Abstract
Background & Aims
The tumor suppressor PTEN inhibits AKT2 signaling; both are aberrantly expressed in liver tumors. We investigated how PTEN and AKT2 regulate liver carcinogenesis. Loss of PTEN leads to spontaneous development of liver tumors from progenitor cells. We investiged how the loss of PTEN activates liver progenitor cells and induces tumorigensis.
Methods
We studied mice with liver-specific disruptions in Pten and the combination of Pten and Akt2 to investigate mechanisms of liver carcinogenesis.
Results
PTEN loss leads to hepatic injury and establishes selective pressure for tumor-initiating cells (TICs), which proliferate to form mixed-lineage tumors. The Pten-null mice had increasing levels of hepatic injury before proliferation of hepatic progenitors. Attenuation of hepatic injury by deletion of Akt2 reduced progenitor cell proliferation and delayed tumor development. In Pten/Akt2-null mice given 3,5-diethoxycarbonyl-1,4 dihydrocollidine, the primary effect of AKT2 loss was attenuation of hepatic injury and not inhibition of progenitor-cell proliferation in response to injury.
Conclusion
Liver carcinogenesis in Pten-null mice requires not only the transformation of TICs but selection pressure from hepatic injury and cell death, which activate TIC. Further research is required to elucidate the mechanism for hepatic injury and its relationship with TIC activation.
Keywords: Liver cancer stem cells, DDC, lipotoxic cell death, mixed-lineage tumors
INTRODUCTION
Recent studies propose tumor progenitor/tumor initiating cells (TIC) are the cause of tumor cellular heterogeneity [1]. Initially identified for leukemia, putative TICs putative TICs have been identified in solid tumors including liver cancers [2–4]. In rodents, facultative liver progenitors are found to be multipotent and express phenotypic markers of hepatocytes, such as epithelial cell adhesion molecule EpCAM, α-fetoprotein (AFP) and ductal lineage markers such as keratins [5,6]. Moreover, these progenitor cells can differentiate into both hepatocytes and cholangiocytes, the two major cell lineages in the liver [3,7]. In humans, liver cancer patients with a “progenitor cell” phenotype demonstrate poor prognosis compared to patients with differentiated “hepatocyte” cancers [8]. A second series of experiments have identified a population of chemo-resistant and tumorigenic TICs within established hepatocellularcarcinoma (HCC) cell lines [9]. Despite these new findings, the mechanism driving the activation of TICs is still not clear.
Our group developed a liver cancer mouse model that carries hepatic deletion of tumor suppressor phosphatase and tensin homologue deleted on chromosome ten (Pten) [10,11]. PTEN is the primary negative regulator of the phosphatidylinositol-3 kinase (PI3K)/AKT pathway. In humans, PTEN loss and upregulation of AKT2, the primary AKT isoform in the liver, are highly associated with liver cancer [12–16]. Deletion of Pten in mouse liver results in hepatosteatosis and inflammation followed by liver cancer [10,11,17]. This two-phase progression paradigm is similar to human liver cancers in which steatohepatitis is a common pre-condition or co-morbidity to HCC development. Using the liver specific Pten deletion model, our group identified a stem cell population that is capable of grafting multilineage tumors and that demonstrates resistance to traditional chemotherapy [18]. In this study, we elucidate the downstream mechanisms leading to the expansion of TICs and tumor formation in vivo using two unique genetic models that we developed, the liver specific Pten null (Pm) mice and a newly developed Pten/Akt2 double mutant (Dm) model. Our study demonstrates that proliferation of liver progenitor cells is a consequence of chronic hepatic injury resulting from Pten deletion in hepatocytes. Deletion of Akt2 abrogates hepatic injury induced with Pten deletion but not the capacity of progenitor cells to proliferate, resulting in the delay of tumor development. Our findings suggest that without the selection pressure from chronic hepatic injury, loss of PTEN in TICs is not sufficient in and of itself to drive the progression of liver cancer. In addition to the 40–50% human liver cancers that carry Pten mutation[12–15], our findings are relevant to the majority of liver cancer patients in which chronic liver injury precedes cancer development.
MATERIALS AND METHODS
Animals
Targeted deletion of Pten was reported previously [10]. Pten/Akt2 double mutant (PtenloxP/loxP; Akt2−/−; Alb-Cre+) (Dm) were generated by crossing the PtenloxP/loxP; Alb-Cre+ (Pm) with the Akt2−/− mice [19]. Control animals are PtenloxP/loxP; Albumin (Alb)-Cre−. Blood samples are collected through cardiac puncture prior to tissue collection. 3,5-dietoxycarbonyl-1,4 dihydrocollidine (DDC, 0.1% w/w diet) treatment was performed in 3-month old mice for 5 weeks. Male animals of C57BL/6 and J129svj background were used for all experiments. Experiments were conducted according to IACUC guidelines of the University of Southern California.
Cell lines
Hepatic progenitor cell lines were established and cultured from Pm liver as described [18]. For growth curve analysis, cells were serum starved overnight before addition of 25 ng/ml platelet derived growth factor (PDGFA) (Invitrogen, Camarillo, CA). Cell growth was quantified by counting the number of cells in vehicle vs. PDGFA treated cultures.
Immunohistochemistry
Liver sections were stained with hematoxylin and eosin (H&E) for morphology analysis, anti-human trans-4-hydroxy-2-nonenal (4-HNE, Northwest Life Science Specialties LLC, Vancouver, WA) for lipid peroxidation product, and hepatocyte paraffin (Hep-Par1) & keratin (DakoCytomation, Denmark A/S) to identify hepatocytes and cholangiocytes respectively. Apoptosis was determined using TUNEL staining (Roche Diagnostics, Manheim Germany). Cell proliferation was evaluated by Ki67 staining (Thermo Fisher Scientific, Fremont, CA). Six sections per group were stained.
Serum alanine aminotransferase (ALT) and liver hydrogen peroxide (H2O2) quantification
Serum ALT was determined using ALT Reagent (Raichem, San Diego, CA). Liver H2O2 was assayed using Amplex Red Hydrogen Peroxide kit (Molecular Probes, Eugene, OR) [17]. H2O2 levels were normalized by protein concentration.
Quantitative PCR
Total RNA (2μg) from liver tissues was used for first strand synthesis of cDNA (Promega). Maxima SYBR Green qPCR Master Mix (Fermentas, Glen Burnie, MD) was used for the qPCR reaction and quantification was determined using the ΔΔCt method. Primers used are: AFP, EpCAM, K-19, Wnt7a and 10a, Fizzled receptor 2 (Fzd2), glutathione peroxidase (GPx), glutathione-S-transferase (GST), PDGFA and GAPDH (Table S1)[20,21].
