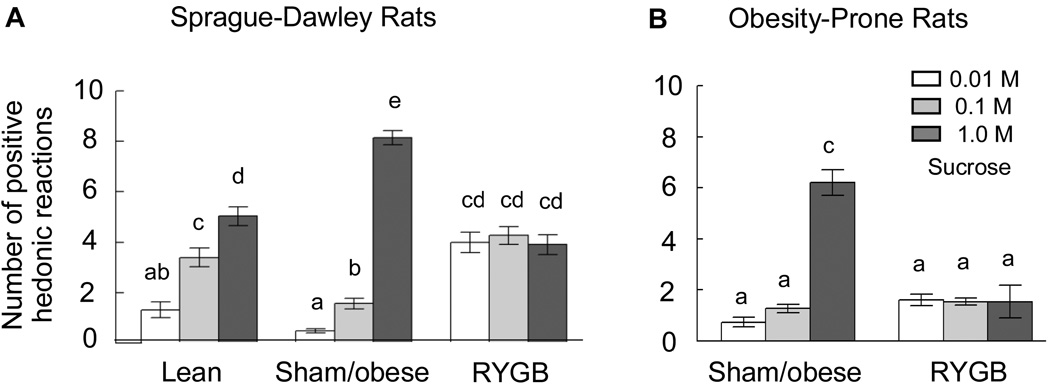
As expected, lean control rats exhibited a linear sucrose concentration-dependent increase in the number of positive hedonic reactions (Fig. 2A). This linear increase was significantly skewed towards higher sucrose concentrations in sham-operated obese rats, with decreased ‘liking’ of 0.1 M, but increased ‘liking’ of 1.0 M sucrose. A similar right-shift was found in sham-operated “obese” OP rats (Fig. 2B), although we have not compared it with genetically selected obesity-resistant rats. Importantly, in both models, RYGB surgery completely flattened the concentration-response curve. After RYGB, SD rats ‘liked’ the two lower sucrose concentrations more (0.01 M: t[20] = 8.0, p < 0.001; 0.1 M: t[20] = 6.1, p < 0.001), but ‘liked’ the highest concentration less (t[20] = 9.67, p < 0.01) compared with sham-operated obese rats. RYGB rats ‘liked’ the lowest sucrose concentration more (t[18] = 5.77, p < 0.001) and the highest concentration marginally less (t[18] = 2.44, p = 0.052) compared with lean rats. In OP rats, ‘liking’ of the highest sucrose concentration was significantly blunted by RYGB. Separate ANOVA for the two models showed a significant treatment × sucrose concentration interaction (SD rats: F[4,56] = 78.52, p < 0.001; OP rats: F[2,16] = 23.3, p < 0.001).
Fig. 2.
Hedonic impact or ‘liking’ of sucrose as measured by the taste reactivity test. The number of positive hedonic reactions in response to tasting 3 different sucrose concentrations is shown. A: outbred Sprague-Dawley rats, either non-operated, chow-fed lean controls (lean, n = 9), sham-operated, obese (sham/obese, n = 11), or RYGB (n =11). B: Genetically select line of obesity-prone (CD–OP) rats, sham-operated, obese (n = 5), and RYGB (n = 5). Bars that do not share the same letter are significantly different from each other (p < 0.05, based on ANOVA and Bonferroni adjusted multiple comparisons).
