Abstract
While an extensive literature is now available on age-related differences in white matter integrity measured by diffusion MRI, relatively little is known about the relationships between diffusion and cognitive functions in older adults. Even less is known about whether these relationships are influenced by the apolipoprotein (APOE) ε4 allele, despite growing evidence that ε4 increases cognitive impairment in older adults. The purpose of the present study was to examine these relationships in a group of community-dwelling cognitively normal older adults. Data were obtained from a sample of 126 individuals (ages 52–92) that included 32 ε4 heterozygotes, 6 ε4 homozygotes, and 88 non-carriers. Two measures of diffusion, the apparent diffusion coefficient (ADC) and fractional anisotropy (FA), were obtained from six brain regions – frontal white matter, lateral parietal white matter, the centrum semiovale, the genu and splenium of the corpus callosum, and the temporal stem white matter – and were used to predict composite scores of cognitive function in two domains, executive function and memory function. Results indicated that ADC and FA differed with increasing age in all six brain regions, and these differences were significantly greater for ε4 carriers compared to noncarriers. Importantly, after controlling for age, diffusion measures predicted cognitive function in a region-specific way that was also influenced by ε4 status. Regardless of APOE status, frontal ADC and FA independently predicted executive function scores for all participants, while temporal lobe ADC additionally predicted executive function for ε4 carriers, but not noncarriers. Memory scores were predicted by temporal lobe ADC but not frontal diffusion for all participants, and this relationship was significantly stronger in ε4 carriers compared to noncarriers. Taken together, age and temporal lobe ADC accounted for a striking 53% of the variance in memory scores within the ε4 carrier group.
The results provide further evidence that APOE ε4 has a significant impact on the trajectory of age-related cognitive functioning in older adults. Possible mechanisms are discussed that could account for the associations between ε4, diffusion, and cognitive function, including the influence of ε4 on neural repair, oxidative stress, and the health of myelin-producing oligodendroglia.
Keywords: diffusion tensor imaging, aging, cognition, APOE
1 Introduction
There is growing evidence of an association between the apolipoprotein (APOE) genotype and cognitive function in healthy older adults. The presence of the APOE ε4 allele has been related to poorer performance on tests of global cognitive functioning, episodic memory, and executive functioning (Nilsson et al., 2006; Adamson et al., 2008; Schultz et al., 2008), although a meta-analysis reviewing 38 cross-sectional studies suggests that these effects are rather small (Small et al., 2004). Longitudinal studies have revealed faster cognitive decline for ε4 carriers in the domains of memory (Mayeux et al., 2001; Caselli et al., 2004; Blair et al., 2005; Swan et al., 2005), executive functioning (Swan et al., 2005), and global cognitive function (Bretsky et al., 2003; Caselli et al., 2007). Additionally, the magnitude of the cognitive decline increased as gene dose increased from one to two copies of the ε4 allele (Caselli et al., 2004, 2007; Blair et al., 2005).
The APOE ε4 allele also has been associated with structural and metabolic differences in many of the same brain regions affected by Alzheimer’s disease (AD; for reviews, see Scarmeas and Stern, 2006; Cherbuin et al., 2007). Compared to noncarriers, ε4 carriers exhibit smaller volumes in the medial temporal lobe including the hippocampus proper (den Heijer et al., 2002; Lemaitre et al., 2005; Wishart et al., 2006), decreased cortical thickness in the entorhinal cortex and subiculum (Burggren et al., 2008), and an accelerated rate of atrophy in the hippocampus (Jak et al., 2007). Several PET studies have also demonstrated a pattern of reduced glucose metabolism in brain regions that are reminiscent of early AD, including regions of frontal, lateral temporal, parietal, and posterior cingulate cortices in ε4 carriers compared to noncarriers (Small et al., 2000; Reiman et al., 2005; Mosconi et al., 2008).
Recent studies have employed diffusion tensor imaging (DTI) as a method for detecting subtle changes in the integrity of brain tissue. DTI provides information about the degree and directionality of water diffusion in tissue and has been shown to be sensitive to microstructural changes in brain tissue, particularly in white matter (Le Bihan, 2003). Diffusion is most frequently reported as the apparent diffusion coefficient (ADC), a measure of mean diffusivity, and fractional anisotropy (FA), measuring the primary directionality of diffusion. In the presence of pathology such as stroke, demyelination, or inflammation, ADC is most likely to increase while FA values will decrease. Thus, DTI is a non-specific but relatively sensitive marker of pathological changes in brain tissue, even in the absence of volumetric changes on MRI (Burgmans et al., 2010).
An extensive literature now exists on diffusion changes associated with normal aging. DTI studies have demonstrated widespread changes in diffusion across the adult lifespan with increases in ADC and decreases in FA in both anterior and posterior white matter regions. Many studies have reported age-related diffusion changes that are most prominent in the frontal lobe with relative sparing of the temporal, parietal, and occipital regions (Head et al., 2004; Hugenschmidt et al., 2008; Kochunov et al., 2007; Madden et al., 2007; Pfefferbaum et al., 2005; Salat et al., 2005; Sullivan and Pfefferbaum, 2006, Abe et al., 2008; Bhagat and Beaulieu, 2004; Deary et al., 2006; Furutani et al., 2005; Hsu et al., 2008; Hugenschmidt et al., 2008; Kochunov et al., 2007; Pagani et al., 2008; Shenkin et al., 2003, 2005; Smith et al., 2008; Zhang et al., 2005). This anterior-posterior gradient has been hypothesized to reflect the development of myelin, such that axons myelinating late in development (i.e., frontal white matter) are most vulnerable to age-related degeneration while axons myelinating early in life (i.e., posterior areas) remain relatively intact (Bartzokis, 2004). It should be noted, however, that age-related diffusion changes are also observed in posterior brain regions, particularly when the samples include a wide range of ages. For example, in a group of 52 healthy adults, ages 19 to 81 years, Kennedy and Raz (2009) reported age-related changes in both FA and ADC that extended through the brain, including frontal, temporal, parietal, and occipital lobe white matter regions.
Diffusion measures obtained from individuals with Alzheimer’s disease (AD) and mild cognitive impairment (MCI) most consistently differentiate from age-matched controls in regions with a more posterior distribution, including in the cingulum bundle (Fellgiebel et al., 2005; Teipel et al., 2007; Zhang et al., 2007), parietal lobe and posterior cingulate (Medina et al., 2006; Firbank et al., 2007; Huang et al., 2007; Ding et al., 2008; Rose et al., 2008), lateral and medial temporal lobe white matter (Fellgiebel et al., 2006; Huang et al., 2007; Yasmin et al., 2008; Xie et al., 2006; Stahl et al., 2007), and the splenium of the corpus callosum (Stahl et al., 2007). Findings in individuals with MCI tend to overlap with AD, particularly in posterior areas (Kantarci et al., 2001). In contrast, only a few studies have reported diffusion differences in the frontal lobe in MCI or AD compared to age-matched controls (Choi et al., 2005; Sydykova et al., 2007). Taken together, these findings have been described as support for the general notion that normal aging most prominently affects frontal regions, while MCI or AD results in additional changes in diffusion with a more posterior distribution (Smith et al., 2008).
Few studies, however, have examined diffusion in cognitively normal older adults who are at genetic risk for AD due to the presence of the APOE ε4 allele. Four studies exist to date, all focusing primarily on fractional anisotropy but interestingly using three different DTI analysis methods, one utilizing anatomically placed regions of interest, two taking a whole-brain voxel-based morphometric approach, and the third using a tract-based analysis method. The studies are remarkably consistent in their findings, showing consistent reductions in FA values in the medial temporal lobe, although the specific region varied somewhat. Nierenberg and colleagues (2005) found reduced FA in the parahippocampal white matter in a sample of 14 ε4 carriers compared to age-matched noncarriers. Persson et al. (2006) reported that ε4 was associated with decreased FA in the splenium of the corpus callosum, anterior frontal lobe white matter, inferior temporal lobe white matter, the hippocampus, and the white matter of the cingulum. Smith et al. (2008), using tract-based spatial statistics, observed similar regions of decreased FA including the inferior temporal lobe white matter bilaterally, the splenium of the corpus callosum, the cingulum bundle, and anterior frontal lobe white matter. Bendlin et al. (in press), using methods similar to Persson et al. (2006) in a middle-aged cohort, did not find a main effect of APOE ε4 on FA, but found that it interacted with parental family history of AD in medial temporal lobe white matter. The regions are similar to those reported in individuals with MCI and AD (Kantarci et al., 2001; Müller et al., 2005; Kantarci et al., 2005; Fellgiebel et al., 2005; Medina et al., 2006; Naggara et al., 2006; Cho et al., 2008; Huang et al., 2007). Smith et al. (2008) suggest that the alterations seen in at-risk individuals may reflect pathological changes associated with preclinical stages of AD.