Protein Electrophoresis
Protein lysates (40 μg) were loaded for each sample for electrophoresis using polyacrylamide gels. Membranes were probed with antibodies for keratin (Millipore, Billerica, MA), AFP (Epitomics, Burlingame, CA), and β-catenin (Sigma-Alderich, St. Louis, MO). Tubulin and β-actin (Sigma) protein expression are used as loading controls.
Microarray
RNA isolated from 9 month-old mice was analyzed using the Illumina Mouse gene chip (Illumina, San Diego, CA) [22,23]. Data analysis was conducted with 2-fold or greater change in expression considered to be different.
Statistics
Data were subjected to Student’s t tests for two sample comparisons. In cases of more than 2 groups, multivariate ANOVA were used to determine the statistical differences followed by pairwise comparison using Fischer’s LSD test. P≤0.05 is considered to be statistically significant. Data are presented as mean+SEM.
RESULTS
Deletion of Akt2 inhibits liver cancer development in the Pten null mice
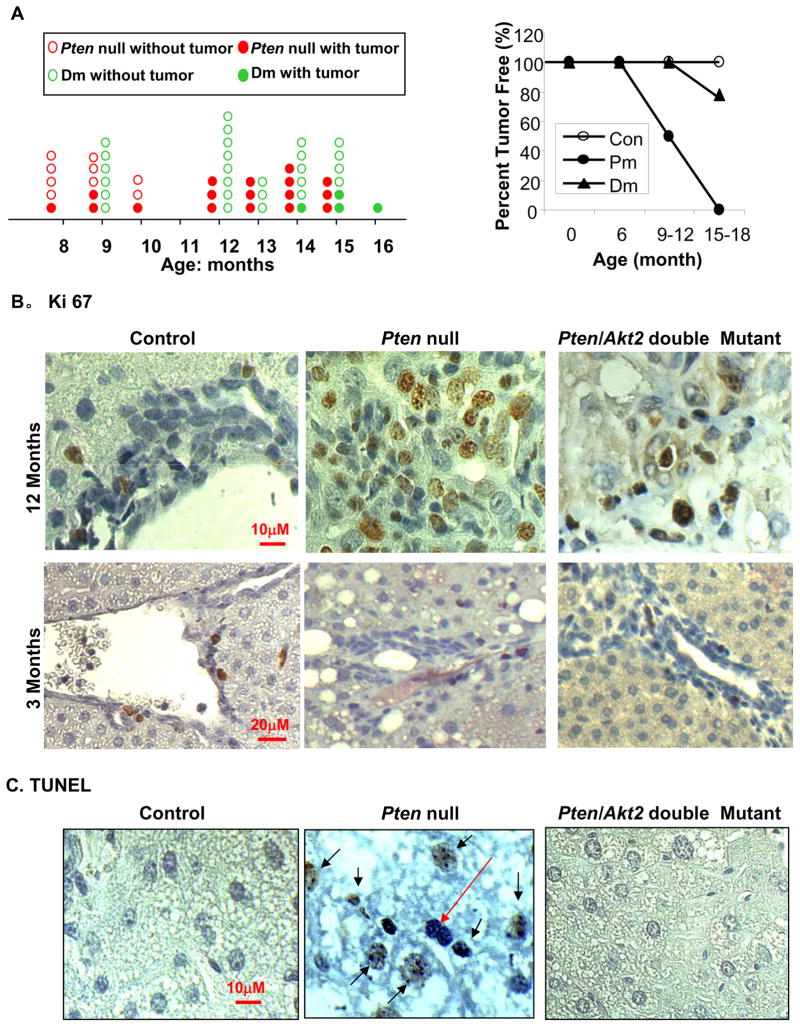
Overexpression of AKT2 is associated with human liver carcinogenesis [16]. How AKT2 contributes to liver cancer development is unclear because germline deletion of Akt2 produced a metabolic but not growth/survival phenotype [24]. We analyzed tumor spectrums in Pten null (Pm) and Pten/Akt2 double mutant (Dm) mice to assess the function of AKT2 in liver carcinogenesis. Pm mice develop tumors starting at approximately 8–9 months of age [10] without significant differences between males and females. A 6-month delay in tumor onset is observed when Akt2 is deleted simultaneously with Pten in Dm mice (Fig 1A, left panel). Between 9–12 months of age, 50% of the Pm mice (5/10) developed tumors compared to 0% (0/14) of the Dm mice (Fig 1A and table s2). 100% of Pm mice 12 months and older (10/10) developed tumors. Only 25% of Dm mice older than 12 months developed liver nodules (4/16), and only 2 had visible tumors.
Figure 1. Akt2 deletion inhibits tumor growth in Pten null mice.
A. Left panel, tumor spectrum. Each red circle represents one Pten null (Pm) mouse. Each green circle represents one Pten/Akt2 double mutant (Dm) mouse. The solid circles represent mice with tumors and open circles represent mice with tumors. Right panel, tumor data presented as percentage of the total number of animals evaluated. B. Liver sections were stained with a cell proliferation marker Ki67 (brown nuclei staining). Top panel, 12 month old mice. Bottom panel, 3 month-old mice. C. Liver sections were stained with TUNEL (brown nuclei staining) to identify apoptotic cells. All sections were counterstained with hematoxylin for nuclei identification. Black arrows: TUNEL positive cells. Red arrow: TUNEL negative cells.
Paradoxical Roles of PTEN and AKT2 in proliferation and survival of liver cells
The observation that Akt2 deletion delays tumor development in the Pm model is not surprising since the canonical role of AKT kinases is pro-growth and pro-survival. We evaluated cell proliferation and survival in Pm and Dm livers. At 12 months of age, we observed a significantly higher Ki67 positive proliferation index in Pm compared to Dm and Control livers (Fig 1B, top panel and supplemental Fig 1). Because Dm and Controls are not tumor bearing at this age, we also analyzed the mitotic index in pre-malignant 3-month old mice. Interestingly, we discovered very limited proliferation activity in the Pm liver (Fig 1B, bottom panel) whereas concurrent deletion of Akt2 had no effect on the proliferation index. Moreover, TUNEL staining showed that simultaneous deletion of Akt2 with Pten inhibits apoptosis rather than causing more apoptotic cells (Fig 1C). Furthermore, Pten deletion leads to progressive hepatocyte cell death as the phenotype progress from steatosis to premalignancy phase (supplemental Fig 2). By 9 months of age, the majority of the hepatocytes are TUNEL positive indicating massive hepatocyte death in the Pm liver (Fig 1C).
