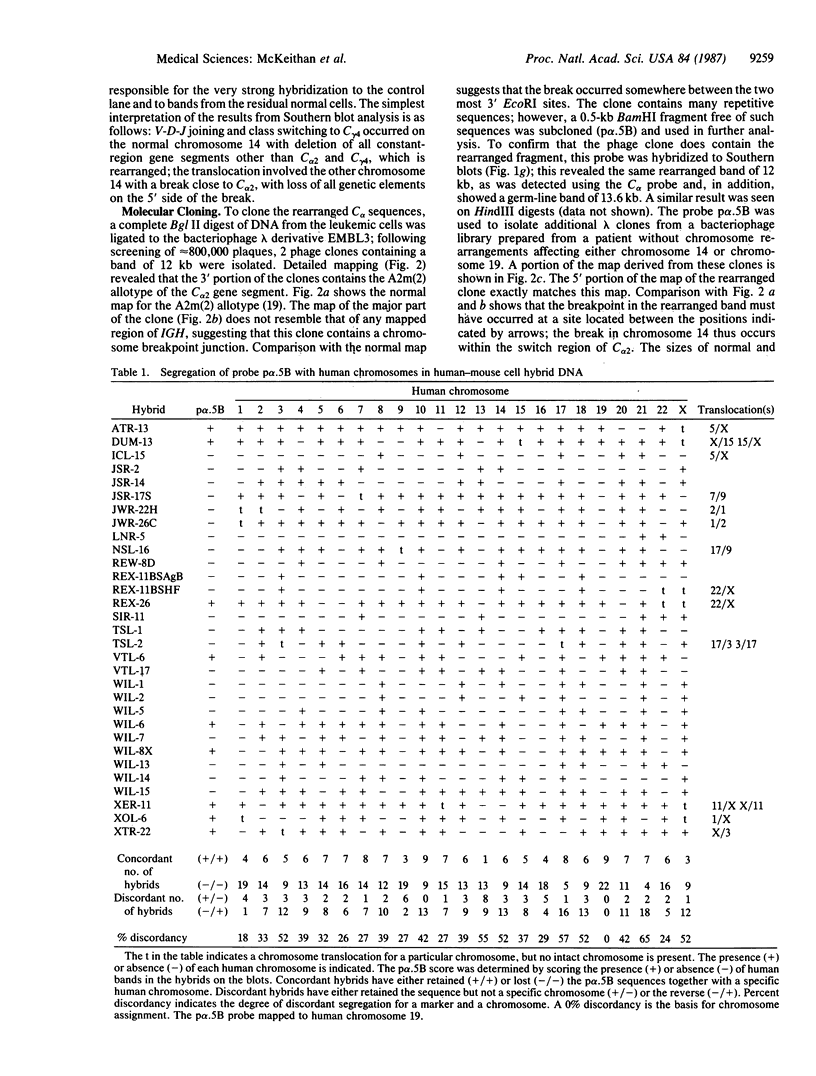
Abstract
Our laboratory has reported that t(14;19)(q32; q13.1) is a recurring translocation in the neoplastic cells of patients with chronic lymphocytic leukemia. In the present study, we have analyzed the leukemic cells from one such patient with probes from the immunoglobulin heavy-chain locus, which is present on band q32 of chromosome 14. Using a probe for the alpha constant-region gene segments, we detected a rearranged band by Southern blot analysis. This rearranged band was cloned and mapped. A subclone free of repetitive sequences was shown to be from chromosome 19 by analysis of human-mouse somatic cell hybrids, confirming that the rearranged band contains the translocation breakpoint junction. This probe may be used to identify a gene on chromosome 19 adjacent to the breakpoint that can contribute to the malignant development of B lymphocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander A., Steinmetz M., Barritault D., Frangione B., Franklin E. C., Hood L., Buxbaum J. N. gamma Heavy chain disease in man: cDNA sequence supports partial gene deletion model. Proc Natl Acad Sci U S A. 1982 May;79(10):3260–3264. doi: 10.1073/pnas.79.10.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Chen K. C., Smith S. D., Rabbitts T. H. Fusion of an immunoglobulin variable gene and a T cell receptor constant gene in the chromosome 14 inversion associated with T cell tumors. Cell. 1985 Dec;43(3 Pt 2):705–713. doi: 10.1016/0092-8674(85)90243-0. [DOI] [PubMed] [Google Scholar]
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Bloomfield C. D., Arthur D. C., Frizzera G., Levine E. G., Peterson B. A., Gajl-Peczalska K. J. Nonrandom chromosome abnormalities in lymphoma. Cancer Res. 1983 Jun;43(6):2975–2984. [PubMed] [Google Scholar]
- Bloomfield C. D., Arthur D. C., Frizzera G., Levine E. G., Peterson B. A., Gajl-Peczalska K. J. Nonrandom chromosome abnormalities in lymphoma. Cancer Res. 1983 Jun;43(6):2975–2984. [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny C. T., Hollis G. F., Hecht F., Morgan R., Link M. P., Smith S. D., Kirsch I. R. Common mechanism of chromosome inversion in B- and T-cell tumors: relevance to lymphoid development. Science. 1986 Oct 10;234(4773):197–200. doi: 10.1126/science.3092355. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Leder P., Battey J., Lenoir G., Moulding C., Murphy W., Potter H., Stewart T., Taub R. Translocations among antibody genes in human cancer. Science. 1983 Nov 18;222(4625):765–771. doi: 10.1126/science.6356357. [DOI] [PubMed] [Google Scholar]
- Lefranc M. P., Rabbitts T. H. Human immunoglobulin heavy chain A2 gene allotype determination by restriction fragment length polymorphism. Nucleic Acids Res. 1984 Feb 10;12(3):1303–1311. doi: 10.1093/nar/12.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C., Vonderheid E. C., Besa E., Hoxie J. A., Moreau L., Finan J. B. The most common chromosome change in 86 chronic B cell or T cell tumors: a 14q32 translocation. Cancer Genet Cytogenet. 1986 Jan 15;19(3-4):219–227. doi: 10.1016/0165-4608(86)90050-6. [DOI] [PubMed] [Google Scholar]