In contrast to the quite extensive literature now available on age-related differences in white matter diffusivity, relatively little is known about the functional implications of these changes and their impact on cognitive abilities in older adults, and even less is known about the additional impact on individuals with the APOE ε4 allele. The studies to date have focused on very different cognitive domains or a single task within a domain, so that a consistent picture has not yet emerged. Associations have been observed on some occasions, but not others, between diffusion measures and executive function tasks (Charlton et al., 2006; Grieve et al., 2007; Shenkin et al., 2005; Sullivan et al., 2006). Speed of processing measures have also been shown to correlate with diffusivity in some samples of older adults (Bucur et al., 2008; Deary et al., 2006; Madden et al., 2004; Sullivan et al., 2006) but not others (Charlton et al., 2008; Grieve et al., 2007; Madden et al., 2007). Few studies have found an association between diffusion measures and memory (Bucur et al., 2008; Persson et al., 2006). In one recent study, Kennedy and Raz (2009) included 52 participants between 19 and 81 years of age and found that diffusion measures were not only associated with cognition, but that the relationship was region-specific: frontal white matter diffusivity predicted processing speed, posterior white matter predicted inhibition and task switching performance, while more central white matter predicted episodic memory performance.
While several studies have reported that diffusion measures predict global cognitive functioning or memory impairments in patients with MCI or AD (Hanyu et al., 1998; Bozzali et al., 2002; Müller et al., 2005; Fellgiebel et al., 2005; Ray et al., 2006; Huang et al., 2007), little is known about whether similar relationships exist for cognitively healthy individuals with genetic risk for AD. The only study to date examining the association between diffusion and cognition as a function of ε4 risk found no relationship between FA in the parahippocampal white matter and performance on a digit span task (Nierenberg, et al. 2005). However, digit span is not generally affected in cognitively healthy APOE carriers (Small et al., 2004) or in individuals with preclinical AD (Bäckman, et al., 2005), thus digit span might not be a particularly sensitive cognitive test to employ with this population. Smith et al. (2008) found changes in speed of processing associated with APOE status along with decreases in FA, but did not report the correlation between the two measures.
The goal of the present paper is to further address the interactions between age, APOE ε4 status, and cognitive function. Specifically, does the presence of the ε4 allele moderate age-related changes in diffusion? Furthermore, does ε4 influence all brain regions equally, or only more posterior regions that have been associated with MCI and AD? Based on the few existing studies described earlier, we hypothesize that the ε4 allele will increase age-related diffusion changes in more posterior regions including the temporal and parietal lobes, but not frontal regions. Finally, what is the relationship between diffusion measures and cognitive function, and does this relationship differ as a function of APOE status? While most studies of AD and MCI have focused on memory performance because of its importance as an early predictor of AD, the predominant cognitive domain associated with normal aging is executive function. Because frontal lobe diffusion changes have been associated with normal aging, we hypothesized that frontal diffusion may be associated with executive function across both ε4 carriers and noncarriers. In contrast, more posterior diffusion measures, including the inferior temporal lobe, may predict memory scores more strongly for ε4 carriers compared to noncarriers, since changes to posterior white matter diffusivity appear to be more specifically associated with the presence of the ε4 allele.
2 Methods
Participants
Volunteers included 126 healthy individuals between the ages of 52 and 92. All participants were living independently in the community and were recruited through newspaper advertisements and an existing database of older individuals who had participated in previous studies in our laboratory. Apolipoprotein E genotype was determined using DNA that was purified from the white blood cell fractions obtained from donated blood samples. DNA was amplified by polymerase chain reaction using primers and reaction conditions described previously (Hixson and Vernier, 1990). Amplified DNAs were digested with restriction endonuclease Hha1 and separated through 9% acrylamide gels. ApoE genotypes from each case were obtained from the patterns of digested fragments on the gels. Of the participants, 32 (25%) were heterozygous for the APOE ε4 allele, 6 participants (5%) were homozygous for ε4, and the remaining 88 participants (70%) were non-carriers, consistent with the distribution of ε4 found in the general population (Gerdes et al., 1992). Participants underwent medical health screening and were free from past or current neurological disorders, head injury with sequelae, psychiatric disorder, or a history of drug or alcohol abuse. No participant was taking medications that might interfere with cognitive function, such as anxiolytics. Hypertension was assessed through self-reported diagnosis of hypertension and/or current use of an anti-hypertensive drug. Participants were also screened for contraindications to MRI, including pacemakers, metal implants, and claustrophia. Informed consent was obtained from each volunteer following procedures approved by the Human Subjects Committee at the University of Arizona.
Neuropsychological testing
Participants underwent a detailed neuropsychological assessment that included the Mini Mental State Exam (MMSE; Folstein et al., 1975) and the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) that included the Vocabulary subtest. Composite measures of memory and executive functioning were computed for each participant based on factor analyses described by Glisky (Glisky and Kong, 2008; Glisky et al., 2001). The memory factor score was based on the following tests: Logical Memory I recall total score, Verbal Paired Associate I, and Face Recognition I (Wechsler Memory Scale-III; Wechsler, 1997), Visual Paired Associates II (Wechsler Memory Scale-Revised; Wechsler, 1987), and Long-Delay Cued Recall from the California Verbal Learning Test (CVLT; Delis et al., 1987). The executive function factor score included the number of correct categories completed in the Wisconsin Card Sorting Test (WCST; Hart et al., 1988), Mental Arithmetic total score (WAIS-R; Wechsler, 1981), total number of correct responses in the Controlled Oral Word Association Test (Spreen and Benton, 1977), Digit Span Backwards total score and the Mental Control total score (WMS-III; Wechsler, 1997). For each participant, test scores were transformed into z scores based on Glisky and Kong’s (2008) normative sample of 227 older adults and averaged to create two composite z scores for memory and executive function.
MRI acquisition and image processing
Magnetic resonance images were acquired on a GE 3.0T Signa VH/I whole body echo-speed scanner equipped with an 8-channel phased array head coil (HD Signa Excite, General Electric, Milwaukee, WI). A set of 3-plane localizer images were obtained in order to align subsequent images. High resolution T1-weighted whole-brain structural images were acquired using a 3D spoiled gradient-echo (3D SPGR) pulse sequence with a section thickness of 0.7mm, no skip (TR/TE/TI = 5.1ms/2ms/500ms; flip angle = 15°; matrix = 256×256; FOV = 260 × 260mm2). T2 Fluid Attenuated Inversion Recovery (T2-FLAIR) images were collected with fifty-eight axial images covering the whole brain (2.6 mm slice thickness, no gap, TE/TR/TI = 120/11000/ 2250ms, Matrix = 256×192, FOV = 260×260mm2). The T2-Flair images were used to obtain a general assessment of the amount of white matter hyperintensity using a clinical rating scale from 0 to 3 (Fazekas et al., 1987).
Diffusion tensor images were acquired with an echo planar imaging sequence corrected for spatial distortion (GE ASSET). Fifty-eight axial sections of 2.6 mm thickness, no gap, covering the whole brain were acquired (TE/TR = 71ms/13000ms, matrix 96×96, FOV = 250 × 250mm2), resulting in isotropic voxels with a resolution of 2.6mm3. Diffusion was measured in 25 directions with 2 averages (B0 = 1000s mm2, 2 NEX). While the scan resolution was somewhat lower than other relevant studies with in-plane resolution of 1.8 × 1.8 mm (for example, Smith et al., 2008; Kennedy & Raz, 2009), the somewhat large voxel size is similar to other recent studies (for example, Persson et al., 2006) and allowed us to obtain more b orientations in a reasonable amount of scanning time.
Diffusion images were resampled prior to downloading at 256 × 256 using sinc interpolation. Images were realigned to remove linear eddy current distortions using the Functional Software Library (FSL) package (www.fmrib.ox.ac.uk/fsl). DTI Studio Version 2.4 (Jiang et al., 2006; https://www.dtistudio.org/) software was used to compute a diffusion tensor for each voxel that included three eigenvalues and eigenvectors. Based on these values, fractional anisotropy (FA) maps and apparent diffusion coefficient (ADC) maps were computed for each participant. Diffusion maps and T1-weighted images from each individual were then oriented parallel to the anterior-posterior commissural line and co-registered to each other using SPM2 and left in native space (Welcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/).
