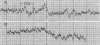Abstract
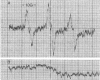
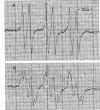
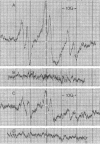
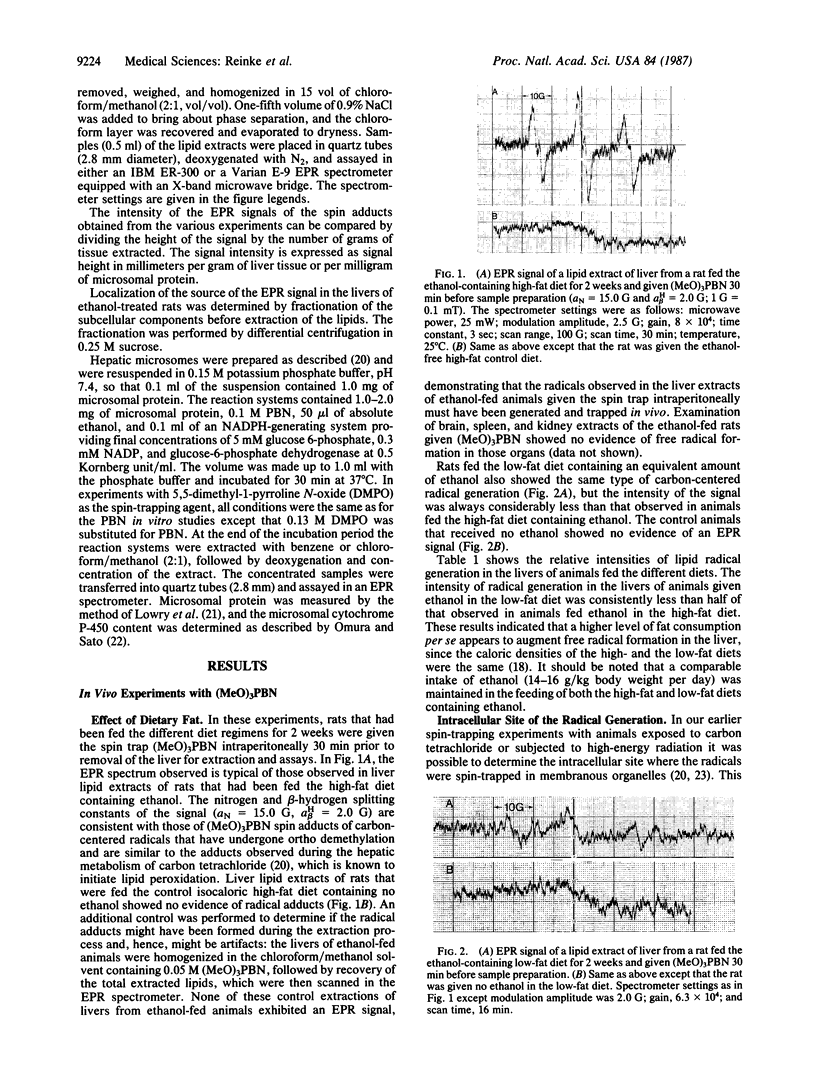
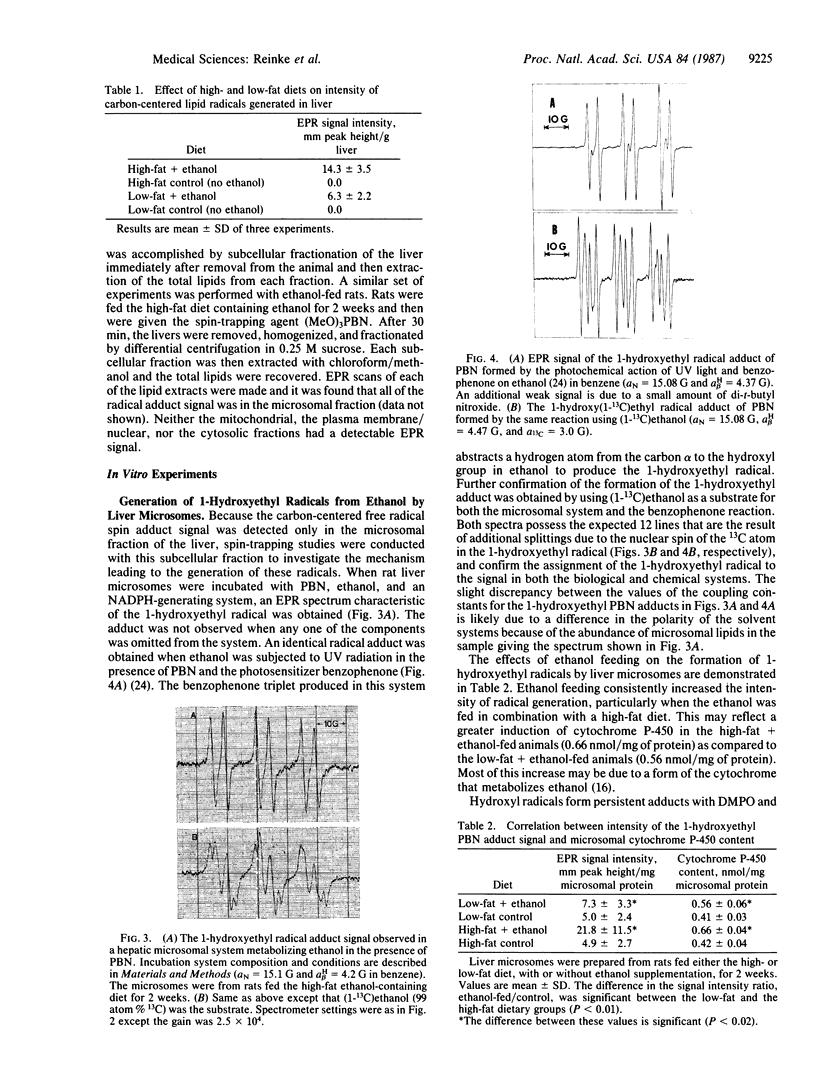
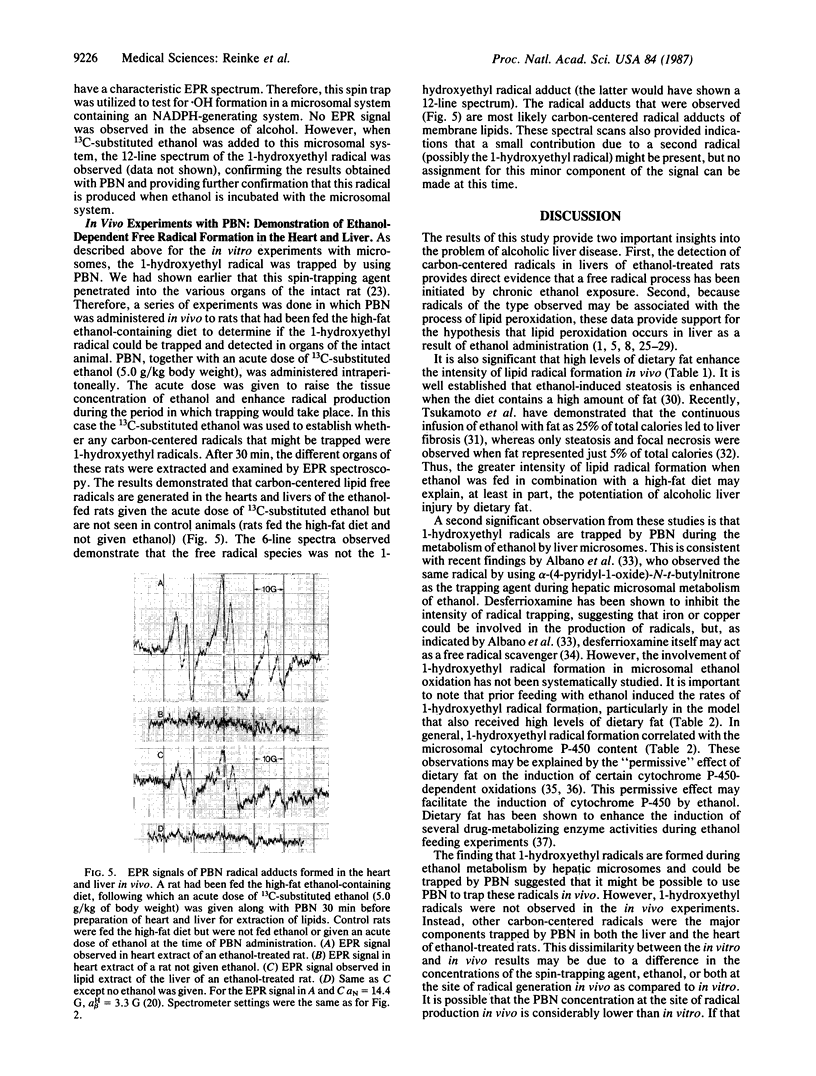
Rats fed a high-fat ethanol-containing diet for 2 weeks were found to generate free radicals in liver and heart in vivo. The radicals are believed to be carbon-centered radicals, were detected by administering spin-trapping agents to the rats, and were characterized by electron paramagnetic resonance spectroscopy. The radicals in the liver were demonstrated to be localized in the endoplasmic reticulum. Rats fed ethanol in a low-fat diet showed significantly less free radical generation. Control animals given isocaloric diets without ethanol showed no evidence of free radicals in liver and heart. When liver microsomes prepared from rats fed the high-fat ethanol diet were incubated in a system containing ethanol, NADPH, and a spin-trapping agent, the generation of 1-hydroxyethyl radicals was observed. The latter was verified by using 13C-substituted ethanol. Microsomes from animals fed the high-fat ethanol-containing diet had higher levels of cytochrome P-450 than microsomes from rats fed the low-fat ethanol-containing diet. The results suggest that the consumption of ethanol results in the production of free radicals in rat liver and heart in vivo that appear to initiate lipid peroxidation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano E., Tomasi A., Goria-Gatti L., Poli G., Vannini V., Dianzani M. U. Free radical metabolism of alcohols by rat liver microsomes. Free Radic Res Commun. 1987;3(1-5):243–249. doi: 10.3109/10715768709069789. [DOI] [PubMed] [Google Scholar]
- Baraona E., Lieber C. S. Effects of ethanol on lipid metabolism. J Lipid Res. 1979 Mar;20(3):289–315. [PubMed] [Google Scholar]
- Century B. A role of the dietary lipid in the ability of phenobarbital to stimulate drug detoxification. J Pharmacol Exp Ther. 1973 May;185(2):185–194. [PubMed] [Google Scholar]