Pten null liver sustains chronic liver injury that is relieved by Akt2 deletion
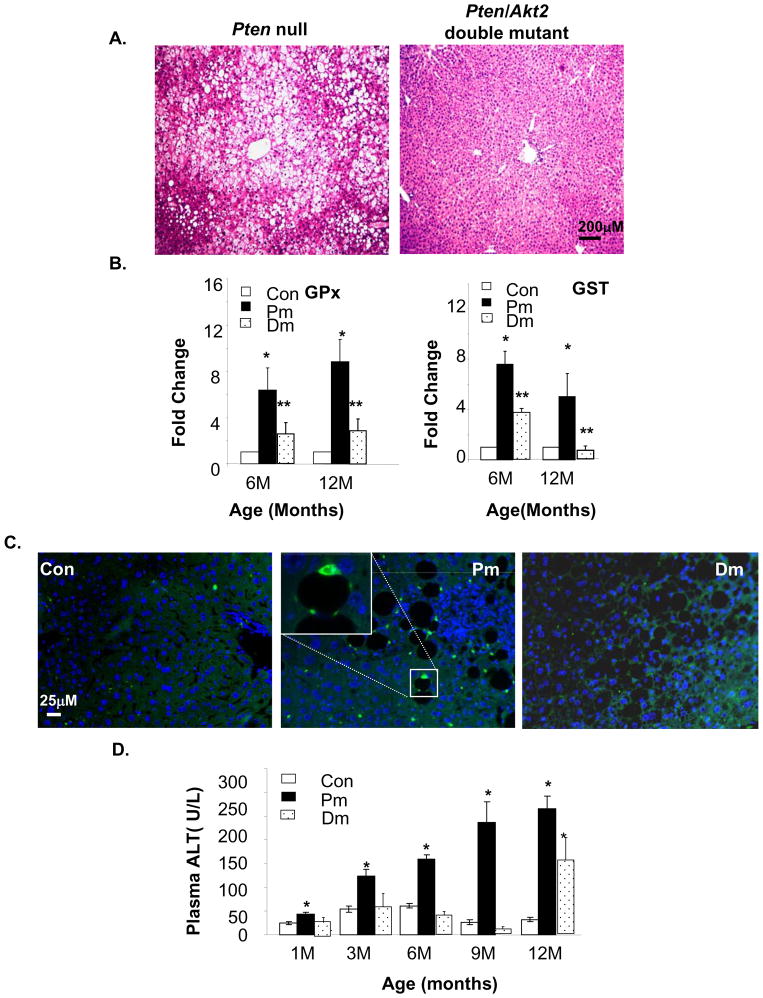
These observations are inconsistent with the roles of AKT as an anti-and PTEN, a pro-apoptotic molecule. Given the presence of progressive and massive steatosis in Pm mice [10] and the lack thereof in the Dm liver (Fig 2A), we hypothesized that steatosis may be causing liver injury in the Pm model. In Dm mice, injury is attenuated due to the inhibitory effect of AKT2 loss on lipogenesis [25]. Liver injury is often accompanied by the loss of liver function and increase in oxidative stress [26,27]. The Pm livers exhibited high H2O2 contents [17] (supplemental Fig 3). H2O2 can transcriptionally activate enzymes responsible for reducing oxidative stress. Expressions of H2O2 scavenger GPx and antioxidant enzyme GST are significantly higher in Pm mice compared to Controls from 6–12 months of age (Fig 2B). In Dm mice, the mRNA expression of GPx and GST are significantly reduced compared to Pm mice. We performed immunostaining for 4-HNE, a lipid peroxidation product and identified 4-HNE aggregates in Pm livers, particularly surrounding lipid droplets (Fig 2C, middle panel inset). Consistent with GPx and GST expression analysis, 4-HNE positive staining is significantly reduced in Control and Dm livers, suggesting oxidative stress and injury conditions are present in the Pm liver.
Figure 2. Akt2 deletion inhibits liver injury induced by Pten deletion in the liver.
A. Representative H&E image show liver steatosis in 3 months old Pten null (Pm) mice and not in the Pten/Akt2 double mutant (Dm) mice. B. qPCR analysis reveals higher expression of GPx and GST in Pm than in Dm and Controls. Values are expressed as fold change vs. Controls (set as 1). C. Immunostaining with anti-4-HNE shows accumulation of lipid peroxidation aggregates (green fluorescent stained spots) in the Pm liver. The sections are also stained with DAPI (blue) for nuclei. D. Liver injury is measured by serum ALT quantification. All values are expressed as the mean ± SEM. * indicates significant difference from Controls (p≤0.05). ** indicates significant difference from Pm (p≤0.05). n=5.
We measured the serum levels of ALT, a clinical marker for liver function and found that it increases progressively as Pm mice age, reaching levels 5 fold higher levels than Controls at 12 months of age (Fig 2D). Continuously rising ALT indicates progressive and chronic liver injury. In contrast, serum ALT levels of Dm mice remained at control levels (approximately 50U/L) until 12 months of age when levels began to increase (Fig 2D). Low levels of serum ALT prior to 12 months of age (Fig 2D) is concurrent with the delayed onset of hepatosteatosis observed in Dm mice(data not shown). Together, these findings suggest that the Pm liver is undergoing chronic injury.