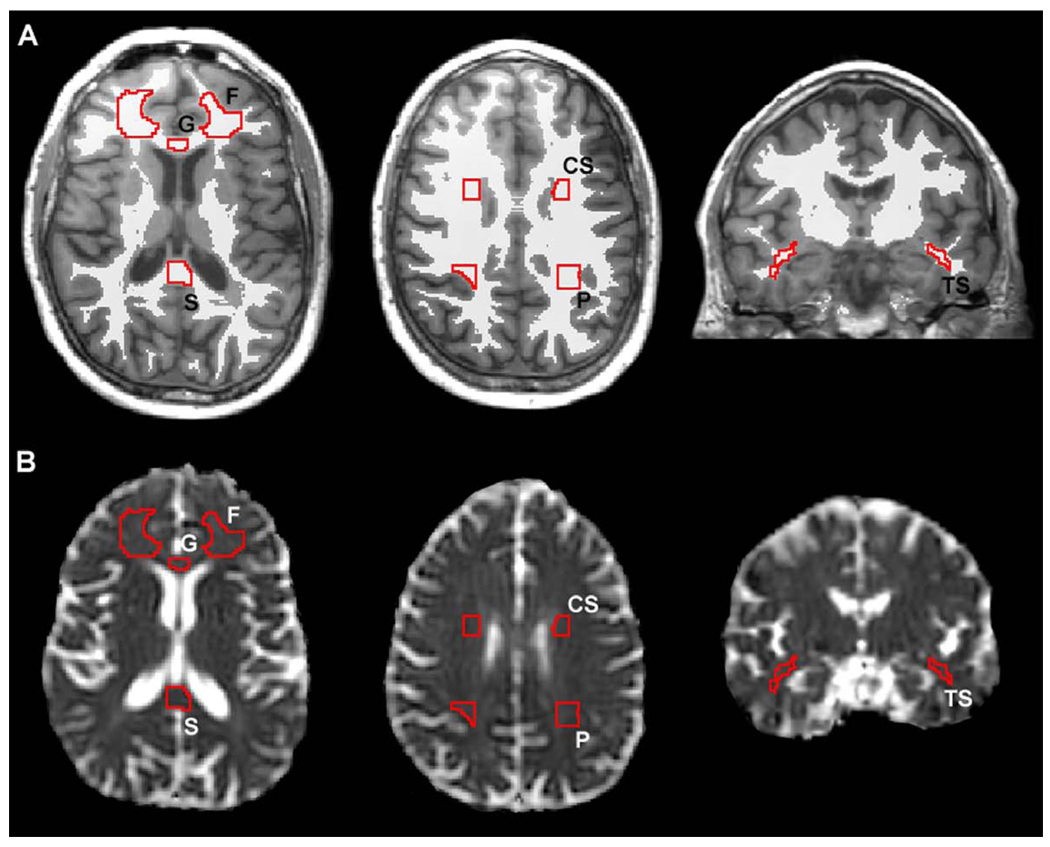
Region of interest (ROI) templates were created using MRIcro software (Rorden, www.mricro.com) and included fully-volumed regions of the frontal white matter, lateral parietal white matter, the centrum semiovale, the genu of the corpus callosum, the splenium of the corpus callosum, and the temporal stem white matter. ROI’s for the frontal and parietal white matter, centrum semiovale, and temporal stem were created bilaterally on a representative T1 brain chosen from the sample (see Figure 1). The frontal white matter was measured on 21 contiguous sections in the axial plane beginning two sections inferior to the anterior commissure. The ROI was placed in the center of the frontal white matter with the most posterior extent of the ROI corresponding to a plane level with the center of the genu. The centrum semiovale was measured on 11 contiguous sections in the coronal plane beginning five sections anterior to the anterior commissure and ending five sections posterior to the anterior commissure. The ROI was placed on fully volumed white matter adjacent to the ventricles and superior to the caudate nucleus. The temporal stem white matter was measured on 23 contiguous coronal sections beginning at the section showing fully-volumed anterior commissure. The parietal white matter was measured on 12 contiguous sections in the coronal plane beginning one section posterior to fully-volumed inferior colliculi. The ROI was placed on the white matter adjacent to the ventricles and superior to the level of the lateral fissure.
Figure 1.
Regions of interest superimposed on an individual MRI in native space. A. Two axial and one coronal T1 weighted sections showing ROIs overlaid on the binarized white matter segmented images. The resulting ROIs include only voxels shared between the ROIs and fully volumed white matter. B. Resulting ROIs placed on the apparent diffusion coefficient images at the same location as the T1 images. F= frontal white matter, G = genu of the corpus callosum, S = splenium of the corpus callosum, CS = centrum semiovale, TS = temporal stem white matter, P = superior parietal.
Rather than using a standardized template for the corpus callosum, ROIs for the genu and splenium of the corpus callosum were drawn manually on the native space SPGRs for each participant because of the large variability in callosal shape and width across participants. The genu ROI was outlined on sagittal images beginning at the midsagittal section and continuing over the next five contiguous lateral sections in both hemispheres for a total of 10 sections. The posterior boundary was set at the most anterior point in the curve of the genu. The splenium ROI was drawn on consecutive sagittal images beginning at the midsagittal section and continuing over the next six lateral sections in both hemispheres for a total of 12 sections. The anterior boundary was set at the posterior commissure. Interrater reliability was determined for these two regions using a random sample of 10 brains. The intraclass correlations for the genu and splenium were 0.95 and 0.97, respectively, demonstrating high reliability for the procedure. The locations of the callosal ROIs are presented in Figure 1.
In order to ensure that diffusion values were extracted only from white matter tissue and not partially volumed gray matter, T1 images were segmented into gray matter, white matter, and cerebrospinal fluid using SPM2. We applied the segmentation procedure used in optimized voxel based morphometry (Good et al., 2001; Gaser, http://dbm.neuro.uni-jena.de/vbm.html) which uses information about the known location of the tissue types (priors) in addition to the signal intensity of voxels obtained from actual scans. The priors were created from a random sample of 100 participants in the present study. To minimize the potential of partial voluming effects, a conservative threshold was applied to the white matter map with a white matter probability of 0.9 to create a binarized white matter mask. The binarized mask was transformed back to native space by applying the inversion of the normalization parameters for a given individual. The ROI templates described earlier were then applied to individual participant’s diffusion maps and were then multiplied by the individualized white matter mask, thereby ensuring that only fully volumed white matter was included in the ROI for a given individual. This segmentation procedure had the added benefit of removing regions of white matter hyperintensity that were visible on T2-flair images. The low signal values of abnormal white matter on T1 images ensured that these regions were excluded from the white matter mask, as evident from visual comparison between the white matter mask and T2-flair images. Thus, diffusion measures were only obtained from normal-appearing white matter. Diffusion values from each ROI were averaged across all extracted voxels.
3 Results
Demographics
Noncarrier, heterozygote, and homozygote participants are characterized in Table 1. The majority of the sample (89%) was Caucasian. The three groups were well matched on demographic and cognitive measures. One-way ANOVAs indicated no significant differences in age, years of education, cognitive functioning (MMSE, memory factor, executive factor), or clinical rating of white matter hyperintensities; all F’s(2,123) < 1.33, ns. Rates of hypertension were similar for noncarriers (28%) and carriers (total 29%; 31% within the heterozygote group and 17% within the homozygote group). Three individuals, all within the noncarrier group, had a diagnosis of diabetes.
Table 1.
Demographics (mean ± standard deviation) and cognitive function scores for APOE ε4 allele noncarrier, heterozygote, and homozygote individuals. Mean hyperintensity ratings are based on a scale of 0 to 4.
| Noncarriers | Heterozygotes | Homozygotes | |
|---|---|---|---|
| Number of participants | 88 | 32 | 6 |
| Age (years) | 71.6 ± 8.8 | 69.2 ± 9.7 | 67.0 ± 12.2 |
| Gender (m/f) | 20/68 | 7/25 | 1/5 |
| Hypertension (%) | 28% | 31% | 17% |
| Education (years) | 15.5 ± 2.8 | 15.5 ± 2.8 | 15.0 ± 2.4 |
| MMSE | 28.8 ± 1.3 | 28.9 ± 1.5 | 28.7 ± 1.2 |
| Vocabulary (raw score) | 69.7 ± 6.4 | 68.9 ± 6.0 | 69.2 ± 3.4 |
| Executive Factor (z-score) | 0.03 ± 0.64 | 0.08 ± 0.66 | 0.09 ± 0.41 |
| Memory Factor (z-score) | 0.28 ± 0.63 | 0.19 ± 0.84 | 0.02 ± 1.06 |
| Mean hyperintensity rating | 1.47± 0.98 | 1.23± 1.02 | 1.58± 1.43 |
For the purpose of analysing the diffusion and cognitive measures, heterozygote and homozygote groups were combined due to the small number of homozygous individuals in the sample. Additionally, because of the high correlations between right and left hemisphere diffusion measures and the lack of a priori hypotheses regarding hemispheric differences, regional measures of FA and ADC were averaged across hemispheres. Other studies have also collapsed diffusion values across left and right hemispheres finding no hemisphere-specific differences in FA or ADC (for example, Kennedy & Raz, 2009). Diffusion measures were analysed using multivariate omnibus tests to control for experiment-wise error, followed by univariate tests where appropriate (detailed in sections below).
