Abstract
It is well-known that electrical field stimulation (EFS)-induced contraction is mediated by a cholinergic mechanism and other neurotransmitters. NO, ATP, calcitonin gene-related peptide (CGRP), and substance P are released by EFS. To investigate the purinergic mechanism involved in the EFS-induced contraction, purinegic receptors antagonists were used. Suramine, a non-selective P2 receptor antagonist, reduced the contraction induced by EFS. NF023 (10-7~10-4 M), a selective P2X antagonist, inhibited the contraction evoked by EFS. Reactive blue (10-6~10-4 M), selective P2Y antagonist, also blocked the contraction in a dose-dependent manner. In addition, P2X agonist α,β-methylene 5'-adenosine triphosphate (αβMeATP, 10-7~10-5 M) potentiated EFS-induced contraction in a dose-dependent manner. P2Y agonist adenosine 5'-[β-thio]diphosphate trilithium salt (ADPβS, 10-7~10-5 M) also potentiated EFS-induced contractions in a dose-dependent manner. Ecto-ATPase activator apyrase (5 and 10 U/ml) reduced EFS-induced contractions. Inversely, 6-N,N-diethyl-D-β,γ-dibromomethylene 5'-triphosphate triammonium (ARL 67156, 10-4 M) increased EFS-induced contraction. These data suggest that endogenous ATP plays a role in EFS-induced contractions which are mediated through both P2X-receptors and P2Y-receptors stimulation in cat esophageal smooth muscle.
Keywords: Electrical field stimulation, Smooth muscle, G protein, P2 receptor, ATP, Calcium
INTRODUCTION
Smooth muscle distributed in the gastrointestinal tract is under the control of the autonomic nervous system, and contraction or relaxation of the muscle plays an important physiological role in the motility of digestion. Recent physiological, pharmacological, and histochemical investigations indicate that neurotransmitters other than acetylcholine or noradrenaline are involved in peripheral autonomic neuro-effector transmission, and these neurotransmitters are generally termed non-adrenergic, non-cholinergic (NANC) neurotransmitters [1]. Putative NANC mediators include the peptides calcitonin gene-related peptide (CGRP) and substance P, and non-peptide mediators including nitric oxide (NO) and ATP [2]. We have investigated the components of EFS-induced responses and the intracellular factors that mediate electrically stimulated responses in feline distal esophageal smooth muscles. Muscle contractions were recorded using an isometric force transducer. Low-frequency EFS induced only off contraction but induced on contraction in the presence of NG-nitro-L-arginine methyl ester, suggesting that NO acts as an inhibitory mediator in smooth muscle [3].
Extracellular purines (adenosine, ADP, and ATP) and pyrimidines (UDP and UTP) are important signaling molecules that mediate diverse biological effects via cell-surface receptors termed purine receptors. There are two main families of purine receptors, adenosine or P1 receptors, and P2 receptors, recognizing primarily ATP, ADP, UTP and UDP. Adenosine/P1 receptors have been further subdivided, according to convergent molecular, biochemical, and pharmacological evidence, into four subtypes, A1, A2A, A2B, and A3, all of which couple to G proteins. Based on differences in molecular structure and signal transduction mechanisms, P2 receptors divide naturally into two families of ligand-gated ion channels and G protein-coupled receptors termed P2X and P2Y receptors [4,5]. Recently, seven mammalian P2X receptors (P2X1-7) and seven mammalian P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11 P2Y12, P2Y13) have been cloned, characterized, and accepted as valid members of the P2 receptor family [6].
P2X1 is the main P2X receptor subtype expressed in visceral and vascular smooth muscle [7], whereas P2X2 and P2X3 are the main receptor subtypes expressed in peripheral sensory ganglia [8,9]. Both P2X1 and P2X3 receptors have high affinity for ATP and α,β-meATP, and are rapidly desensitized [9,10]. P2X2, P2X4, and P2X6 receptors are the predominant receptor subtypes expressed in the adult brain where they are present in various heteromeric combinations; these receptor subtypes exhibit lower affinity for ATP, are insensitive to α,β-meATP, and are not readily desensitized [8,11,12]. Insensitivity to α,β-meATP restricts the usefulness of this analog as a radioligand for all but the P2X1 and P2X3 receptor subtypes [10].
P2Y receptors exhibit variable affinity for purine and pyrimidine nucleotides. P2Y1 are purinoceptors and are adenine nucleotide-specific [10,13], whereas P2Y2 receptors have equal affinity for adenosine and uridine nucleotide triphosphates (UTP≥ATP) [14,15]. P2Y4 and P2Y6 are pyrimindinoceptors; P2Y4 is UTP selective whereas P2Y6 is UDP selective [15,16]. The functional status of P2Y5, which has low homology to other P2Y receptors, has not been resolved [16] while the P2Y7 receptor has now been identified as the leukotriene B4 receptor [17]. P2Y receptors are variously coupled to pertussis toxin-sensitive and -insensitive G proteins which activate or inhibit various effectors enzymes including phospholipase C-β (PLC-β), phospholipase D [18,19], phospholipase A2 [20], and adenylyl cyclase [21,22].
Adenosine and ATP receptors have been suggested to play important roles in the modulation of motility in the gastrointestinal tract. Adenosine can directly activate receptors located on smooth muscle [23,24] or act prejunctionally, suppressing the release of excitatory neurotransmitters such as acetylcholine and substance P [25,26]. ATP is a neurotransmitter or co-transmitter in the central and peripheral nervous systems. In 1970, Burnstock and colleagues proposed that ATP was a transmitter involved in NANC nerve-mediated responses of smooth muscle in the gut [1]. Since then, evidence has accumulated in support of the hypothesis that ATP is a NANC transmitter in the enteric nerve system. In most species, ATP activates P2 receptors on smooth muscle to produce relaxation or contraction in the gastrointestinal tract [27].