Accumulation of hepatic progenitor cells in Pten null mice is inhibited by AKT2 loss
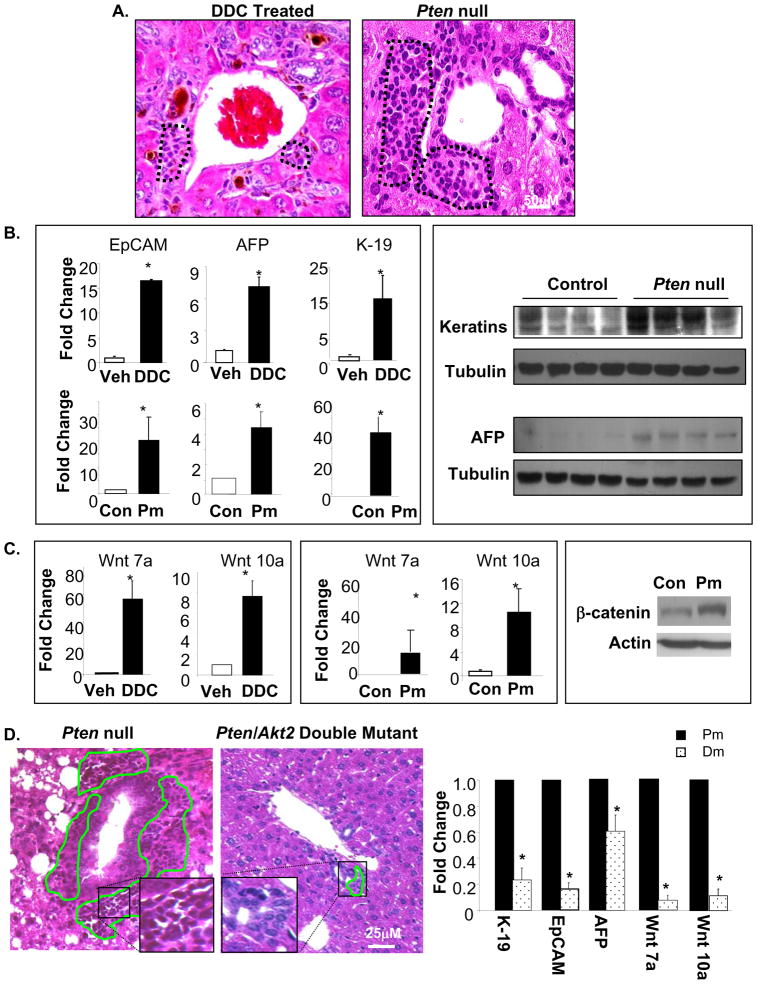
Concomitant with hepatosteatotic injury, we observed an accumulation of cells in the periductal region of Pm livers from 9–12 months of age. These cells are morphologically similar to liver progenitor cells observed in hepatotoxin DDC induced liver [28] injury models (Fig 3A, dotted areas). Similar to DDC treatment models, the expression of several hepatic progenitor markers including K-19, EpCAM, and AFP [29,30] is significantly higher in Pm mice compared to Controls (Fig 3B). Protein expression of pan-keratins and AFP are also higher in Pm mice compared to Controls (Fig 3B). In addition, Wnt7a, and 10a have been identified as inducible mediators of hepatic progenitor cells [31]. Expression of Wnt7a and 10a are also upregulated in Pm mice (Fig 3C). Increased protein expression of β-Catenin from cell lines isolated from Pm mice corroborates upregulation of Want/β-Catenin Pathway components (Fig 3C, right panel). The histological similarities between Pm and DDC mice and the gene expression profiles suggest that hepatic progenitor cells are present in the livers of the Pm mice. We analyzed Dm liver sections and found significantly reduced progenitor cell accumulation in the periodical region, the putative progenitor cell niche (Fig 3D, left panel). Accumulation of progenitor cells occurs later when injury conditions are present in mice 12 months and older (supplemental Fig 4), indicating that the effect of AKT2 on progenitor cell activation may be secondary to liver injury. Expression of progenitor cell markers K-19, EpCAM, and AFP are also significantly reduced by 5, 5 and 2 fold respectively in the Dm vs. Pm mice (Fig 3D, right panel). Expression of Wnt7a and 10a is reduced approximately by 10 fold (Fig 3C, right panel). The morphological and expression analysis suggests that deletion of Akt2 inhibits progenitor cell accumulation.
Figure 3. Deletion of Akt2 inhibits expansion of liver progenitor cells observed in Pten null mice.
A. H&E stains of liver sections from DDC treated mice demonstrate progenitor cell accumulation at the portal vein (circled, left panel). Pten deletion (Pm) causes a similar accumulation of progenitor cells in portal areas at 9 months of age (circled, right panel). B. Left panel, qPCR analysis of hepatic progenitor markers EpCAM (left), AFP (middle), and K-19 (right). Top, DDC treated vs. vehicle; Bottom, Pm vs. Controls (Con). Right panel, Western analysis of AFP and Keratin protein levels in Control and Pm mice. Tubulin is detected as loading control. C. Left panel, qPCR analysis of Wnt 7a and 10a in DDC vs. vehicle treated mice. Middle panel, qPCR analysis of Wnt 7a and 10a in Pm vs. Control (Con) mice. Right panel, analysis of β-catenin protein levels in cells isolated from livers of Con and Pm mice. β-actin is detected as loading control. D. Left panel, H&E staining shows multiple layers of progenitor cells surrounding the ductal structures in the Pm liver (left). Progenitor cells accumulation is limited in the Dm liver (middle). Expression of EpCAM, AFP, K-19, and Wnt7a/10a are reduced in Dm vs. Pm livers (right). Data expressed as fold change over Pm (set as 1). All values expressed as the mean ± SEM. * indicates significant difference between the two groups (p≤0.05). n=5.
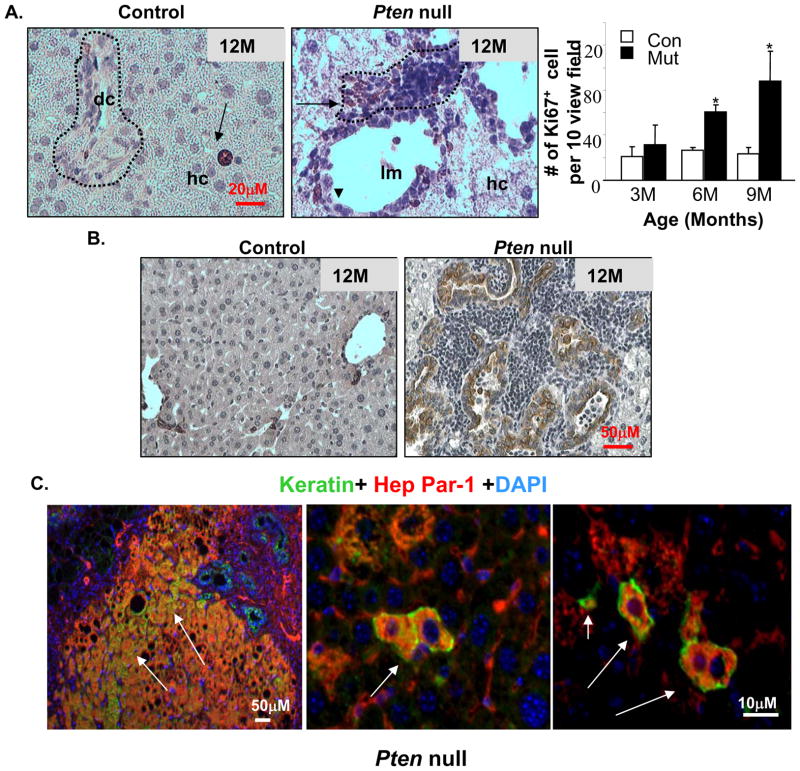
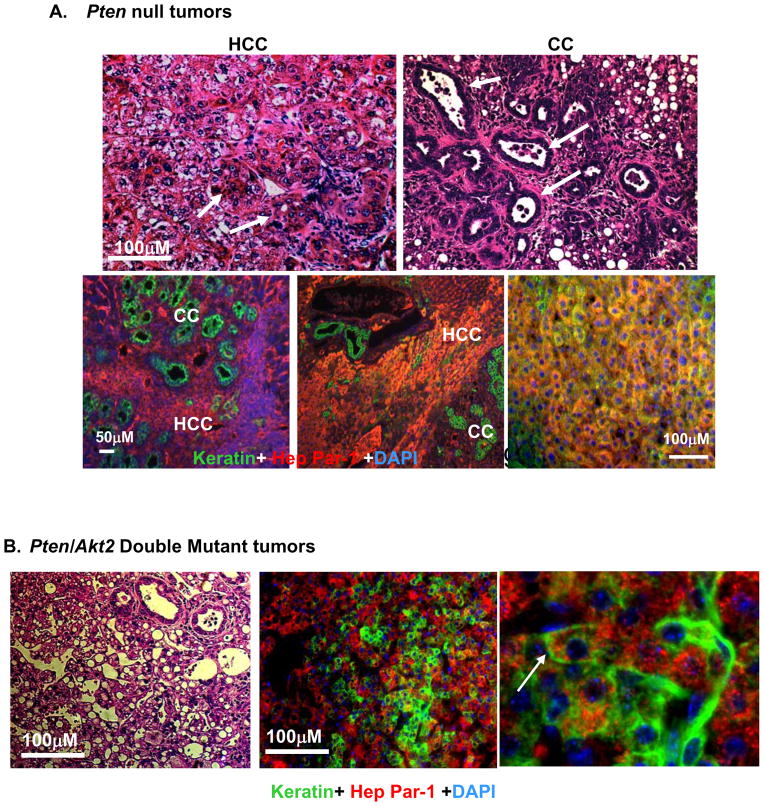