Diffusion measure omnibus tests
Omnibus tests were carried out for ADC and FA separately using a multivariate general linear model (GLM) that included the values for six white matter regions (centrum semiovale, genu, splenium, frontal lobe, parietal lobe, and temporal stem), and two between-subjects factors, age (continuous variable) and APOE ε4 status (carriers, noncarriers).
The omnibus test for FA showed a main effect of age, F(1,122)=12.51, p<.001, demonstrating the previously well documented decrease in FA with increasing age. Interestingly, age did not interact with region, suggesting that age-related FA differences were generally similar across all six regions. Across all regions, FA values were lower for the ε4 carriers compared to noncarriers, indicated by a main effect of APOE status, F(5,118)=2.79, p<.05. Importantly, a three-way interaction between region, age, and APOE status was obtained, F(5,118)=3.78, p<.01, suggesting that APOE status moderated age-related differences in some but not other regions.
The omnibus test for ADC showed that this measure increased with age in all regions, indicated by the main effect of age, F(1,122)=91.57, p<.0001, and this effect did not interact with region. In contrast to FA, however, no main effect of APOE status was observed. Instead, age and APOE status interacted significantly, F(1,122)=4.14, p<.05, but did not further interact with region, suggesting that APOE status moderated age-related differences in ADC similarly across all six brain regions.
Diffusion measure univariate tests
To further elucidate the interactions between age and APOE status obtained from the omnibus tests, univariate GLM analyses were carried out separately for ADC and FA in each brain region. Although the omnibus test for ADC suggested that this step was not necessary as the APOE by age interaction applied to all regions, the univariate tests provided an estimate of the variance explained (R2) and the related p value for each region. The univariate interaction terms and R2 values are provided in Table 2. As expected, the univariate tests indicated that age-related increases in ADC were significantly greater for the ε4 carriers than noncarriers in all brain regions. For FA values, ε4 carriers showed significantly greater age-related decreases only within the frontal lobe and temporal lobe white matter compared to noncarriers. The genu also showed a significant interaction between age and APOE status for FA, but the interaction explained a small percentage of variance (5%) compared to the frontal and temporal lobe regions (22% and 17%, respectively). The univariate tests also showed a main effect of APOE status for FA in two regions, the frontal lobe white matter, F(1,122)=6.20, p<.05, and the splenium, F(1,122)=4.76, p<.05, indicating overall lower average FA values for ε4 carriers compared to noncarriers in these two regions.
Table 2.
Univariate GLM interaction terms (F) between age and APOE status, and the percentage of variance explained (R2) by the interaction term for each brain region.
| F | p | R2 | |
|---|---|---|---|
| ADC | |||
| Frontal | 21.32 | .0001 | 26% |
| Centrum | 25.24 | .0001 | 29% |
| Genu | 22.58 | .0001 | 27% |
| Temporal | 30.22 | .0001 | 33% |
| Parietal | 19.15 | .0001 | 24% |
| Splenium | 17.78 | .0001 | 22% |
| FA | |||
| Frontal | 16.63 | .001 | 22% |
| Centrum | 1.45 | ns | |
| Genu | 3.04 | .05 | 5% |
| Temporal | 12.74 | .001 | 17% |
| Parietal | 2.09 | ns | |
| Splenium | 2.35 | ns |
Pearson correlations are listed in Table 3, showing the simple linear correlations between age and diffusion measures for ε4 carriers and noncarriers separately. Consistent with the GLM analyses described above, the table demonstrates the general increase in correlation between ADC and age across all regions, and the increasingly negative correlation between FA and age in frontal, temporal stem, and genu white matter regions for APOE ε4 positive individuals compared to non-carriers.
Table 3.
Pearson correlations between age and diffusion measures for APOE ε4 carriers (ε4+) and noncarriers (ε4−).
| ADC |
FA |
|||
|---|---|---|---|---|
| APOE ε4− | APOE ε4+ | APOE ε4− | APOE ε4+ | |
| Frontal | 0.38 | 0.73 | −0.31 | −0.71 |
| Centrum | 0.52 | 0.58 | −0.07 | −0.35 |
| Genu | 0.44 | 0.65 | −0.22 | −0.01 |
| Temporal | 0.42 | 0.78 | −0.30 | −0.59 |
| Parietal | 0.45 | 0.55 | 0.18 | 0.14 |
| Splenium | 0.38 | 0.67 | −0.28 | 0.07 |
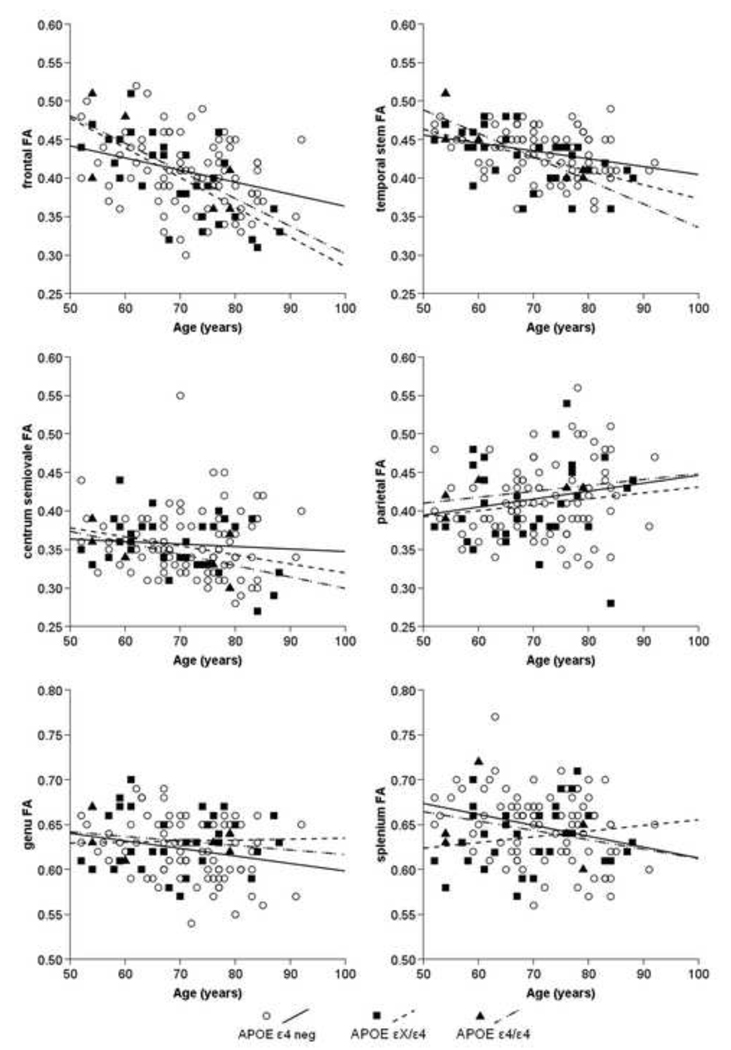
Figures 2 and 3 depict the simple correlations between age and ADC (Figure 2) and age and FA (Figure 3) in all regions. While the statistical tests were carried out comparing noncarriers to a combined group of heterozygote and homozygote individuals, the slopes of the correlation lines for the three groups are provided separately. Consistent with the GLM analyses described earlier, the dominant pattern across all brain regions appears to be an interaction between age and APOE status, such that age-related changes in diffusion values increase in the presence of the ε4 allele. Importantly, as depicted in Figures 2 and 3, the increase in correlation is not due solely to the inclusion of the homozygote group, but applies to those individuals with one copy of the ε4 allele as well.
Figure 2.
Scatter plots showing the relation between age and Apparent Diffusion Coefficient (ADC) in the frontal, temporal stem, centrum semiovale, and parietal white matter as well as the genu and splenium of the corpus callosum as a function of APOE status.
Figure 3.
Scatter plots showing the relation between age and Fractional Anisotropy (FA) in the frontal, temporal stem, centrum semiovale, and parietal white matter as well as the genu and splenium of the corpus callosum as a function of APOE status. Note that the FA scale for the genu and splenium was changed due to the higher diffusion directionality in these regions.
APOE status, diffusion, and cognition
Cognitive scores were predicted from diffusion measures from two brain regions that have been most closely associated with executive function and memory, namely the frontal white matter and temporal lobe white matter, respectively. Table 4 lists the Pearson linear correlations between the two cognitive measures and frontal ADC, frontal FA, temporal stem ADC, and temporal stem FA. Diffusion measures in both regions were consistently correlated with both memory function and executive function for the ε4 carriers. For the noncarriers, only frontal and temporal stem ADC measures correlated with memory scores and did not predict executive function scores.
Table 4.