- Comporti M., Benedetti A., Chieli E. Studies on in vitro peroxidation of liver lipids in ethanol-treated rats. Lipids. 1973 Sep;8(9):498–502. doi: 10.1007/BF02531984. [DOI] [PubMed] [Google Scholar]
- DILUZIO N. R., COSTALES F. INHIBITION OF THE ETHANOL AND CARBON TETRACHLORIDE INDUCED FATTY LIVER BY ANTIOXIDANTS. Exp Mol Pathol. 1965 Apr;28:141–154. doi: 10.1016/0014-4800(65)90030-4. [DOI] [PubMed] [Google Scholar]
- Dianzani M. U. Lipid peroxidation in ethanol poisoning: a critical reconsideration. Alcohol Alcohol. 1985;20(2):161–173. [PubMed] [Google Scholar]
- Ekström G., Cronholm T., Ingelman-Sundberg M. Hydroxyl-radical production and ethanol oxidation by liver microsomes isolated from ethanol-treated rats. Biochem J. 1986 Feb 1;233(3):755–761. doi: 10.1042/bj2330755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista S., Meli A. Influence of antioxidants and radical scavengers on ethanol-induced gastric ulcers in the rat. Gen Pharmacol. 1985;16(3):285–286. doi: 10.1016/0306-3623(85)90085-0. [DOI] [PubMed] [Google Scholar]
- Feierman D. E., Winston G. W., Cederbaum A. I. Ethanol oxidation by hydroxyl radicals: role of iron chelates, superoxide, and hydrogen peroxide. Alcohol Clin Exp Res. 1985 Mar-Apr;9(2):95–102. doi: 10.1111/j.1530-0277.1985.tb05525.x. [DOI] [PubMed] [Google Scholar]
- Joly J. G., Hétu C. Effects of chronic ethanol administration in the rat: relative dependency on dietary lipids--I. Induction of hepatic drug-metabolizing enzymes in vitro. Biochem Pharmacol. 1975 Aug 15;24(16):1475–1480. doi: 10.1016/0006-2952(75)90021-0. [DOI] [PubMed] [Google Scholar]
- Klein S. M., Cohen G., Lieber C. S., Cederbaum A. I. Increased microsomal oxidation of hydroxyl radical scavenging agents and ethanol after chronic consumption of ethanol. Arch Biochem Biophys. 1983 Jun;223(2):425–432. doi: 10.1016/0003-9861(83)90606-9. [DOI] [PubMed] [Google Scholar]