In Pm liver sections, we observed multiple layers of progenitor cells surrounding a single layer of ductal cells. These progenitor cells exhibit high mitotic activity indicated by Ki-67 staining (Fig 4A). This phenotype becomes prominent after 9 months of age, after extensive hepatocyte apoptosis occurs. We investigated the differentiation of progenitor cells to cholangiocytes using keratin staining (Fig 4B and supplemental Fig 5) and observed a simultaneous increase of keratin positive ductal cells when extensive progenitor cell proliferation occurs (Fig 4B). Transient progenitors can give rise to the two major liver cell types and are expected to express markers for both. To identify the hepatic progenitor cells in vivo, we performed keratin and Hep Par-1 (hepatocyte marker) double staining. In Pm liver sections, we found large clusters of as well as isolated progenitor cells co-expressing both lineage markers (Fig 4C).
Figure 4. Proliferation and differentiation of progenitor cells in the Pten null mice.
A. Immunohistochemical staining with Ki67 (brown stained nucleus) shows that mitotic activity is predominantly found in the peri-ductal region (arrows) in the liver progenitor cell niche (dotted circle) in Pm mice (middle). Arrow heads, ductal cells. Control livers contain few proliferating hepatocytes (arrow in left panel), and low mitotic activity at the portal triad (circled area, left panel). Right, quantification of ki67 positive cells. Values expressed as the mean ± SEM. * indicates values that are significantly different at p≤0.05. n=5. B. Immunohistochemical staining with keratin (brown stain) revealed an increase in ductal lineage cells associated with the expansion of hepatic progenitors. C. Identification of bi-lineage progenitor cells (arrows) coexpressing hepatocyte (HepPar-1) and cholangiocyte (keratin) markers in the Pm liver. Red, Hep-Par; Green, Keratin; Blue, DAPI.
Deletion of Akt2 does not alter the mixed cell characteristics of the tumors
The tumors developed in Pm mice displayed mixed cell characteristics (Fig 5A). Staining of tumor sections with HepPar-1 and keratin confirmed that the tumors are composed of three major cell types: cholangiocytes, hepatocytes, and bi-lineage cells (Fig 5A, bottom panels). Histologic and immunohistochemical analysis of HepPar-1 and keratin demonstrate that deletion of Akt2 did not alter the mixed cell characteristics of the tumor in Dm mice (Fig 5B). In addition, we found cells coexpressing HepPar-1 and keratin (Fig 5B, right panel), suggesting that bi-lineage progenitors are also the likely sources of tumors in the Dm mice. In addition, expression of hepatic progenitor markers AFP, K-19 and EpCAM from the two Dm mice that developed macroscopic tumors is similar to Pm mice (data not shown), supporting the progenitor cell characteristics of the tumors.
Figure 5. Deletion of Akt2 does not alter the mixed cell tumor phenotype in liver specific Pten null mice.
A. Top, H&E images of tumors developed in the Pten null mice demonstrate compact trabecular growth patterns and pseudoglandular structures of HCC (arrows, left panel); and tubular features of CC (arrows, right panel). Bottom, Immunohistochemistry of liver tissue with HepPar-1 (red) and keratin (green) in Pm mice identifies HCC and CC respectively. Blue, DAPI. B. H&E section of one of the two tumors formed in the Pten/Akt2 double mutant liver (left panel). Immunochemical staining confirms bilineage tumor development with both Hep Par-1 (red) and keratin (green) (right two panels). Arrow point to bilineage cells expressing both markers.