Pearson correlations between frontal and temporal stem diffusion measures and cognitive function for noncarriers (ε4−) and carriers (ε4+) of the APOE ε4 allele.
| Memory Functioning | Executive Functioning | |||
|---|---|---|---|---|
| APOE ε4− | APOE ε4+ | APOE ε4− | APOE ε4+ | |
| Frontal ADC | −.18* | −.49* | .16 | −.42* |
| Frontal FA | .14 | .43* | .04 | .46* |
| Temporal stem ADC | −.26* | −.65* | .11 | −.56* |
| Temporal stem FA | .14 | .57* | .04 | .50* |
p<.05, two-tailed
In the present study, age was correlated with the ADC and FA measures in both groups (described earlier), and age was also correlated with cognitive function (age and memory functioning: noncarrier r=−.39, ε4 carrier r=−.70; age and executive functioning: noncarriers r=−.04, ε4 carriers r=−.47). One way to interpret the results is to consider the noncarrier group as reflecting the age-related correlations between cognition and diffusivity without the influence of APOE, which are statistically significant only between ADC and memory function (see Table 4), while the increase in correlations within the carrier group indicates the additional effect of the ε4 allele. In order to assess statistical differences between groups, the correlations were assessed with two univariate GLM models predicting memory and executive function. Predictors included the four diffusion measures as continuous variables (frontal ADC, frontal FA, temporal stem ADC, and temporal stem FA), and APOE status as a categorical variable (ε4 carriers, noncarriers). To control for demographic influences, gender and years of education were added as covariates along with age as a mean-centered covariate. The models tested all main effects and the interaction terms between APOE status and the four diffusion measures. Two variables, gender and temporal stem FA, did not enter into the models significantly either as main effects or as an interaction term, and were therefore dropped from the analyses in order to maximize degrees of freedom. The revised model included APOE status and three diffusion measures as predictors (frontal ADC, frontal FA, and temporal stem ADC) while age and education remained as covariates.
Predicting memory scores
Results of the GLM predicting memory performance are summarized in Table 5. The model accounted for 32% of the total variance (adjusted R2 =. 32, F(9,116)=7.34, p<.0001). Two important findings were observed. First, controlling for age and education, temporal stem ADC predicted memory scores while the frontal diffusion measures did not, suggesting that the relationship between memory and diffusivity is region-specific, rather than global. Second, while temporal stem ADC predicted memory significantly for both groups, APOE status interacted significantly with this factor, indicating that temporal stem ADC was a stronger predictor of memory performance for ε4 carriers than noncarriers, consistent with the simple correlations described earlier. The model produced a main effect of age, F(1,116)=18.15, p<.001, temporal stem ADC, F(1,116)=10.23, p<.001, and a significant interaction between APOE status and temporal stem ADC, F(1,116)=5.59, p<.02. The main effect of APOE status was marginally significant, F(1,117)=2.68, p=.10, as was the main effect of education, F(1,116)=3.74, p=.056. Frontal ADC and frontal FA did not approach significance either as main effects or in interactions with APOE status.
Table 5.
Univariate GLM models showing significant predictors of memory and executive function scores for APOE ε4 carriers and noncarriers.
| Executive function | Adjusted R2 = .22, p<.0001 | |
|---|---|---|
| Predictor | F | p |
| Yrs education | 13.83 | .001 |
| Frontal ADC | 5.23 | .01 |
| Frontal FA | 3.85 | .05 |
| APOE status | 6.67 | .01 |
| APOE x temporal ADC | 5.74 | .02 |
| Memory function | Adjusted R2 = .32, p<.0001 | |
| Predictor | F | p |
| Age | 18.15 | .001 |
| Temporal ADC | 10.23 | .002 |
| APOE x temporal ADC | 5.59 | .02 |
Based on these results, a post hoc model predicting memory in ε4 carriers alone was constructed including three predictors: age, temporal lobe ADC, and the interaction between age and temporal lobe ADC. This model predicted 53% of the variance in memory scores within the ε4 carrier group (adjusted R2=.53, F(4,33)=11.59, p<.001). Importantly, the interaction term (age by temporal lobe ADC) was the only significant predictor in the model, demonstrating the importance of both variables in determining memory scores for ε4 carriers.
Predicting executive function scores
As summarized in Table 5, the GLM predicting executive function accounted for 22% of the variance, a significant but somewhat smaller amount of variance than was accounted for in memory performance (adjusted R2=.22, F(9,116)=4.95, p<.0001). The model showed several interesting results that differed from the memory model. First, while controlling for age and education, executive function scores were predicted independently by both frontal ADC, F(1,116)=5.22, p<.05, and frontal FA, F(1,116)=3.85, p<.05, while the main effect of temporal stem ADC did not approach significance. APOE status also predicted overall executive functioning scores, indicating that ε4 carriers obtained overall lower executive scores than noncarriers once age was accounted for, F(1,116)=6.67, p<.01. Finally, although the main effect of temporal stem ADC was not significant, APOE status and temporal stem ADC interacted significantly, F(1,116)=5.74, p<.05, suggesting that temporal stem ADC values predicted executive function scores for the ε4 carriers, but not the noncarriers, consistent with the Pearson correlations. The main effect of years of education was also significant, F(1,116)=13.83, p<.001 but did not interact with other variables.
4 Discussion
In summary, the present study demonstrated that ADC and FA diffusion measures differ with increasing age, replicating numerous prior studies. Age-related differences in ADC and FA diffusion measures were more pronounced in ε4 carriers than noncarriers across the whole brain, including frontal, temporal, and parietal white matter regions, as well as the corpus callosum and the centrum semiovale. Importantly, after controlling for age, region-specific diffusion measures predicted executive function and memory function, and these relationships were significantly influenced by APOE status. The results are discussed in more detail below.
Aging, APOE ε4, and diffusion
Three studies to date have found differences in white matter diffusion between ε4 carriers and noncarriers. Using a region of interest approach similar to the present study, Nierenberg (2005) found lower FA as well as higher radial diffusivity in ε4 carriers compared to noncarriers, ages 60–75, in a region of parahippocampal white matter that was similar to the temporal stem region used in the present study. Persson et al. (2006) also found lower FA values in ε4 carriers compared to noncarriers in the splenium of the corpus callosum, but no differences between individuals with one or two copies of the ε4 allele. In addition, a voxelwise analysis revealed decreased FA in the body and splenium of the corpus callosum, the hippocampus, the occipitofrontal fasciculus, and the cingulum. Smith et al. (2008), using whole brain spatial statistics analysis methods, compared two groups of women ranging in age from 40 to 90 years; 42 women were ε4 positive with a family history of Alzheimer’s disease, and 23 women were noncarriers. Similar to the results from Persson et al. (2006), they found lower FA values in the inferior temporal lobe, splenium, anterior cingulum, and frontal white matter. Common across all three studies, despite large methodological differences, was the finding of lower FA values in the parahippocampal/inferior temporal lobe white matter for ε4 carriers relative to noncarriers.
While our results are generally consistent with previous literature, the present study is unique in several ways. First, the three studies described above focused primarily on FA (radial diffusion was considered in Nierenberg et al.,2005), while the present study evaluated both FA and ADC. These two measures tend to be negatively correlated to a moderate degree within white matter tracts, but they may be sensitive to different aspects of pathological change in white matter (Song et al., 2002). We found that ADC, as well as FA, differed across all six regions of the brain sampled here as a function of age and APOE status. Second, rather than comparing mean differences across groups, the present study focused on the trajectory of age-related changes in diffusion measures. Taking this approach highlights the finding that the ε4 effect does not apply equally across all older adults, but rather appears to affect individuals differentially in increasing decades, as indicated by the interactions between APOE status, age, and diffusion values. The differential effect of ε4 over the life span may account in part for the lack of a main effect of ε4 on diffusion found in Bendlin et al. (in press), where participants were largely middle-aged. Although the cross-sectional design of the present study precludes making strong statements regarding longitudinal change as a function of age, the pattern of results suggests that the ε4 allele does not impart an additive effect on diffusion across the entire older adult age range, but rather changes the trajectory of normal aging.
The present results suggest that age-related differences in diffusion were observed in all regions of the brain, both anterior and posterior. Previous studies have suggested that aging results in more pronounced changes in frontal white matter (e.g., Bartzokis et al., 2004) with relative sparing of posterior regions, although more recent studies including Kennedy and Raz (2009) reported age-related changes in both FA and ADC that extended throughout both anterior and posterior brain regions. In our study, ADC was similarly correlated with age in all six brain regions, while FA was most strongly correlated with age within the frontal lobe and the temporal stem. The results are consistent with recent work by Zhang et al. (2008), comparing the pattern of age-related differences for FA and mean diffusivity (similar to ADC) across various brain regions. They found that both measures differed with age (FA decreased while mean diffusivity increased) in frontal areas, the corpus callosum, periventricular white matter, and brain stem. However, mean diffusivity differences were more widely distributed than FA differences encompassing both anterior and posterior brain regions, and several regions showed significant mean diffusivity differences without decreases in FA (bilateral cingulum, bilateral thalamus, caudate nuclei, the centrum semiovale, and the medial temporal lobe). The relatively large sample size in the present study and separating the variance associated with both aging and APOE status likely increased the power to detect differences in all regions. The present results suggest that age-related differences in diffusion are best described as affecting the whole brain, rather than frontal regions only.