RYGB reverses obesity-induced changes in ‘wanting’ of a food reward in the incentive runway
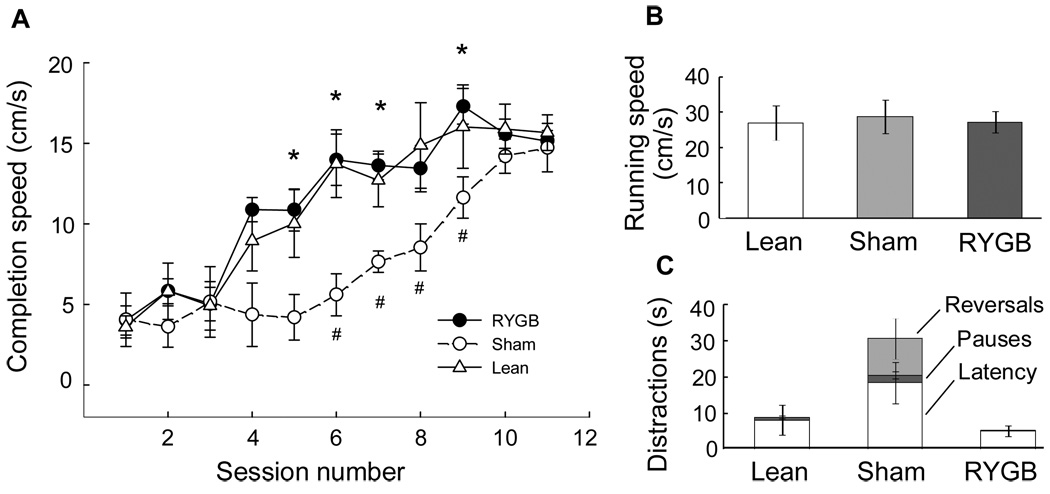
Completion speed in the incentive runway, a measure of motivation or ‘wanting’, was significantly decreased in sham-operated obese SD rats compared with lean, chow-fed controls (Fig. 3A). The deficit was significant in sessions 6–9, with completion speed only about half that of lean rats. The increased time to get to the reward was not due to inability to run fast, as the net running speed was similar for obese and lean rats. The entire difference was due to increased duration of distractions such as the latency to get out of the start box, pauses, and reversals. With overtraining, the obese rats eventually learned to get to the goal box just as fast as lean rats.
Fig. 3.
Motivation to obtain food or ‘wanting’ as measured in the incentive runway. Rats were required to run from a start box to a goal box where they were rewarded with a palatable treat (sweet tasting Fruit-Loops). A: Completion speed (based on time required to go from start to goal box) for non-operated, chow-fed lean controls (lean, n = 4), sham-operated, obese (sham/obese, n = 6), and RYGB (n = 4) outbred Sprague-Dawley rats. Completion speed (‘wanting’) is significantly suppressed in obese rats with sham-surgery compared with both RYGB (* p < 0.05) and lean rats (# p < 0.05). B: Net running speed is not different for the three groups. C: Obese, sham-operated rats spend significantly more time being distracted by latency to leave the start box, pauses, and reversals.
Importantly, the reduced completion speed of obese rats was fully reversed by RYGB surgery. RYGB rats completed the task significantly faster compared with sham-operated obese rats in sessions 4–9 (Fig. 3A). Completion speed, running speed, and duration of distractions were identical to lean controls (Fig. 3B). As expected, there was a highly significant effect of trial order (F[1,11] = 110.6, p < 0.001), with completion speed for the second trial each session significantly faster than for the first trial (data not shown), suggesting that recent memory of being reinforced is an important factor determining runway performance. However, absence of a trial × treatment interaction (F[2,11] = 1.41, n.s.) showed that it was not differentially affected in sham-operated obese and RYGB rats.
RYGB shifts the sucrose and corn oil concentration-response curves to lower concentrations in the brief access test
In the brief access lick test, both ‘liking’ and low-effort ‘wanting’ are combined into the number of licks emitted in the short time frame that minimizes postingestive feedback, and normal animals show a typical sigmoid concentration-response curve. Strikingly, sham-operated, obese rats of both strains almost completely abstained from lower concentrations but responded strongly to the higher concentrations of either sucrose or corn oil (Fig. 4). For sucrose, the transition from abstaining to going-for-it was between 0.03 and 0.06 M in SD rats and between 0.06 and 1.0 M in OP rats. For corn oil, it was between 4% and 16% in both strains. This reduced responding to low sweet and oily stimuli was completely reversed after RYGB surgery in both strains (although we did not test the selected line of obesity-resistant rats). At the highest concentrations, RYGB rats responded significantly less than their sham-operated, obese counterparts, and there was a strong tendency for lower responding compared to lean controls in SD rats.