Chemical injury in Dm mice leads to activation of liver progenitors and development of premalignant lesions
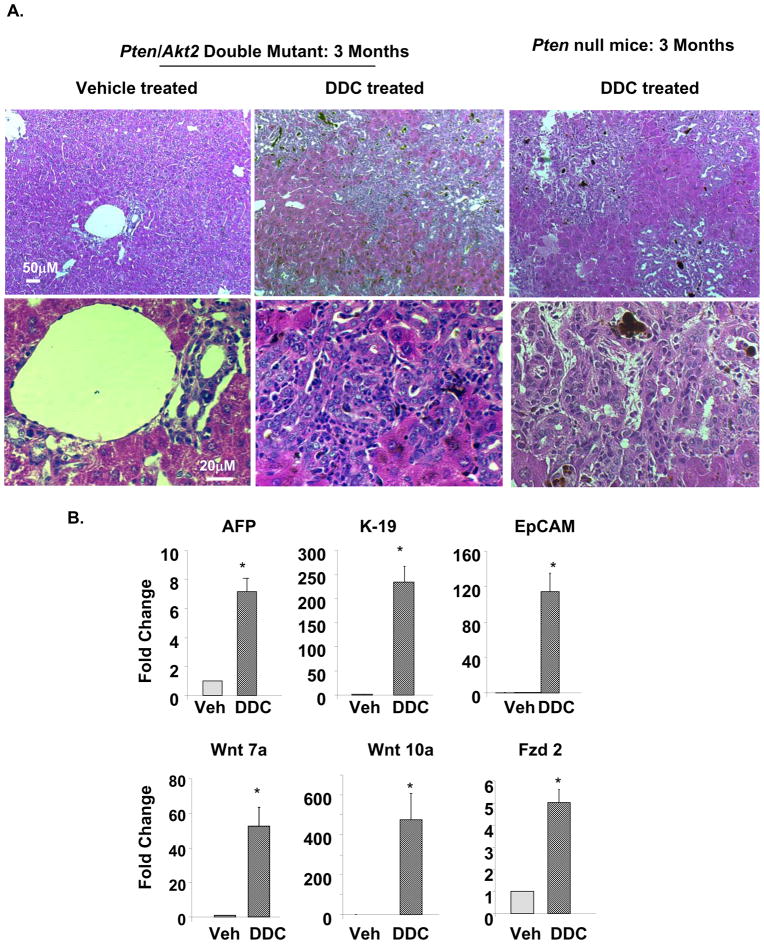
Our observations suggest that liver injury may be a crucial component for tumor development in the Pten deletion model. To determine if liver injury is necessary for the development of tumors, we treated the Dm mice with DDC to induce liver injury. Our data demonstrates that deletion of Akt2 in the Dm model does not inhibit the ability of hepatic progenitors to respond to liver injury. DDC treatment in 3 month-old Dm mice induced massive expansion of hepatic progenitor cells and premalignant lesions (Fig 6A). These lesions are morphologically similar to those observed in the premalignant 9–12-month livers. Comparison of DDC treatment in Dm and Pm mice showed identical morphology with focal expansion of progenitors and cholangiocytes. This data suggest that Akt2 deletion does not inhibit the ability of progenitor cells to proliferate. Expression of AFP, K-19 and EpCAM is also robustly induced by 7, 226 and 116 fold respectively in Dm mice treated with DDC vs. vehicle (Fig 6B, top panel). Expression of Wnt7a and 10a and receptor Fzd2 are all significantly elevated in DDC vs. vehicle treated Dm mice (Fig 6B, bottom), correlating with the activation of hepatic progenitor niche. These expression data support the notion that the ability of hepatic progenitors to respond to injury is not inhibited by AKT2 loss.
Figure 6. Deletion of Akt2 in the Pten null liver does not hinder the proliferation of progenitor cells in response to DDC treatment.
A. Three months Pten null (Pm) and Pten/Akt2 double mutant (Dm) mice were treated with DDC to induce the expansion of progenitor cells. Both groups of mice responded to DDC treatment with progenitor cell expansion phenotypes. Top panel, low magnification images showing the extent of liver damage and progenitor cell expansion. Bottom panel, high magnification images show progenitor cell morphology. B. Markers for progenitor cells, AFP, K-19 and EpCAM are induced when Dm mice are treated with DDC vs. vehicle (Veh, top panel). The markers for progenitor cell niche Wnt 7a and 10a and Fzd2, a receptor for Wnt are also induced in the DDC treated Dm mice (bottom panel). Data presented as mean±SEM. * indicates values that are significantly different from that of vehicle controls at p≤0.05. n=5.
Growth factors may mediate the activation of hepatic progenitor cells
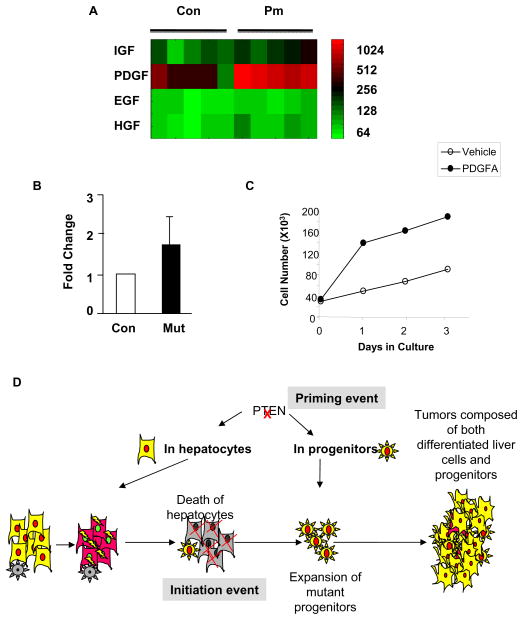
To determine the potential molecular mechanism leading to the activation of hepatic progenitors, we performed transcriptome profile analysis of 9 month- old Control and Pm mice. This analysis revealed that PDGF, a mesenchymal growth factor is robustly upregulated in the Pm compared to Control mice (Fig 7A). qPCR analysis confirmed that PDGF expression is 50% higher in Pm mice vs. Control mice (Fig 7B). Furthermore, we treated the liver progenitor cells that we established from the Pm mice with PDGF. Our data showed that addition of PDGF stimulated the growth of these progenitor cells (Fig 7C) and validate that PDGF is one of the factors that stimulate the growth of progenitor cells in this model. PDGF has received significant attention recently for its association with liver carcinogenesis [32,33]. Our findings suggest that PDGF may be one of the promoting factors for the growth and expansion of liver progenitor cells. Other growth factors such as IGF, HGF and EGF were not robustly induced by PTEN loss (Fig 7A).
Figure 7. PDGF is potential growth factor mediating the progenitor cell expansion in the Pten null mice.
A. Heatmap of microarray analysis of expression for IGF, PDGF, EGF and HGF. Expression of PDGF is robustly induced in the Pten null (Pm) mice vs. the Controls. Each square represents one mouse. Relative level of expression is indicated with color intensity. Color scale (log scale) is provided as reference. B. Quantitative PCR data confirming the upregulation of PDGF in the Pm liver. C. Growth curve of PDGF and vehicle treated progenitor cell cultures. Solid circle, PDGF (25ng/ml) treated culture; Open circle, vehicle treated culture. D. Schematic representation of tumor progression in the Pten null mice.
DISCUSSION
In this study, we investigated AKT2, a major downstream effector molecule of PTEN regulated pathways and its role in the activation of TICs. Using in vivo markers identified in human liver cancer (EpCAM, AFP, and K-19) [30,34] and morphological analysis [4,35–37], we analyzed the proliferation and accumulation of liver progenitor cells. Our analyses demonstrate that the activation of TICs and development of liver tumors in our model system is a multi-stage process that requires two critical events (Fig 7D). The first is transformation of progenitor cells after deletion of Pten, an event that is NOT inhibited by AKT2 loss in our model. This primary transformation provides a growth advantage for the mutant progenitors and primes them for proliferation when the proper signals are present. The second event is chronic injury and impaired hepatocyte regeneration, which is due to liver steatohepatitis in the Pten null model. This second event is inhibited by AKT2 loss. This inflammation and impaired hepatocyte replication response provides the signal impetus for progenitor cells to proliferate.