Importantly, we found that ε4 exacerbated age-related diffusion differences in all regions of the brain that were sampled, both anterior and posterior. This is consistent with a recent study (Smith et al., 2008) showing lower FA values in the white matter of the frontal lobe, inferior temporal lobe, splenium, and anterior cingulum bundle. The results of these two studies are somewhat at odds with the majority of diffusion studies of MCI and AD patients suggesting that MCI/AD primarily affects posterior regions with relative sparing of frontal lobes. Smith et al. (2008) have suggested that the alterations observed in cognitively normal ε4 carriers reflect pathological changes associated with preclinical stages of AD, rather than normal aging. One might expect, then, that ε4 differences in diffusion should be most prominent in posterior regions rather than across the whole brain, reflecting the known distribution of early AD pathology. However, at least two studies have reported differences in frontal diffusion in MCI and AD groups compared to age-matched controls (Choi et al., 2005; Sydykova et al., 2007). The findings of this study and Smith et al. (2008) suggest that ε4 has widespread effects on diffusion that include both posterior regions associated with early AD pathology as well as more anterior regions of frontal white matter typically associated with normal aging. Some of the difference across studies may arise because few studies have separated participants based on APOE status. Although we did not have sufficient homozygotic individuals in the study to assess the differential effect of one versus two copies of ε4, the present study demonstrated that the effect of ε4 was not being driven solely by the homozygotic indivdiuals. At the very least, we can say that having even a single copy of the ε4 allele is sufficient to increase the amount of age-related diffusion difference in the white matter regions, and that the pattern of results is as strong, if not stronger, for those individuals with two copies of the ε4 allele.
The relation between diffusion and cognitive function
While many studies have found that diffusion measures differ with increasing age, fewer studies have focused on the association between diffusion and cognition, and even fewer on whether the association between diffusion and cognition is influenced by the presence of the ε4 allele. In the present study, we found that diffusion measures predicted cognitive function in a region-specific way, and that ε4 had a significant influence on these associations. More specifically, regardless of APOE status, frontal white matter diffusion measures (ADC and FA) independently predicted executive function scores for all participants, while temporal lobe ADC additionally predicted executive function for ε4 carriers, but not noncarriers. Memory scores were predicted specifically by temporal lobe ADC but not frontal diffusion measures for all participants, although the relationship between temporal lobe ADC and memory scores was significantly strengthened in ε4 carriers compared to noncarriers. Taken together, age and temporal lobe ADC accounted for a striking 53% of the variance in memory scores within the ε4 carrier group. It should be noted that ADC and FA values in the temporal lobe were highly correlated with one another. Once ADC was included in the multivariate models, FA did not add much predictive value to the model.
The results of previous studies on the influence of ε4 on cognitive function are mixed. Adamson et al. (2008) found no age by APOE interaction for memory function, but participants in the study were younger (ages 50–76) compared to the present study, an age range where ε4 had relatively limited effect on memory function. Consistent with our findings, Nilsson et al. (2006) found episodic memory decreased in ε4 carriers compared to noncarriers, but only in the old-old (ages 70 to 85), not young-old (ages 50–65) or young (ages 35–50). Somewhat inconsistent with our findings, a recent paper by Raz et al. (2009) found that ε4 resulted in greater age-related differences in executive function but not memory function. However, their sample included 189 individuals from ages 18 to 82 years. Such a sample with fewer participants in the older age range would be less likely to observe ε4 effects that may be present only in the 7th decade and beyond, as suggested by the present findings.
An important question is the extent to which the results are driven by the inclusion of individuals in preclinical stages of AD, or whether the APOE effects observed here are influencing the trajectory of normal aging. In other studies, the term “presymptomatic” has been applied to all individuals who are ε4 positive but otherwise cognitively healthy (e.g., Smith et al., 2008). However, this term implies that each person who is ε4 positive will go on to develop AD. Recent studies suggest that ε4 carries the greatest AD risk for individuals between 60 and 69 years. In this age group, nearly 50% of patients diagnosed with AD are ε4 carriers. At age 70 and beyond, the frequency of ε4 allele decreases in cognitively normal populations (Sando et al., 2008; Davidson et al., 2007). In the present study, more than half of the sample was age 70 or above, and this is the group in which ε4 had the strongest effect on cognitive functioning. We suggest that APOE may have dual effects. In earlier years, particularly in the 5th and 6th decades, the presence of ε4 may be related more directly to the development of pathology that results in a diagnosis of MCI or probable AD. In later decades, the influence of ε4 on the trajectory of normal aging may become more clearly visible, because a significant number of individuals who will develop AD as a function of ε4 risk have already been removed from normal aging samples. While this question can never be completely answered without longitudinal data, the present results suggest that ε4 may have a negative effect on the normal development of age-related changes in executive function and memory per se that may be separate from the development of the pathology associated with early AD. We are now following the sample longitudinally to determine the rate of decline in cognition as a function of APOE status and diffusion, and whether some of these individuals go on within a 2–3 year period to develop MCI or AD.
What could be the underlying mechanism that accounts for the associations observed here between ε4, diffusion, and normal age-related changes in cognitive function? We consider two possibilities, but clearly others could exist and in fact it is likely that several of these mechanisms interact with one another to produce cognitive decline. Separate from its role in the development of beta amyloid, we first consider the influence of ε4 on the function of neurons, and second, the influence of ε4 on inflammation and the health of myelin-producing oligodendroglia.
APOE is critical for maintaining the health and function of neurons through its role in redistributing lipids among CNS cells for normal lipid homeostasis, neuronal repair, synapse maintenance, and scavenging toxins. For example, through its lipid transport function, APOE ε3 and ε2 are effective in remodeling and repairing synapto-dendritic connections, but ε4 is much less effective (Mahley and Rall, Jr., 2000; reviewed in Mahley et al., 2006). The presence of ε4 therefore results in increased injury to cells in the presence of stressors such as oxidative stress, ischemia, excess beta amyloid production, SOD1 mutations, inflammation, and the aging process itself. In addition, APOE ε4 disrupts mitochondrial regulation of energy and glucose metabolism in neurons. An association between ε4 genotype and altered CNS glucose metabolism has been demonstrated in both AD patients and cognitively normal older adults (Small et al., 2000; Reiman et al., 2001; Reiman et al., 2004). Thus, even in adults without significant beta amyloid deposition, ε4 is likely to result in reduced neuronal glucose utilization due to altered mitochondrial activity and increased levels of cell damage due to a variety of CNS stressors that are known to increase with normal aging.
In comparison to ε2 and ε3, APOE ε4 results in an imbalance between pro-and anti-inflammatory cytokines that promotes a pro-inflammatory state and increases oxidative stress (reviewed in Jofre-Monseny et al., 2008). Oligodendrocytes that are responsible for the production and maintenance of myelin are very vulnerable to oxidative stress, inflammatory cytokines, ischemia, and excitotoxic neurotransmitters (Ludwin, 1997). APOE ε4 could contribute to accelerated myelin breakdown that underlies age-related changes in diffusion. The death of oligodendrocytes can activate the generation of new oligodendrocytes and remyelinization of the axon, but the resulting myelin sheaths are thinner with shorter internodes than the original myelin (Peters, 2002; 2009). Such alterations in myelin would result in decreased conduction velocity and disrupt the coordinated timing of neuronal signals across multiple brain regions. Damage to the myelin sheath would likely result in decreased FA diffusion and increased ADC, and possibly contribute to the cognitive impairments observed in older adults.
The notion that ε4 is related to inflammatory stress is consistent with several findings, both from AD and normal aging. For instance, meta-analyses of epidemiological studies show a 50% or better decrease in risk for developing AD among users of nonsteroidal anti-inflammatories (NSAIDs) compared to non-users (Etminan et al., 2003; McGeer et al., 1996; Szekely et al., 2004). In contrast to the overwhelmingly positive results of epidemiological studies, clinical trials of NSAIDs for the treatment of probable AD have yielded disappointing results (Aisen et al., 2002, 2003; Reines et al., 2004; Scharf et al., 1999). These studies suggested that NSAIDs may be useful prophylactically, rather than as a treatment for the disorder after the first symptoms appear. However, a large-scale primary prevention study that enrolled over 2,500 participants (ADAPT Research Group, 2007) also yielded negative results, although the study included only individuals over the age of 70 years. Few studies have considered anti-inflammatories in the context of normal aging. A recent study from our laboratory focusing on cognitively normal older adults demonstrated that NSAID drug users had significantly less age-related decreases in gray and white matter volumes compared to age-matched controls (Walther et al., 2009). These results suggest that drug treatments or life-style changes that combat inflammation may slow age-related changes in brain structure and function (Jofre-Monseny et al., 2008). This is a promising line of research that warrants further investigation.
