Abstract
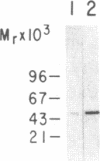
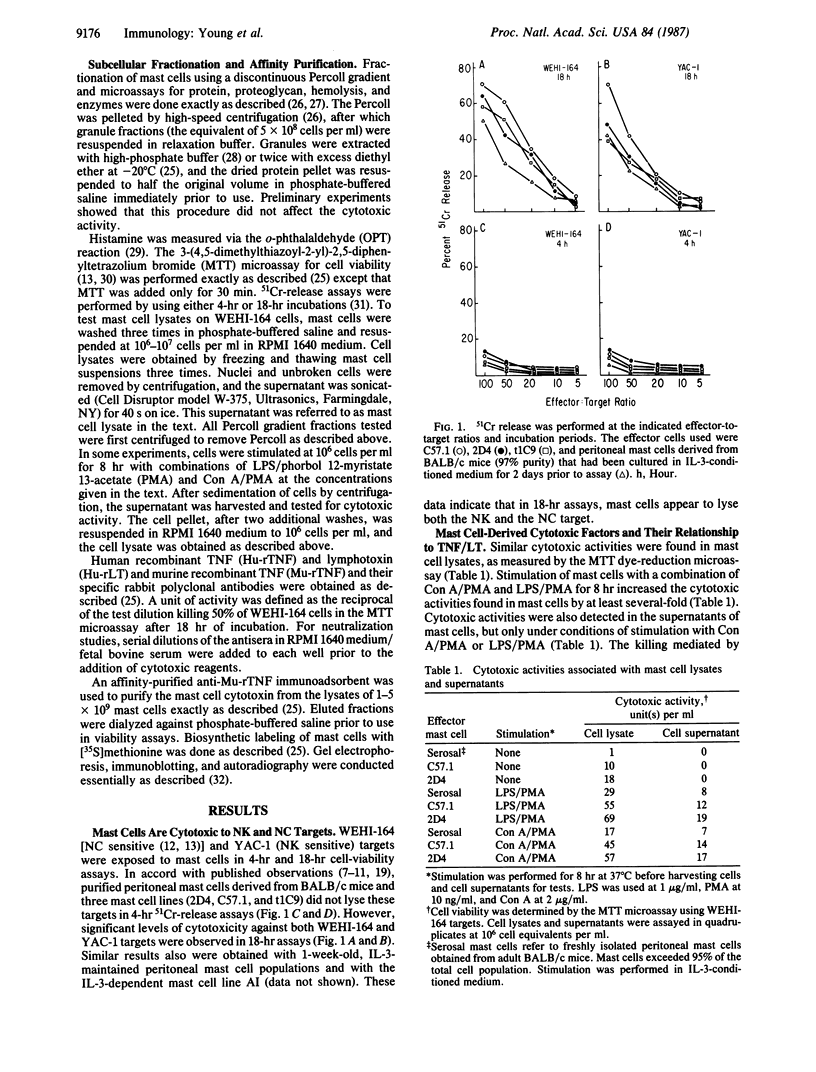
The role of mast cells and mast-cell-derived factors in natural cytotoxic reactions was investigated. Cultured and freshly isolated murine mast cells are shown to be cytotoxic to WEHI-164 and YAC-1 targets in 18-hr viability assays but not in 4-hr assays. Here, we describe a cytotoxic factor in murine mast cells that is immunologically related to tumor necrosis factor (TNF). This TNF-like factor lyses WEHI-164 cells with a slow time course requiring 16-20 hr for the lytic reaction to complete. Antibodies specific for human and murine TNF and human lymphotoxin partially block mast cell lysis of WEHI-164 cells. These antibodies react on immunoblots with one major mast cell protein band of 50 kDa. Immunoblot analysis shows this factor in cloned and uncloned cultured mouse mast cells and in mature "connective tissue-type" mast cells freshly purified from rat or mouse peritoneal cavities. The amount of this factor is greatly enhanced in cells that have been stimulated with a combination of phorbol ester/concanavalin A or bacterial lipopolysaccharide. Subcellular fractionation analysis of mast cells with Percoll gradients reveals two pools of TNF-related cytotoxic activity that are associated with free cytosolic material and granule fractions. In contrast to cytotoxic T lymphocytes and natural killer cells, granule-enriched fractions of mast cells do not contain any hemolytic activity. The localization of the TNF-like molecule in mast cell granules may play a strategical role in the rapid delivery of this mediator to the target cell membrane following cell surface stimulation and degranulation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Blakely A., Gorman K., Ostergaard H., Svoboda K., Liu C. C., Young J. D., Clark W. R. Resistance of cloned cytotoxic T lymphocytes to cell-mediated cytotoxicity. J Exp Med. 1987 Oct 1;166(4):1070–1083. doi: 10.1084/jem.166.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom B. C., Alexander P. Mast cell and anaphylactic antibody responses in inbred rats to syngeneic fibrosarcomas. Int Arch Allergy Appl Immunol. 1975;49(5):627–631. doi: 10.1159/000231444. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A. M., Galli S. J., Galli A. S., Hammond M. E., Churchill W. H., Jr, Dvorak H. F. Tumor-basophil interactions in vitro--a scanning and transmission electron microscopic study. J Immunol. 1979 Jun;122(6):2447–2457. [PubMed] [Google Scholar]
- Dvorak H. F., Dvorak A. M., Churchill W. H. Immunologic rejection of diethylnitrosamine-induced hepatomas in strain 2 guinea pigs: participation of basophilic leukocytes and macrophage aggregates. J Exp Med. 1973 Mar 1;137(3):751–775. doi: 10.1084/jem.137.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerbäck L., Svensson I. Isolation of rat peritoneal mast cells by centrifugation on density gradients of Percoll. J Immunol Methods. 1980;39(1-2):135–145. doi: 10.1016/0022-1759(80)90302-6. [DOI] [PubMed] [Google Scholar]