Our analysis shows that PTEN may have distinct functions in hepatocytes vs. liver progenitors. In hepatocytes, deletion of Pten leads to lipid accumulation and cell death. In humans, hepatocyte cell death is a morphological and pathological feature of non-alcoholic steatohepatitis [38]. It is conceivable that the anti-apoptosis effect of PTEN loss in hepatocytes is overshadowed by the toxicity effects of lipid accumulation resulting in hepatocyte death as a net effect. Experimentally, free fatty acids have been shown to induce death of multiple cell types including hepatocytes [39]. In our model, lipid accumulation is the immediate and primary effect of PTEN loss [10]. AKT2 is the major regulatory molecule for hepatic lipogenesis when Pten is deleted. [25]. AKT2 loss inhibited lipogenesis and inhibited cell death, suggesting that lipid toxicity is the primary cause of hepatocyte death in the Pm mice. Among the AKT kinases, AKT2 is characterized for its role in metabolic regulation. Genetic manipulation of AKT2 by itself has failed to show any effect on cell growth and survival [24]. However, upregulation of AKT2 is associated with human HCC suggesting that AKT2 may have a pro-growth and pro-survival role in tumor transformation [16]. Our analysis showed that in a tumor model where PI3K/AKT signaling is upregulated by Pten loss, Akt2 deletion still does not induce any cell death. On the contrary, we observed reversion of fatty liver, recovery of injury, and inhibition of apoptosis determined by TUNEL analysis. Thus, the primary function of AKT2 in this context of tumor transformation is still not pro-survival or pro-growth but rather metabolic regulation.
In liver progenitor cells, PTEN loss resulted in induction of proliferation as predicted. However, this effect was preceded by chronic liver injury. In Pm mice, this condition is brought about at 9–12 months of age after massive lipid accumulation and severe liver damage. Chemical injury in young mice (3 months of age) with hepatotoxin DDC induces expansion of progenitor cell population and the development of premalignant lesions. This observation suggests that progenitor cells in the Pm mice are primed for proliferation and that their proliferation is initiated only after injury is induced either by lipid toxicity in older Pm mice or DDC treatment in young mice. Similarly, DDC treatment also led to expansion of progenitor cells in the Dm mice. Indeed, the phenotypes of the DDC treated Pm and Dm mice are identical, suggesting that pro-growth signals downstream of PTEN in progenitors are not compromised by AKT2 loss. These observations are consistent with loss of function studies of AKT2 versus other AKT kinases [24,40–42]. Thus, the fact that Akt2 deletion attenuated tumor growth is not due to inhibition of cell growth/survival but rather its effect on liver injury, a key element for the activation of the primed progenitor cells.
As hypothesized by Sell and others, liver injury creates a niche for the activation of liver progenitor cells [43]. In DDC induced injury models, , Wnt7a and 10a are upregulated and may contribute to the activation of hepatic progenitor cells [44]. We demonstrate here that these Wnt ligands are also induced in the Pm liver. Wnt/β-catenin is a signaling pathway associated with maintenance of progenitor cell niche. It is upregulated when liver progenitor cell marker (keratin 7) is induced [45]. Topical expression of constitutively active β-catenin, the core molecule in Wnt signaling, promotes the expansion of OV6 positive liver progenitor cells [46]. Thus, Wnt/β-catenin signaling, likely regulates the liver progenitor cell compartment in our Pm model.
In addition, our microarray analysis identified PDGF as a potential growth factor that may induce progenitor cell proliferation. Expression of PDGF receptor is associated with HCC in humans [32]. Furthermore, expression of PDGF in the liver accelerated chemical carcinogen induced tumor growth [33]. The primary function of the PDGF family members is controlling the growth of mesenchymal cells. Upregulation of PDGF in the Pm mice likely alters the progenitor cell niche and allows the proliferation of hepatic progenitor cells and their subsequent progression to liver cancer. A potential link between overexpression of PDGF with liver steatosis is oxidative stress [47], a condition that is present in the Pm liver. Whether oxidative stress induces the expression of PDGF in our model remains to be clarified.
In summary, our findings suggest that liver cancer development may follow a multistage process similar to skin and colon cancers where well defined basal/progenitor cell compartments are present. In chemically induced liver cancer models as well as in our Pten deletion model, a hepatic insult causes hepatocyte death (Fig 7D). Under the pressure of hepatic injury, Pten loss confers progenitor cells the ideal environment to become activated TICs. Thus, loss of PTEN in the liver not only directly contributes to the transformation of progenitors but also creates a niche that favors proliferation of progenitor cells.
Supplementary Material
Acknowledgments
We thank Drs. Lee, Okamoto, Hamm-Alvarez and B.W. Stiles for their help in proof reading the manuscript.
Funding Sources: Pilot grants from USC Liver Disease Center and TREC (B.L.S.), NIDDK (R21 DK075928-02, B.L.S.), NIDDK (R01-DK56886, M.J.B.), NIAAA (K08AA016290-03, K. W.), and the American Cancer Society, Research Scholar Award (RSG-10-073-01-TBG, C.B.R)
Footnotes
There are no conflicts of interest to disclose for all authors.
Author Contributions: V.G.: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript.
L. H.: study concept and design; acquisition of data; analysis and interpretation of data.
H.D.: Acquisition of data; analysis and interpretation of data
G. K.: analysis and interpretation of data.
C. V.: acquisition of data.
B. F.: acquisition of data.
N. Z.: acquisition of data.
J. B.: acquisition of data.
W.D.: acquisition of data.
K. W.: material support, critical revision of the manuscript for important intellectual content.
S. F.: Acquisition of data; Analysis and interpretation of data.
M.J.B.: material support, critical revision of the manuscript for important intellectual content.
C.B.R.: data analysis and acquisition, critical revision of the manuscript for important intellectual content.