In summary, the present study provides evidence that age-related differences in white matter diffusion interact with APOE status, which together predict cognitive functions of older adults in the areas of executive function and memory. We suggest that the presence of ε4 plays an important role in the trajectory of normal aging, possibly via mechanisms related to neural repair, inflammation, and oxidative stress. Our study suggests that the combination of ε4 and diffusion MRI is a good predictor of who will experience cognitive impairments as they age. Future studies may determine whether ε4 also results in faster rates of decline, and whether drug or life-style interventions can ameliorate these cognitive effects.
Acknowledgements
The project was supported by the Arizona Biomedical Research Commission, the Arizona Alzheimer’s Research Consortium (HB 2354, Dept. of Health Services, Arizona), the Evelyn F. McKnight Brain Institute, the National Institute on Aging (P30 AG19610 Arizona Alzheimer's Disease Core Center), and the National Institute on Neurologic Disorder and Stroke (RO1 NS044107). We thank Ms. Kristina Irwin for coordinating and administering neuropsychological testing, and Dr. Thomas Beach at Banner Sun Health Research Institute for his generous support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol. Aging. 2008;29:102–116. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Adamson MM, Landy KM, Duong S, Fox-Bosetti S, Ashford JW, Murphy GM, Weiner M, Taylor JL. Apolipoprotein E epsilon4 influences on episodic recall and brain structures in aging pilots. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.07.017. doi:10.1016/j.neurobiolaging.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Breitner JC, Green RC, Martin BK, Meinert C, Piantadosi S, Sabbagh M ADAPT Research Group. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schmeidler J, Pasinetti GM. Randomized pilot study of nimesulide treatment in Alzheimer's disease. Neurology. 2002;58:1050–1054. doi: 10.1212/wnl.58.7.1050. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol. Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Ries ML, Canu E, Lazar M, Alexander AL, Carlsson CM, Sager MA, Asthana S, Johnson SC. White matter is altered with parental family history of Alzheimer's disease. Alzheimer's & Dementia. doi: 10.1016/j.jalz.2009.11.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat YA, Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: changes with aging and the role of CSF-suppression. J. Magn. Reson. Imaging. 2004;20:216–227. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- Blair CK, Folsom AR, Knopman DS, Bray MS, Mosley TH, Boerwinkle E. Atherosclerosis Risk in Communities (ARIC) Study Investigators, 2005. APOE genotype and cognitive decline in a middle-aged cohort. Neurology. 2005;64:268–276. doi: 10.1212/01.WNL.0000149643.91367.8A. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Comi G, Filippi M. White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry. 2002;72:742–746. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2003;60:1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, Huettel SA. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol. Aging. 2008;29:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage. 2008;41:1177–1183. doi: 10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Locke DE, Hutton ML, Hentz JG, Hoffman-Snyder C, Woodruff BK, Alexander GE, Osborne D. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch. Neurol. 2007;64:1306–1311. doi: 10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62:1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiol. Aging. 2008;29:1547–1555. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Leach LS, Christensen H, Anstey KJ. Neuroimaging and APOE genotype: a systematic qualitative review. Dement. Geriatr. Cogn. Disord. 2007;24:348–362. doi: 10.1159/000109150. [DOI] [PubMed] [Google Scholar]
- Cho H, Yang DW, Shon YM, Kim BS, Kim YI, Choi YB, Lee KS, Shim YS, Yoon B, Kim W, Ahn KJ. Abnormal integrity of corticocortical tracts in mild cognitive impairment: a diffusion tensor imaging study. J. Korean. Med. Sci. 2008;23:477–483. doi: 10.3346/jkms.2008.23.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Lim KO, Monteiro I, Reisberg B. Diffusion tensor imaging of frontal white matter microstructure in early Alzheimer's disease: a preliminary study. J. Geriatr. Psychiatry Neurol. 2005;18:12–19. doi: 10.1177/0891988704271763. [DOI] [PubMed] [Google Scholar]
- Davidson Y, Gibbons L, Pritchard A, Hardicre J, Wren J, Stopford C, Julien C, Thompson J, Payton A, Pickering-Brown SM, Pendleton N, Horan MA, Burns A, Purandare N, Lendon CL, Neary D, Snowden JS, Mann DM. Apolipoprotein E epsilon4 allele frequency and age at onset of Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2007;23:60–66. doi: 10.1159/000097038. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, Wardlaw JM. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer J, Kaplan E, Ober BA. Psychological Corporation. San Antonio, TX: 1987. The California Verbal Learning Test. [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59:746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Ding B, Chen KM, Ling HW, Zhang H, Chai WM, Li X, Wang T. Diffusion tensor imaging correlates with proton magnetic resonance spectroscopy in posterior cingulate region of patients with Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2008;25:218–225. doi: 10.1159/000113948. [DOI] [PubMed] [Google Scholar]
- Etminan M, Gill S, Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer's disease: systematic review and meta-analysis of observational studies. BMJ. 2003;327:128. doi: 10.1136/bmj.327.7407.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MRI signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJNR. 1987;8:421–426. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Dellani PR, Greverus D, Scheurich A, Stoeter P, Müller MJ. Predicting conversion to dementia in mild cognitive impairment by volumetric and diffusivity measurements of the hippocampus. Psychiatry Res. 2006;146:283–287. doi: 10.1016/j.pscychresns.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Müller MJ, Wille P, Dellani PR, Scheurich A, Schmidt LG, Stoeter P. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol. Aging. 2005;26:1193–1198. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Blamire AM, Krishnan MS, Teodorczuk A, English P, Gholkar A, Harrison R, O’Brien JT. Atrophy is associated with posterior cingulate white matter disruption in dementia with Lewy bodies and Alzheimer's disease. Neuroimage. 2007;36:1–7. doi: 10.1016/j.neuroimage.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Furutani K, Harada M, Minato M, Morita N, Nishitani H. Regional changes of fractional anisotropy with normal aging using statistical parametric mapping (SPM). J. Med. Invest. 2005;52:186–190. doi: 10.2152/jmi.52.186. [DOI] [PubMed] [Google Scholar]
- Gerdes LU, Klausen IC, Sihm I, Faergeman O. Apolipoprotein E polymorphism in a Danish population compared to findings in 45 other study populations around the world. Genet. Epidemiol. 1992;9:155–167. doi: 10.1002/gepi.1370090302. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Kong LL. Do young and older adults rely on different processes in source memory tasks? A neuropsychological study. J. Exp. Psychol. Learn. Mem. Cogn. 2008;34:809–822. doi: 10.1037/0278-7393.34.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Rubin SR, Davidson PS. Source memory in older adults: an encoding or retrieval problem? J. Exp. Psychol..Learn. Mem. Cogn. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am. J. Neuroradiol. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Hanyu H, Sakurai H, Iwamoto T, Takasaki M, Shindo H, Abe K. Diffusion-weighted MR imaging of the hippocampus and temporal white matter in Alzheimer's disease. J. Neurol. Sci. 1998;156:195–200. doi: 10.1016/s0022-510x(98)00043-4. [DOI] [PubMed] [Google Scholar]
- Hart RP, Kwentus JA, Wade JB, Taylor JR. Modified Wisconsin Card Sorting Test in elderly normal, depressed and demented patients. Clin. Neuropsychol. 1988;2:49–56. [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb. Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J. Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- Hsu JL, Leemans A, Bai CH, Lee CH, Tsai YF, Chiu HC, Chen WH. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage. 2008;39:566–577. doi: 10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Huang J, Friedland RP, Auchus AP. Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am. J. Neuroradiol. 2007;28:1943–1948. doi: 10.3174/ajnr.A0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ. Relating imaging indices of white matter integrity and volume in healthy older adults. Cereb. Cortex. 2008;18:433–442. doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, Bondi MW. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dement. Geriatr. Cogn. Disord. 2007;23:382–389. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput. Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol. Nutr. Food Res. 2008;52:131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR, Jr, Xu YC, Campeau NG, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Mild cognitive impairment and Alzheimer disease: regional diffusivity of water. Radiology. 