Fig. 4.
Lickometer responding for different concentrations of sucrose (A,C) and corn oil (B,D) as a measure of combined taste-guided ‘liking’ and low-effort ‘wanting’. The number of licks/10s was measured in series of ascending concentrations of sucrose solutions and corn oil emulsions. A,B: Outbred Sprague-Dawley rats with either Roux-en-Y gastric bypass surgery (RYGB, filled circles; n = 9) or sham surgery (sham, open circles; n = 11), and non-operated, chow-fed, lean controls (lean, open triangles; n = 7). C,D: Genetically select obesity-prone (CD–OP) rats with RYGB (n = 4) or sham-surgery (n = 4). *p < 0.05, RYGB compared with sham/obese and, # p < 0.05, lean compared with sham/obese rats, based on ANOVA and Bonferroni adjusted multiple comparisons.
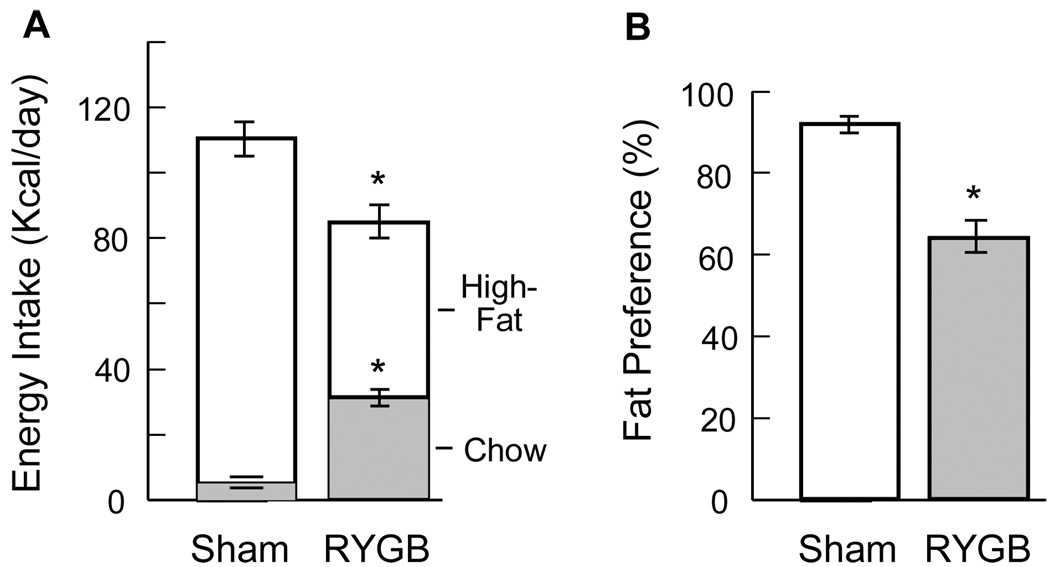
RYGB shifts preference from high-fat to chow
Measurement of chow and high-fat diet intake over 3 consecutive 24 h periods revealed a significantly decreased preference for the high-fat diet. While sham-operated, obese SD rats ate 95.1 % of their caloric intake from high-fat diet, RYGB rats showed only 63.3 % preference (P < 0.01) (Fig. 5).
Fig. 5.
Food choice of sham-operated (Sham, n = 6) and RYGB (n = 4) outbred Sprague-Dawley rats, 8 months after surgery. Two-choice diet (chow and high-fat) was available throughout postsurgical period. A: Intake of calories from chow and high-fat diet measured during 3 consecutive days. B: Fat preference in percent of total intake. * P < 0.01, RYGB compared with sham/obese, based on t-test.