- Pittman S., Catovsky D. Prognostic significance of chromosome abnormalities in chronic lymphocytic leukaemia. Br J Haematol. 1984 Dec;58(4):649–660. doi: 10.1111/j.1365-2141.1984.tb06112.x. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Siebenlist U., Korsmeyer S., Waldmann T., Leder P. Structure of the human immunoglobulin mu locus: characterization of embryonic and rearranged J and D genes. Cell. 1981 Dec;27(3 Pt 2):583–591. doi: 10.1016/0092-8674(81)90400-1. [DOI] [PubMed] [Google Scholar]
- Shima E. A., Le Beau M. M., McKeithan T. W., Minowada J., Showe L. C., Mak T. W., Minden M. D., Rowley J. D., Diaz M. O. Gene encoding the alpha chain of the T-cell receptor is moved immediately downstream of c-myc in a chromosomal 8;14 translocation in a cell line from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1986 May;83(10):3439–3443. doi: 10.1073/pnas.83.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe L. C., Ballantine M., Nishikura K., Erikson J., Kaji H., Croce C. M. Cloning and sequencing of a c-myc oncogene in a Burkitt's lymphoma cell line that is translocated to a germ line alpha switch region. Mol Cell Biol. 1985 Mar;5(3):501–509. doi: 10.1128/mcb.5.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows T. B., Brown J. A., Haley L. L., Byers M. G., Eddy R. L., Cooper E. S., Goggin A. P. Assignment of the beta-glucuronidase structural gene to the pter leads to q22 region of chromosome 7 in man. Cytogenet Cell Genet. 1978;21(1-2):99–104. doi: 10.1159/000130882. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Sakaguchi A. Y., Naylor S. L. Mapping the human genome, cloned genes, DNA polymorphisms, and inherited disease. Adv Hum Genet. 1982;12:341–452. doi: 10.1007/978-1-4615-8315-8_5. [DOI] [PubMed] [Google Scholar]
- Shows T., Eddy R., Haley L., Byers M., Henry M., Fujita T., Matsui H., Taniguchi T. Interleukin 2 (IL2) is assigned to human chromosome 4. Somat Cell Mol Genet. 1984 May;10(3):315–318. doi: 10.1007/BF01535253. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Finger L. R., Yunis J., Nowell P. C., Croce C. M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984 Nov 30;226(4678):1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Jaffe E., Cossman J., Gorham J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985 May 23;315(6017):340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Ueshima Y., Bird M. L., Vardiman J. W., Rowley J. D. A 14;19 translocation in B-cell chronic lymphocytic leukemia: a new recurring chromosome aberration. Int J Cancer. 1985 Sep 15;36(3):287–290. [PubMed] [Google Scholar]
- ar-Rushdi A., Nishikura K., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983 Oct 28;222(4622):390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]