2001;219:101–107. doi: 10.1148/radiology.219.1.r01ap14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Petersen RC, Boeve BF, Knopman DS, Weigand SD, O’Brien PC, Shiung MM, Smith GE, Ivnik RJ, Tangalos EG, Jack CR., Jr DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology. 2005;64:902–904. doi: 10.1212/01.WNL.0000153076.46126.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Dufouil C, Grassiot B, Tzourio C, Alperovitch A, Mazoyer B. No epsilon4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. Neuroimage. 2005;24:1205–1213. doi: 10.1016/j.neuroimage.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Ludwin SK. The pathobiology of the oligodendrocyte. J..Neuropathol. Exp. Neurol. 1997;56:111–124. doi: 10.1097/00005072-199702000-00001. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol. Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, Small SA, Tang M, Tycko B, Stern Y. Memory performance in healthy elderly without Alzheimer's disease: effects of time and apolipoprotein-E. Neurobiol. Aging. 2001;22:683–689. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- Medina D, Toledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol. Aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Mosconi L, De SS, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, Rich KE, Switalski R, Mehta PD, Pratico D, Zinkowski R, Blennow K, de Leon MJ. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol. Psychiatry. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MJ, Greverus D, Dellani PR, Weibrich C, Wille PR, Scheurich A, Stoeter P, Fellgiebel A. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage. 2005;28:1033–1042. doi: 10.1016/j.neuroimage.2005.06.029. [DOI] [PubMed] [Google Scholar]
- Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla BG, Meder JF. Diffusion tensor imaging in early Alzheimer's disease. Psychiatry Res. 2006;146:243–249. doi: 10.1016/j.pscychresns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Nierenberg J, Pomara N, Hoptman MJ, Sidtis JJ, Ardekani BA, Lim KO. Abnormal white matter integrity in healthy apolipoprotein E epsilon4 carriers. Neuroreport. 2005;16:1369–1372. doi: 10.1097/01.wnr.0000174058.49521.16. [DOI] [PubMed] [Google Scholar]
- Nilsson LG, Adolfsson R, Backman L, Cruts M, Nyberg L, Small BJ, Van BC. The influence of APOE status on episodic and semantic memory: data from a population-based study. Neuropsychology. 2006;20:645–657. doi: 10.1037/0894-4105.20.6.645. [DOI] [PubMed] [Google Scholar]
- Pagani E, Agosta F, Rocca MA, Caputo D, Filippi M. Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage. 2008;41:657–667. doi: 10.1016/j.neuroimage.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van BC, Adolfsson R, Nilsson LG, Nyberg L. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology. 2006;66:1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. J. Neurocytol. 2002;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Front. Neuroanat. 2009 doi: 10.3389/neuro.05.011.2009. doi: 10.3389/neuro.05.011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Ray KM, Wang H, Chu Y, Chen YF, Bert A, Hasso AN, Su MY. Mild cognitive impairment: apparent diffusion coefficient in regional gray matter and white matter structures. Radiology. 2006;241:197–205. doi: 10.1148/radiol.2411051051. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S. Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology. 2009;23:105–116. doi: 10.1037/a0013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc. Natl. Acad. Sci. U.S. A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines SA, Block GA, Morris JC, Liu G, Nessly ML, Lines CR, Norman BA, Baranak CC. Rofecoxib: no effect on Alzheimer's disease in a 1-year, randomized, blinded, controlled study. Neurology. 2004;62:66–71. doi: 10.1212/wnl.62.1.66. [DOI] [PubMed] [Google Scholar]
- Rose SE, Janke AL, Chalk JB. Gray and white matter changes in Alzheimer's disease: a diffusion tensor imaging study. J. Magn. Reson. Imaging. 2008;27:20–26. doi: 10.1002/jmri.21231. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol. Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Sando SB, Melquist S, Cannon A, Hutton ML, Sletvold O, Saltvedt I, White LR, Lydersen S, Aasly JO. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer's disease; a case control study from central Norway. BMC Neurology. 2008;8:9. doi: 10.1186/1471-2377-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y. Imaging studies and APOE genotype in persons at risk for Alzheimer's disease. Curr. Psychiatry Rep. 2006;8:11–17. doi: 10.1007/s11920-006-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer's disease. Neurology. 1999;53:197–201. doi: 10.1212/wnl.53.1.197. [DOI] [PubMed] [Google Scholar]
- Schultz MR, Lyons MJ, Franz CE, Grant MD, Boake C, Jacobson KC, Xian H, Schellenberg GD, Eisen SA, Kremen WS. Apolipoprotein E genotype and memory in the sixth decade of life. Neurology. 2008;70:1771–1777. doi: 10.1212/01.wnl.0000286941.74372.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkin SD, Bastin ME, MacGillivray TJ, Deary IJ, Starr JM, Rivers CS, Wardlaw JM. Cognitive correlates of cerebral white matter lesions and water diffusion tensor parameters in community-dwelling older people. Cerebovasc. Dis. 2005;20:310–318. doi: 10.1159/000087930. [DOI] [PubMed] [Google Scholar]
- Shenkin SD, Bastin ME, MacGillivray TJ, Deary IJ, Starr JM, Wardlaw JM. Childhood and current cognitive function in healthy 80-year-olds: a DT-MRI study. Neuroreport. 2003;14:345–349. doi: 10.1097/00001756-200303030-00010. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychol. Aging. 2004;19:592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, Mazziotta JC, Saxena S, Wu HM, Mega MS, Cummings JL, Saunders AM, Pericak-Vance MA, Roses AD, Barrio JR, Phelps ME. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Chebrolu H, Andersen AH, Powell DA, Lovell MA, Xiong S, Gold BT. White matter diffusion alterations in normal women at risk of Alzheimer's disease. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.006. doi:10.1016/j.neurobiolaging.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia, Revised Edition. Victoria, British Columbia, Canada: University of Victoria Neuropsychology Laboratory; 1977. [Google Scholar]
- Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO. White matter damage in Alzheimer disease and mild cognitive impairment: assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology. 2007;243:483–492. doi: 10.1148/radiol.2432051714. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb. Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neurosci. Biobehav. Rev. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN, Carmelli D, Schellenberg GD, La Rue A. Apolipoprotein E epsilon4 and change in cognitive functioning in community-dwelling older adults. J. Geriatr. Psychiatry Neurol. 2005;18:196–201. doi: 10.1177/0891988705281864. [DOI] [PubMed] [Google Scholar]
- Sydykova D, Stahl R, Dietrich O, Ewers M, Reiser MF, Schoenberg SO, Moller HJ, Hampel H, Teipel SJ. Fiber connections between the cerebral cortex and the corpus callosum in Alzheimer's disease: a diffusion tensor imaging and voxel-based morphometry study. Cereb. Cortex. 2007;17:2276–2282. doi: 10.1093/cercor/bhl136. [DOI] [PubMed] [Google Scholar]
- Szekely CA, Thorne JE, Zandi PP, Ek M, Messias E, Breitner JC, Goodman SN. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer's disease: a systematic review. Neuroepidemiology. 2004;23:159–169. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Stahl R, Dietrich O, Schoenberg SO, Perneczky R, Bokde AL, Reiser MF, Moller HJ, Hampel H. Multivariate network analysis of fiber tract integrity in Alzheimer's disease. Neuroimage. 2007;34:985–995. doi: 10.1016/j.neuroimage.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Walther K, Bendlin BB, Glisky EL, Trouard TP, Lisse JR, Posever JO, Ryan L. Anti-inflammatory drugs reduce age-related decreases in brain volume in cognitively normal older adults. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.006. doi:10.1016/j.neurobiolaging.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- Wechsler D. Wechsler Memory Scale - III. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio: Psychological Corporation; 1999. [Google Scholar]
- Wishart HA, Saykin AJ, McAllister TW, Rabin LA, McDonald BC, Flashman LA, Roth RM, Mamourian AC, Tsongalis GJ, Rhodes CH. Regional brain atrophy in cognitively intact adults with a single APOE epsilon4 allele. Neurology. 2006;67:1221–1224. doi: 10.1212/01.wnl.0000238079.00472.3a. [DOI] [PubMed] [Google Scholar]
- Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66:1845–1849. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]
- Yasmin H, Nakata Y, Aoki S, Abe O, Sato N, Nemoto K, Arima K, Furuta N, Uno M, Hirai S, Masutani Y, Ohtomo K. Diffusion abnormalities of the uncinate fasciculus in Alzheimer's disease: diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiology. 2008;50:293–299. doi: 10.1007/s00234-007-0353-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Du AT, Hayasaka S, Jahng GH, Hlavin J, Zhan W, Weiner MW, Schuff N. Patterns of age-related water diffusion changes in human brain by concordance and discordance analysis. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.009. doi:10.1016/j.neurobiolaging.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YT, Zhang CY, Zhang J, Li W. Age-related changes of normal adult brain structure: analysed with diffusion tensor imaging. Chin. Med. J. (Engl.) 2005;118:1059–1065. [PubMed] [Google Scholar]