Discussion
The remarkable success of RYGB in reversing obesity and most of its associated comorbidities makes understanding of the underlying mechanisms an important goal that could eventually lead to “knifeless” behavioral and/or pharmacological approaches. Although the present results do not identify any molecular or cellular mechanisms, they strongly point to a role of neural mechanisms involved in taste processing, hedonic evaluation, and motivation to eat. In our rat model that closely replicates the major effects of RYGB seen in obese human patients on food intake, body weight loss, and secretion of gut hormones, we show a shift from high to low concentrations of sweet and oily food stimuli in their capacity to generate pleasure and stimulate intake, compared with sham-operated obese animals. In addition, we find that under non food-deprived conditions, diet-induced obese rats largely ignore low concentrations of sweet and oily stimuli and are less motivated to work for a food reward compared to chow-fed lean rats. Rather, obese rats seem to only respond to very sweet and oily stimuli that are easily accessible. All these obesity-induced changes were completely reversed after RYGB. These changes are reminiscent of observations in RYGB patients preferring vegetables and fruit over energy dense sweet and fatty foods (16–18). The present results do not determine whether these behavioral changes are due to weight loss or specific changes in gut-brain communication, or a combination of both. Determination of reward behaviors at earlier time points or even before surgery and in pair-fed controls, as well as assessment of potentially underlying neural functions will be necessary to provide answers.
RYGB reverses obesity-induced increases in ‘liking’ of highly concentrated nutrient solutions and enhances ‘liking’ of low concentrations even compared with lean rats
To determine positive affect or ‘liking’, we measured the number of positive hedonic orofacial reactions in response to tasting a drop of sucrose solution. These evolutionarily conserved reactions reflecting neural mechanisms essentially organized at the brainstem level (20, 26) have been validated in many species, including rodents, monkeys, and humans, and are thought to more or less reflect the experience of human conscious pleasure (27, 28). The typical concentration-dependent increase of sucrose ‘liking’ in chow-fed lean rats (20) was more or less shifted to the right in sham-operated, diet-induced, obese SD and OP rats, and completely abolished 4–6 months after RYGB surgery in both models. The slightly increased ‘liking’ of sucrose in obese rats is consistent with findings in obese humans. Although there are conflicting results on sweet detection and obesity, when using a relative scale that takes into account individual differences in the strongest sensation of any kind, obese subjects report higher ‘liking’ for a given sweetness than normal and underweight subjects (29). Interestingly, this is in spite of decreased perceived sweetness in obese subjects (29). Thus, as concluded by Bartoshuk et al., ‘liking’ increases as a function of sweetness more in obese subjects and more as BMI increases, and for the same perceived sweetness, ‘liking’ increases as BMI increases. Importantly, in underweight subjects with a BMI of <18.5, ‘liking’ did not increase as a function of perceived sweetness (29). Such a flat dose-response relationship between sucrose concentration and ‘liking’ is exactly what we find in RYGB rats, after severe weight loss.
Dieting-induced weight loss in obese subjects is typically followed by weight regain, and it has recently been shown that this relapse may be caused by a similar natural biological reaction to weight loss that is part of the homeostatic regulation in normal weight individuals. Neural activity in a number of brain areas evoked by pictures of food is changed after weight loss, and some of these changes are restored after leptin-treatment (30). Thus, even though plasma leptin levels are still higher than in lean subjects, the relative decrease of leptin signaling in weight-reduced individuals is perceived as a threat by the brain, causing it to switch to the “hungry” mode. In spite of a drastic fall in leptin levels, such a mechanism does not appear to kick in after RYGB-induced weight loss. The flat dose-response for sucrose-induced ‘liking’ after RYGB suggests that the brain is not in a “hungry” mode. Food deprivation-induced hunger clearly increases ‘liking’, while caloric, and particularly sensory specific satiety decreases ‘liking’ in lean rats (31), and fasting increases the appeal (rating) of food pictures, particularly those that show high calorie foods in normal weight subjects (32).