- Koes M., Ward T., Pennington S. Lipid peroxidation in chronic ethanol treated rats: in vitro uncoupling of peroxidation from reduced nicotine adenosine dinucleotide phosphate oxidation. Lipids. 1974 Nov;9(11):899–904. doi: 10.1007/BF02532616. [DOI] [PubMed] [Google Scholar]
- Koop D. R., Morgan E. T., Tarr G. E., Coon M. J. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J Biol Chem. 1982 Jul 25;257(14):8472–8480. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai E. K., Crossley C., Sridhar R., Misra H. P., Janzen E. G., McCay P. B. In vivo spin trapping of free radicals generated in brain, spleen, and liver during gamma radiation of mice. Arch Biochem Biophys. 1986 Jan;244(1):156–160. doi: 10.1016/0003-9861(86)90104-9. [DOI] [PubMed] [Google Scholar]
- Lieber C. S. Alcohol and the liver: 1984 update. Hepatology. 1984 Nov-Dec;4(6):1243–1260. doi: 10.1002/hep.1840040625. [DOI] [PubMed] [Google Scholar]
- Lieber C. S. Alcohol and the liver: metabolism of ethanol, metabolic effects and pathogenesis of injury. Acta Med Scand Suppl. 1985;703:11–55. doi: 10.1111/j.0954-6820.1985.tb08903.x. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res. 1982 Fall;6(4):523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- Litov R. E., Irving D. H., Downey J. E., Tappel A. L. Lipid peroxidation: a mechanism involved in acute ethanol toxicity as demonstrated by in vivo pentane production in the rat. Lipids. 1978 Apr;13(4):305–307. doi: 10.1007/BF02533677. [DOI] [PubMed] [Google Scholar]
- Marshall W. J., McLean A. E. The effect of oral phenobarbitone on hepatic microsomal cytochrome P-450 and demethylation activity in rats fed normal and low protein diets. Biochem Pharmacol. 1969 Jan;18(1):153–157. doi: 10.1016/0006-2952(69)90020-3. [DOI] [PubMed] [Google Scholar]
- McCay P. B., Lai E. K., Poyer J. L., DuBose C. M., Janzen E. G. Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism. Observation of lipid radicals in vivo and in vitro. J Biol Chem. 1984 Feb 25;259(4):2135–2143. [PubMed] [Google Scholar]