- Farram E., Nelson D. S. Mouse mast cells as anti-tumor effector cells. Cell Immunol. 1980 Oct;55(2):294–301. doi: 10.1016/0008-8749(80)90162-8. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Brooks C. G., Dvorak A. M., Ishizaka T. Lack of detectable immunoglobulin E receptor expression on 33 of 34 cell lines with natural killer-like or cytotoxic-T-lymphocyte activity. Cell Immunol. 1985 Nov;96(1):223–230. doi: 10.1016/0008-8749(85)90353-3. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Marcum J. A., Ishizaka T., Nabel G., Der Simonian H., Pyne K., Goldin J. M., Rosenberg R. D., Cantor H. Mast cell clones: a model for the analysis of cellular maturation. J Cell Biol. 1982 Nov;95(2 Pt 1):435–444. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S. J. New approaches for the analysis of mast cell maturation, heterogeneity, and function. Fed Proc. 1987 Apr;46(5):1906–1914. [PubMed] [Google Scholar]
- Ghiara P., Boraschi D., Villa L., Scapigliati G., Taddei C., Tagliabue A. In vitro generated mast cells express natural cytotoxicity against tumour cells. Immunology. 1985 Jun;55(2):317–324. [PMC free article] [PubMed] [Google Scholar]
- Green L. M., Reade J. L., Ware C. F. Rapid colorimetric assay for cell viability: application to the quantitation of cytotoxic and growth inhibitory lymphokines. J Immunol Methods. 1984 May 25;70(2):257–268. doi: 10.1016/0022-1759(84)90190-x. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y., Jong E. C., Klebanoff S. J. Mast cell-mediated tumor-cell cytotoxicity. Role of the peroxidase system. J Exp Med. 1981 Mar 1;153(3):520–533. doi: 10.1084/jem.153.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadus M. R., Schmunk G., Djeu J. Y., Parkman R. Morphology and lytic mechanisms of interleukin 3-dependent natural cytotoxic cells: tumor necrosis factor as a possible mediator. J Immunol. 1986 Nov 1;137(9):2774–2783. [PubMed] [Google Scholar]
- Kido H., Fukusen N., Katunuma N. Chymotrypsin- and trypsin-type serine proteases in rat mast cells: properties and functions. Arch Biochem Biophys. 1985 Jun;239(2):436–443. doi: 10.1016/0003-9861(85)90709-x. [DOI] [PubMed] [Google Scholar]
- Lagunoff D., Martin T. W., Read G. Agents that release histamine from mast cells. Annu Rev Pharmacol Toxicol. 1983;23:331–351. doi: 10.1146/annurev.pa.23.040183.001555. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Perussia B., Cohn Z. A., Young J. D. Identification and characterization of a pore-forming protein of human peripheral blood natural killer cells. J Exp Med. 1986 Dec 1;164(6):2061–2076. doi: 10.1084/jem.164.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. C., Steffen M., King F., Young J. D. Identification, isolation, and characterization of a novel cytotoxin in murine cytolytic lymphocytes. Cell. 1987 Nov 6;51(3):393–403. doi: 10.1016/0092-8674(87)90635-0. [DOI] [PubMed] [Google Scholar]