As to the mechanisms responsible for these changes in sucrose sensitivity in obese and RYGB rats, we can only speculate. They could involve processing of gustatory information at the most peripheral to the most central level, and experiments specifically addressing each level will be necessary to answer this question.
RYGB restores obesity-induced deficits in ‘wanting’
The more a food is ‘liked’, the more it is usually ‘wanted’. As discussed above, sucrose ‘liking’ is differentially affected by RYGB, increased by low concentrations and decreased by high concentrations compared with both obese and lean rats. Therefore, ‘wanting’ could be expected to follow similar trends; increased ‘wanting of low- and decreased ‘wanting’ of high concentrations. In the incentive runway test Fruit Loops served as reinforcement and RYGB rats clearly ‘wanted’ this reward more compared to the sham-operated obese rats, although there was no difference compared to lean rats. With 38% (w/v) sugar, Fruit Loops can be considered as very sweet and one could have expected that they were less wanted by RYGB rats compared with obese rats. However, the opposite was found, RYGB rats ‘wanted’ Fruit Loops more than obese rats. Thus, under the assumption that more ‘liking’ leads to more ‘wanting’, RYGB rats treated Fruit Loops as if they were low in sweetness. Although it is possible that solid sweets behave differently from sucrose solutions, using the same treats in both test paradigms will be necessary to resolve this issue.
Although it is generally agreed that runway performance is a measure of motivation, various running schedules have been employed and different interpretations offered regarding the specific sub-process involved (24, 33, 34). If in each daily session more than one run is performed, completion speed for the first trial is mainly determined by the motivation to obtain the palatable food reinforcer (‘wanting”), while in the second trial it is more determined by the impact of reinforcement on subsequent motivation (24). Thus, separate analysis of performance in the first and subsequent runs has the potential to differentiate among these sub-processes. Although in the present study, rats were moving significantly faster to the goal box in the second run, obesity and RYGB significantly and equally modulated performance in the 1st and 2nd run. Therefore, obesity and RYGB affected not just reinforcement learning but also ‘wanting’.
Alternative explanations of the incentive runway results implicate changes in more general motivation processes and/or in learning ability in obese and RYGB rats. First, the results could be interpreted as a general deficit in motivation in the obese state and reversal by RYGB. For example, obese subjects are not easily motivated to adhere to dietary and exercise regimens (35), and such compliance was positively correlated to weight loss after RYGB (36). Second, it could be argued that obese rats ‘want’ the food reward just as much as lean rats, but they simply learn the task slower, and RYBG reverses this learning deficit. This is indicated by the fact that with enough training they eventually attain the same completion speed as controls and gains additional support from a number of studies showing cognitive impairment in obese animals (37) and humans (38). Of particular interest is the recent demonstration that GLP-1 receptor agonism seems to specifically rescue obesity and diabetes-induced cognitive decline (37, 39). Although increased levels of circulating GLP-1 are consistently observed after RYGB in rats (40) and humans (41), it is not yet clear whether the beneficial effects on cognition are due to direct effects on certain brain areas or secondarily through amelioration of insulin resistance (42). Because measurement of motivation (‘wanting’) is intricately linked to learning, it will always be difficult to experimentally dissociate the two processes. One possibility is to reduce the learning component with a test that requires very little or no effort to obtain reinforcement as in the brief-access lick test discussed next.