B.L.S.: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding, technical, or material support; study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature reviews. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 2.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature reviews. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 3.Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem cell reviews. 2005;1:253–260. doi: 10.1385/SCR:1:3:253. [DOI] [PubMed] [Google Scholar]
- 4.Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Seminars in liver disease. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 5.Dunsford HA, Karnasuta C, Hunt JM, Sell S. Different lineages of chemically induced hepatocellular carcinoma in rats defined by monoclonal antibodies. Cancer research. 1989;49:4894–4900. [PubMed] [Google Scholar]
- 6.Germain L, Goyette R, Marceau N. Differential cytokeratin and alpha-fetoprotein expression in morphologically distinct epithelial cells emerging at the early stage of rat hepatocarcinogenesis. Cancer research. 1985;45:673–681. [PubMed] [Google Scholar]
- 7.Mishra L, Banker T, Murray J, et al. Liver stem cells and hepatocellular carcinoma. Hepatology (Baltimore, Md. 2009;49:318–329. doi: 10.1002/hep.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JS, Heo J, Libbrecht L, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nature medicine. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 9.Ma S, Chan KW, Hu L, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Stiles B, Wang Y, Stahl A, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected] Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Kobayashi S, Qiao W, et al. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. The Journal of clinical investigation. 2006;116:1843–1852. doi: 10.1172/JCI27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae JJ, Rho JW, Lee TJ, et al. Loss of heterozygosity on chromosome 10q23 and mutation of the phosphatase and tensin homolog deleted from chromosome 10 tumor suppressor gene in Korean hepatocellular carcinoma patients. Oncology reports. 2007;18:1007–1013. [PubMed] [Google Scholar]
- 13.Dong-Dong L, Xi-Ran Z, Xiang-Rong C. Expression and significance of new tumor suppressor gene PTEN in primary liver cancer.[erratum appears in J Cell Mol Med. 2003 Apr-Jun;7(2):1 p proceeding table of contents] Journal of Cellular & Molecular Medicine. 2003;7:67–71. doi: 10.1111/j.1582-4934.2003.tb00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu TH, Huang CC, Lin PR, et al. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer. 2003;97:1929–1940. doi: 10.1002/cncr.11266. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura N, Nagai H, Bando K, et al. PTEN/MMAC1 mutations in hepatocellular carcinomas: somatic inactivation of both alleles in tumors. Jpn J Cancer Res. 1999;90:413–418. doi: 10.1111/j.1349-7006.1999.tb00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, Sakon M, Nagano H, et al. Akt2 expression correlates with prognosis of human hepatocellular carcinoma. Oncology reports. 2004;11:25–32. [PubMed] [Google Scholar]
- 17.Horie Y, Suzuki A, Kataoka E, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. The Journal of clinical investigation. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rountree CB, Ding W, He L, Stiles B. Expansion of CD133-expressing liver cancer stem cells in liver-specific phosphatase and tensin homolog deleted on chromosome 10-deleted mice. Stem cells (Dayton, Ohio) 2009;27:290–299. doi: 10.1634/stemcells.2008-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. The Journal of biological chemistry. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhari M, Jayaraj R, Santhosh SR, Rao PV. Oxidative damage and gene expression profile of antioxidant enzymes after T-2 toxin exposure in mice. Journal of biochemical and molecular toxicology. 2009;23:212–221. doi: 10.1002/jbt.20282. [DOI] [PubMed] [Google Scholar]
- 21.Foretz M, Ancellin N, Andreelli F, et al. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331–1339. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- 22.Ding W, You H, Dang H, et al. Epithelial-to-mesenchymal transition of murine liver tumor cells promotes invasion. Hepatology (Baltimore, Md. doi: 10.1002/hep.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding W, Mouzaki M, You H, et al. CD133+ liver cancer stem cells from methionine adenosyl transferase 1A-deficient mice demonstrate resistance to transforming growth factor (TGF)-beta-induced apoptosis. Hepatology (Baltimore, Md. 2009;49:1277–1286. doi: 10.1002/hep.22743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho H, Mu J, Kim JK, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science (New York, NY) 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 25.He L, Hou X, Kanel G, et al. The critical role of AKT2 in hepatic steatosis induced by PTEN loss. The American journal of pathology. 176:2302–2308. doi: 10.2353/ajpath.2010.090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen JI, Roychowdhury S, DiBello PM, Jacobsen DW, Nagy LE. Exogenous thioredoxin prevents ethanol-induced oxidative damage and apoptosis in mouse liver. Hepatology (Baltimore, Md) 2009;49:1709–1717. doi: 10.1002/hep.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rountree CB, Barsky L, Ge S, et al. A CD133-expressing murine liver oval cell population with bilineage potential. Stem cells (Dayton, Ohio) 2007;25:2419–2429. doi: 10.1634/stemcells.2007-0176. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Foster M, Al-Dhalimy M, et al. The origin and liver repopulating capacity of murine oval cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100 (Suppl 1):11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem cells (Dayton, Ohio) 2006;24:1852–1858. doi: 10.1634/stemcells.2006-0036. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita T, Ji J, Budhu A, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itoh T, Kamiya Y, Okabe M, Tanaka M, Miyajima A. Inducible expression of Wnt genes during adult hepatic stem/progenitor cell response. FEBS letters. 2009;583:777–781. doi: 10.1016/j.febslet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 32.Stock P, Monga D, Tan X, et al. Platelet-derived growth factor receptor-alpha: a novel therapeutic target in human hepatocellular cancer. Molecular cancer therapeutics. 2007;6:1932–1941. doi: 10.1158/1535-7163.MCT-06-0720. [DOI] [PubMed] [Google Scholar]
- 33.Maass T, Thieringer FR, Mann A, et al. Liver specific overexpression of platelet-derived growth factor-B accelerates liver cancer development in chemically induced liver carcinogenesis. International journal of cancer. doi: 10.1002/ijc.25469. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita T, Forgues M, Wang W, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer research. 2008;68:1451–1461. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 35.Roskams T, Yang SQ, Koteish A, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. The American journal of pathology. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–3822. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- 37.Preisegger KH, Factor VM, Fuchsbichler A, et al. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Laboratory investigation; a journal of technical methods and pathology. 1999;79:103–109. [PubMed] [Google Scholar]
- 38.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Seminars in liver disease. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuttle RL, Gill NS, Pugh W, et al. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nature medicine. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 41.Easton RM, Cho H, Roovers K, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Molecular and cellular biology. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. The Journal of biological chemistry. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 43.Sell S. Comparison of liver progenitor cells in human atypical ductular reactions with those seen in experimental models of liver injury. Hepatology (Baltimore, Md. 1998;27:317–331. doi: 10.1002/hep.510270202. [DOI] [PubMed] [Google Scholar]
- 44.Hu M, Kurobe M, Jeong YJ, et al. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology. 2007;133:1579–1591. doi: 10.1053/j.gastro.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 45.Spee B, Carpino G, Schotanus BA, et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 59:247–257. doi: 10.1136/gut.2009.188367. [DOI] [PubMed] [Google Scholar]
- 46.Yang W, Yan HX, Chen L, et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer research. 2008;68:4287–4295. doi: 10.1158/0008-5472.CAN-07-6691. [DOI] [PubMed] [Google Scholar]
- 47.Liu MY, Eyries M, Zhang C, Santiago FS, Khachigian LM. Inducible platelet-derived growth factor D-chain expression by angiotensin II and hydrogen peroxide involves transcriptional regulation by Ets-1 and Sp1. Blood. 2006;107:2322–2329. doi: 10.1182/blood-2005-06-2377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.