- Nabel G., Galli S. J., Dvorak A. M., Dvorak H. F., Cantor H. Inducer T lymphocytes synthesize a factor that stimulates proliferation of cloned mast cells. Nature. 1981 May 28;291(5813):332–334. doi: 10.1038/291332a0. [DOI] [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985 Sep 1;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno T., Takagaki Y., Pluznik D. H., Djeu J. Y. Natural cytotoxic (NC) cell activity in basophilic cells: release of NC-specific cytotoxic factor by IgE receptor triggering. J Immunol. 1986 Jun 15;136(12):4652–4658. [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason L. H., Mathieson B. J., Liang S. M., Flick D. A., Herberman R. B. Mediation of mouse natural cytotoxic activity by tumour necrosis factor. Nature. 1986 Jun 12;321(6071):700–702. doi: 10.1038/321700a0. [DOI] [PubMed] [Google Scholar]
- Otsu K., Nakano T., Kanakura Y., Asai H., Katz H. R., Austen K. F., Stevens R. L., Galli S. J., Kitamura Y. Phenotypic changes of bone marrow-derived mast cells after intraperitoneal transfer into W/Wv mice that are genetically deficient in mast cells. J Exp Med. 1987 Mar 1;165(3):615–627. doi: 10.1084/jem.165.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Figarella E. F., Cuttito M. J., Cahan A., Stutman O. Natural cytotoxic cells against solid tumors in mice. II. Some characteristics of the effector cells. J Immunol. 1978 Nov;121(5):1827–1835. [PubMed] [Google Scholar]
- Podack E. R., Young J. D., Cohn Z. A. Isolation and biochemical and functional characterization of perforin 1 from cytolytic T-cell granules. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8629–8633. doi: 10.1073/pnas.82.24.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin D. C., Adelman S., Austen K. F., Stevens R. L., Hein A., Caulfield J. P., Woodbury R. G. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutman O., Paige C. J., Figarella E. F. Natural cytotoxic cells against solid tumors in mice. I. Strain and age distribution and target cell susceptibility. J Immunol. 1978 Nov;121(5):1819–1826. [PubMed] [Google Scholar]
- Tanooka H., Kitamura Y., Sado T., Tanaka K., Nagase M., Kondo S. Evidence for involvement of mast cells in tumor suppression in mice. J Natl Cancer Inst. 1982 Dec;69(6):1305–1309. [PubMed] [Google Scholar]
- Young J. D., Damiano A., DiNome M. A., Leong L. G., Cohn Z. A. Dissociation of membrane binding and lytic activities of the lymphocyte pore-forming protein (perforin). J Exp Med. 1987 May 1;165(5):1371–1382. doi: 10.1084/jem.165.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Leong L. G., Liu C. C., Damiano A., Wall D. A., Cohn Z. A. Isolation and characterization of a serine esterase from cytolytic T cell granules. Cell. 1986 Oct 24;47(2):183–194. doi: 10.1016/0092-8674(86)90441-1. [DOI] [PubMed] [Google Scholar]