In the brief access lick test the animal tastes and consumes the reinforcer almost simultaneously – the hedonic experience (‘liking’) temporally coincides with the motivational process. In distinction to the incentive runway, very little effort is required to obtain the reward and there is minimal learning involved. The brief access test could be best characterized as a combination of ‘liking’ and taste-guided, low-effort ‘wanting’. There were both similarities and dissimilarities between the outcome in the brief access lick test and the incentive runway test. At low sucrose and corn oil concentrations the outcome in both tests was similar in that obesity reduced, and RYGB restored performance (‘wanting’) to levels in lean controls. At high sucrose and corn oil concentrations the outcome was different. In the brief access test obesity increased responding or ‘wanting’ compared with lean controls, and this effect was reversed by RYGB, while in the runway test the reverse was the case, obesity decreased ‘wanting’ and RYGB reversed it. At the highest concentrations of each macronutrient stimulus, RYGB significantly reduced taste-guided low-effort ‘wanting’ compared to sham-operated obese rats of both strains. The findings in another rat model of obesity, the CCK1-receptor deficient rat, that taste-responsive neurons in the parabrachial nucleus had a reduced overall sensitivity to sucrose, with decreased responsiveness to lower concentrations and augmented responses to higher concentrations (26) suggest that at least this pontine taste relay participates in the underlying neural circuitry.
Overall, ‘wanting’ was clearly reduced in obese (sham-operated) rats compared with lean rats. This seems intuitively puzzling given the higher ‘liking’ of sucrose in obese rats and humans discussed above, but it is in line with other studies using different test paradigms to measure ‘wanting’. Both high-fat diet-induced obese Long-Evans rats and genetically selected obesity-prone (OP or DIO) rats exhibited drastically reduced break points of progressive lever press responding for food reward (43). In addition, there is considerable evidence in the obese for decreased signaling through the mesolimbic dopamine/D2-receptor system both in rodents (43–49) and humans (50–55), thought to play a pivotal role in both the motivational (‘wanting’) and reinforcement learning component of food and drug reward (56, 57). The incongruity between decreased signaling through this system on one hand and the fact that activation of the system is driving overindulgence leading to obesity on the other hand (51, 57) has been explained by the reward-deficit hypothesis (55, 58, 59). With regard to food reward, this hypothesis suggests that individuals with low dopamine signaling make up by engaging in more eating, thereby restoring a set point for reward generation. Under this scenario, dopamine signaling could be considered to provide negative feedback to a defended level of reward, just as nutrient sensing mechanisms provide negative feedback to a defended level or set point of body weight. Dopamine signaling would then not constitute reward by itself; it would be more like a sensing mechanism leading to reward generation, just like nutrient sensing mechanisms lead to satiation. Our finding that ‘wanting’ is restored to normal levels after RYGB may thus suggest that it somehow restores obesity-induced defective dopamine signaling. A recent PET imaging study in RYGB patients supports this suggestion. In five female obese patients, dopamine D2-receptor availability increased six months after surgery and the increase was roughly proportional to the amount of weight loss (60).
It is not clear whether the loss of ‘liking’ of the high concentration is more important than the increased ‘liking’ of the low concentration for long-term consequences on food choice. With the exception of the middle sucrose concentration (0.1 M), the ‘liking’ profile of the taste reactivity test was well translated into brief access responding, but it is difficult to draw parallels to the long-term choice, because we did not offer the rats a choice of food items with different sucrose content. However, in the case of fat ingestion, it is quite clear that the decreased ‘liking’ of the highest concentration of corn oil (as assessed by the brief access test) translated into reduced consumption of high fat pellets in the long term. This decreased ‘liking’ of high corn oil concentrations after RYGB was accentuated in OP rats and it is unfortunate that we were unable to assess long-term food choice in this group.