- Morgan E. T., Koop D. R., Coon M. J. Catalytic activity of cytochrome P-450 isozyme 3a isolated from liver microsomes of ethanol-treated rabbits. Oxidation of alcohols. J Biol Chem. 1982 Dec 10;257(23):13951–13957. [PubMed] [Google Scholar]
- Müller A., Sies H. Role of alcohol dehydrogenase activity and the acetaldehyde in ethanol- induced ethane and pentane production by isolated perfused rat liver. Biochem J. 1982 Jul 15;206(1):153–156. doi: 10.1042/bj2060153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Oei H. H., Stroo W. E., Burton K. P., Schaffer S. W. A possible role of xanthine oxidase in producing oxidative stress in the heart of chronically ethanol treated rats. Res Commun Chem Pathol Pharmacol. 1982 Dec;38(3):453–461. [PubMed] [Google Scholar]
- Oei H. H., Zoganas H. C., McCord J. M., Schaffer S. W. Role of acetaldehyde and xanthine oxidase in ethanol-induced oxidative stress. Res Commun Chem Pathol Pharmacol. 1986 Feb;51(2):195–203. [PubMed] [Google Scholar]
- Redetzki J. E., Griswold K. E., Nopajaroonsri C., Redetzki H. M. Amelioration of cardiotoxic effects of alcohol by vitamin E. J Toxicol Clin Toxicol. 1983 Jun;20(4):319–331. doi: 10.3109/15563658308990599. [DOI] [PubMed] [Google Scholar]
- Reitz R. C. A possible mechanism for the peroxidation of lipids due to chronic ethanol ingestion. Biochim Biophys Acta. 1975 Feb 20;380(2):145–154. doi: 10.1016/0005-2760(75)90001-6. [DOI] [PubMed] [Google Scholar]
- Report of the American Institute of Nurtition ad hoc Committee on Standards for Nutritional Studies. J Nutr. 1977 Jul;107(7):1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- Rosenblum E., Gavaler J. S., Van Thiel D. H. Lipid peroxidation: a mechanism for ethanol-associated testicular injury in rats. Endocrinology. 1985 Jan;116(1):311–318. doi: 10.1210/endo-116-1-311. [DOI] [PubMed] [Google Scholar]
- Shaw S., Jayatilleke E., Lieber C. S. The effect of chronic alcohol feeding on lipid peroxidation in microsomes: lack of relationship to hydroxyl radical generation. Biochem Biophys Res Commun. 1984 Jan 13;118(1):233–238. doi: 10.1016/0006-291x(84)91091-x. [DOI] [PubMed] [Google Scholar]