Conclusions and perspectives
The results suggest that changes in taste and reward processing may underlie the beneficial effects of gastric bypass surgery on food choice and caloric intake. They confirm earlier findings that obesity is associated with increased ‘liking’ of energy dense foods and a paradoxical decrease in the willingness to work for food rewards, and show for the first time that RYGB reverses these obesity-associated malfunctions. Although it appears plausible that changes in gut hormone secretion cause these beneficial effects after RYGB, the specific mechanisms involved are completely unknown at this point. If hormones are involved, their peripheral and/or central site(s) of action will have to be identified. It is equally likely that changes in neural communication to the brain initiated by hormones, nutrients, and/or mechanical factors are involved. However, because in the present study, behavioral changes were seen several months after surgery, it is unclear whether they are simply due to weight loss or specific changes in gut-brain communication. Future studies with assessment of reward behaviors at earlier time points or even before surgery and in pair-fed controls, as well as assessment of potentially underlying neural functions will be necessary. Such longitudinal studies may also reveal possible learning effects on taste and reward processing, including aversive conditioning, in the course of adaptation to the rearranged gut. A careful and open minded mechanistic elucidation should be very rewarding in that it will eventually result in “knifeless surgery”.
Acknowledgements
We thank Leigh Townsend for technical help and Laurel Patterson for help with the manuscript. Supported by National Institutes of Health Grants DK047348 and DK 071082 (HRB).
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 484-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 2008;31(Suppl 2):S290–S296. doi: 10.2337/dc08-s271. [DOI] [PubMed] [Google Scholar]
- 3.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 4.Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Guven S, et al. Executive summary of the recommendations of the American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14:318–336. doi: 10.4158/EP.14.3.318. [DOI] [PubMed] [Google Scholar]
- 5.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. discussion 350-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 8.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stylopoulos N, Davis P, Pettit JD, Rattner DW, Kaplan LM. Changes in serum ghrelin predict weight loss after Roux-en-Y gastric bypass in rats. Surg Endosc. 2005;19:942–946. doi: 10.1007/s00464-004-8825-x. [DOI] [PubMed] [Google Scholar]
- 10.Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meirelles K, Ahmed T, Culnan DM, Lynch CJ, Lang CH, Cooney RN. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg. 2009;249:277–285. doi: 10.1097/SLA.0b013e3181904af0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.le Roux CW, Batterham RL, Aylwin SJ, Patterson M, Borg CM, Wynne KJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147:3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 13.Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8:201–211. doi: 10.1016/j.cmet.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients' taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc. 1995;95:666–670. doi: 10.1016/S0002-8223(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 15.Scruggs DM, Buffington C, Cowan GS., Jr Taste Acuity of the Morbidly Obese before and after Gastric Bypass Surgery. Obes Surg. 1994;4:24–28. doi: 10.1381/096089294765558854. [DOI] [PubMed] [Google Scholar]
- 16.Naslund E, Melin I, Gryback P, Hagg A, Hellstrom PM, Jacobsson H, et al. Reduced food intake after jejunoileal bypass: a possible association with prolonged gastric emptying and altered gut hormone patterns. Am J Clin Nutr. 1997;66:26–32. doi: 10.1093/ajcn/66.1.26. [DOI] [PubMed] [Google Scholar]
- 17.Thirlby RC, Bahiraei F, Randall J, Drewnoski A. Effect of Roux-en-Y gastric bypass on satiety and food likes: the role of genetics. J Gastrointest Surg. 2006;10:270–277. doi: 10.1016/j.gassur.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1273–R1282. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 21.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunnecke B, Verry P, Benardeau A, von Kienlin M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res. 2004;12:1604–1615. doi: 10.1038/oby.2004.200. [DOI] [PubMed] [Google Scholar]
- 23.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 24.Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spector AC, Redman R, Garcea M. The consequences of gustatory nerve transection on taste-guided licking of sucrose and maltose in the rat. Behav Neurosci. 1996;110:1096–1109. [PubMed] [Google Scholar]
- 26.Hajnal A, Norgren R, Kovacs P. Parabrachial coding of sapid sucrose: relevance to reward and obesity. Ann N Y Acad Sci. 2009;1170:347–364. doi: 10.1111/j.1749-6632.2009.03930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgdorf J, Panksepp J. The neurobiology of positive emotions. Neurosci Biobehav Rev. 2006;30:173–187. doi: 10.1016/j.neubiorev.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006;361:1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–2591. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991;16:103–120. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- 32.Goldstone AP, de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30:1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 33.Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McFarland K, Ettenberg A. Haloperidol does not affect motivational processes in an operant runway model of food-seeking behavior. Behav Neurosci. 1998;112:630–635. doi: 10.1037//0735-7044.112.3.630. [DOI] [PubMed] [Google Scholar]
- 35.Foreyt JP, Poston WS., 2nd The challenge of diet, exercise and lifestyle modification in the management of the obese diabetic patient. Int J Obes Relat Metab Disord. 1999;23(Suppl 7):S5–S11. doi: 10.1038/sj.ijo.0800955. [DOI] [PubMed] [Google Scholar]
- 36.Pontiroli AE, Fossati A, Vedani P, Fiorilli M, Folli F, Paganelli M, et al. Post-surgery adherence to scheduled visits and compliance, more than personality disorders, predict outcome of bariatric restrictive surgery in morbidly obese patients. Obes Surg. 2007;17:1492–1497. doi: 10.1007/s11695-008-9428-8. [DOI] [PubMed] [Google Scholar]
- 37.Gault VA, Porter WD, Flatt PR, Holscher C. Actions of exendin-4 therapy on cognitive function and hippocampal synaptic plasticity in mice fed a high-fat diet. Int J Obes (Lond) doi: 10.1038/ijo.2010.59. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson LG, Nilsson E. Overweight and cognition. Scand J Psychol. 2009;50:660–667. doi: 10.1111/j.1467-9450.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- 39.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 40.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-Induced Hormone Responses in a Rat Model of Roux-en-Y Gastric Bypass Surgery. Endocrinology. doi: 10.1210/en.2009-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hallschmid M, Schultes B. Central nervous insulin resistance: a promising target in the treatment of metabolic and cognitive disorders? Diabetologia. 2009;52:2264–2269. doi: 10.1007/s00125-009-1501-x. [DOI] [PubMed] [Google Scholar]
- 43.Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, et al. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122:1257–1263. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang XF, Yu Y, Zavitsanou K, Han M, Storlien L. Differential expression of dopamine D2 and D4 receptor and tyrosine hydroxylase mRNA in mice prone, or resistant, to chronic high-fat diet-induced obesity. Brain Res Mol Brain Res. 2005;135:150–161. doi: 10.1016/j.molbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159:1193–1199. doi: 10.1016/j.neuroscience.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hajnal A, Acharya NK, Grigson PS, Covasa M, Twining RC. Obese OLETF rats exhibit increased operant performance for palatable sucrose solutions and differential sensitivity to D2 receptor antagonism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1846–R1854. doi: 10.1152/ajpregu.00461.2007. [DOI] [PubMed] [Google Scholar]
- 48.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Thanos PK, Ramalhete RC, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Leptin receptor deficiency is associated with upregulation of cannabinoid 1 receptors in limbic brain regions. Synapse. 2008;62:637–642. doi: 10.1002/syn.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis C, Levitan RD, Kaplan AS, Carter J, Reid C, Curtis C, et al. Reward sensitivity and the D2 dopamine receptor gene: A case-control study of binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:620–628. doi: 10.1016/j.pnpbp.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 51.Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, et al. Dopamine for "wanting" and opioids for "liking": a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 2009;17:1220–1225. doi: 10.1038/oby.2009.52. [DOI] [PubMed] [Google Scholar]
- 52.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 55.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 58.Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, Blum SH, et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model. 2008;5:24. doi: 10.1186/1742-4682-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blum K, Chen TJ, Meshkin B, Downs BW, Gordon CA, Blum S, et al. Reward deficiency syndrome in obesity: a preliminary cross-sectional trial with a Genotrim variant. Adv Ther. 2006;23:1040–1051. doi: 10.1007/BF02850224. [DOI] [PubMed] [Google Scholar]
- 60.Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg. 20:369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]