- Shaw S., Jayatilleke E., Ross W. A., Gordon E. R., Leiber C. S. Ethanol-induced lipid peroxidation: potentiation by long-term alcohol feeding and attenuation by methionine. J Lab Clin Med. 1981 Sep;98(3):417–424. [PubMed] [Google Scholar]
- Tsukamoto H., French S. W., Benson N., Delgado G., Rao G. A., Larkin E. C., Largman C. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology. 1985 Mar-Apr;5(2):224–232. doi: 10.1002/hep.1840050212. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H., Towner S. J., Ciofalo L. M., French S. W. Ethanol-induced liver fibrosis in rats fed high fat diet. Hepatology. 1986 Sep-Oct;6(5):814–822. doi: 10.1002/hep.1840060503. [DOI] [PubMed] [Google Scholar]
- Videla L. A., Fernandez V., Ugarte G., Valenzuela A. Effect of acute ethanol intoxication on the content of reduced glutathione of the liver in relation to its lipoperoxidative capacity in the rat. FEBS Lett. 1980 Feb 25;111(1):6–10. doi: 10.1016/0014-5793(80)80749-6. [DOI] [PubMed] [Google Scholar]
- Videla L. A., Valenzuela A. Alcohol ingestion, liver glutathione and lipoperoxidation: metabolic interrelations and pathological implications. Life Sci. 1982 Nov 29;31(22):2395–2407. doi: 10.1016/0024-3205(82)90743-3. [DOI] [PubMed] [Google Scholar]
- Winston G. W., Cederbaum A. I. NADPH-dependent production of oxy radicals by purified components of the rat liver mixed function oxidase system. I. Oxidation of hydroxyl radical scavenging agents. J Biol Chem. 1983 Feb 10;258(3):1508–1513. [PubMed] [Google Scholar]
- Winston G. W., Cederbaum A. I. NADPH-dependent production of oxy radicals by purified components of the rat liver mixed function oxidase system. II. Role in microsomal oxidation of ethanol. J Biol Chem. 1983 Feb 10;258(3):1514–1519. [PubMed] [Google Scholar]
- Zimmerman H. J. Effects of alcohol on other hepatotoxins. Alcohol Clin Exp Res. 1986 Jan-Feb;10(1):3–15. doi: 10.1111/j.1530-0277.1986.tb05605.x. [DOI] [PubMed] [Google Scholar]