In vitro studies of human esophageal strips using direct activation of enteric motor neurons by electrical field stimulation (EFS) have shown that lower esophageal smooth muscle relaxation is mainly caused by NO released from inhibitory enteric motor neurons, whereas esophageal contraction is mainly caused by acetylcholine released from excitatory enteric motor neurons acting on muscarinic receptors located in the smooth muscle [3, 28-30]. Except for acetylcholine, other neurotransmitters influence EFS-induced contraction in esophageal smooth muscle. In this study, we examined the role of P2X and P2Y-purinoceptors in EFS-induced contraction using cat esophageal smooth muscle.
METHODS
Animals
Adult cats of either sex weighing between 2.5 and 4 kg were used in this study. The cats were anesthetized with ketamine (50 mg/ml/kg) and killed by bleeding. The chest and abdomen were opened with a mid-line incision exposing the esophagus and stomach. The esophagus and stomach were removed together and pinned onto a wax block in their in vivo dimensions and orientation. The esophagus and stomach were opened along the lesser curvature. The location of the squamo-columnar junction was identified and the mucosa was peeled away. The high-pressure zone is characterized by a visible thickening of the circular muscle layer in correspondence of the squamo-columnar junction, and is immediately proximal to the sling fibers of the stomach. We have previously shown that a 5~8 mm band of tissue coinciding with the thickened area constitutes the LES. Esophageal body was selected from a region extending from 1 to 3 cm above the LES. This constituted the most distal portion of the esophagus and comprised ~15% of the length of the whole esophagus.
Tissue bath studies
Transversely oriented muscle strips measuring 2 mm wide and 7 mm long were taken from esophageal body. Such preparations were then cut into 4~5 minor strips and silk-ligatures were tied at both ends. The muscle strips were mounted in separated 1 ml muscle chambers. One wire was fixed and the other attached to a force transducer (FT03 Grass Instruments Co., Quincy, MA, U.S.A.). Changes in isometric force were recorded on a polygraph (Grass model 79).
The strips were initially stretched to 2 g to bring them to near conditions of optimal force development and were equilibrated for 2 hr while being perfused continuously with oxygenated Krebs. During this time the tension in the muscle strips decreased rapidly and stabilized at less than 0.5 g. The solution was equilibrated with a gas mixture containing 95% O2 and 5% CO2 at pH 7.4 and 37℃.
Electrical field stimulation
The strips were stimulated with pulse trains of 80 V in amplitude and 10 s in duration, with a pulse duration of 1 ms at a frequency of 4 Hz using a stimulator (Model S 88; Grass Instruments) through platinum wire electrodes placed longitudinally on either side of the strips. After a stable resting tone of muscle strips was obtained, frequency-response relationships (4 Hz) were constructed, and the rings were washed three times and allowed to equilibrate for 30 min after EFS to permit the strips to recover completely from the contractile responses to EFS.
Assessment of drug responses
The control responses of EFS on the resting tension of circular smooth muscle were investigated. To blocking adrenergic response, all muscle strips are pretreated with guanethidine (1 µM) before 15 min every experiments. To determine whether P2 purinoceptors were involved in EFS-induced contraction, strips were pretreated with suramine (a nonselective P2 receptor antagonist) for 10 min at each concentration (10-6~10-4 M). To assess the effects of P2X purinoceptor on the contraction of circular smooth muscle, the contraction amplitude was measured in muscle strips which were exposed to α,β-methylene ATP (α,β-meATP, P2X agonist) and 8,8'-[Carbonylbis(imino-3, 1-phenylenecarbonyl-imino)]-bis-(1,3,5-naphthalenetrisulphonate; NF023; a P2X antagonist). In addition, to determine whether P2Y purinoceptors were involved in the response to EFS, strips were pretreated with adenosine 5'-O-(2-thiodiphosphate; ADPβS; a P2Y agonist) and Reactive blue (a P2Y antagonist). To investigate the effect of ecto-ATPase on the EFS-induced contraction, strips were pretreated with apyrase (exogenous ecto-ATPase, 5 and 10 U/ml) for 10 min and 6-N,N-diethyl-D-β,γ-dibromomethylene 5'-triphosphate triammonium (ARL 67156, an ecto-ATPase inhibitor) for 15 min.
Solutions and drugs
Tissues were maintained in Krebs buffer of the following composition [19]: NaCl 116.6 mM, NaHCO3 21.9 mM, NaH2PO4 1.2 mM, KCl 3.4 mM, CaCl2 2.5 mM, glucose 5.4 mM and MgCl2 1.2 Mm. α,β-meATP, ADPβS, NF023, ARL 67156, apyrase, suramine, and guanethidine; all reagents were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A). Reactive blue was purchased from Tocris Cookson Ltd. (Langford, UK). The solutions were prepared on the day of experimentation. Doses of all compounds were given in molar concentrations and are referred to their final concentration in the organ bath.
Data analysis
The amplitude of the contraction induced by electrical field stimulation was expressed as gram (g). Data are expressed as means±S.E.M. of different experiments. Student's t-test for grouped data was used to determine statistical significance with p<0.05.
RESULTS
Effects of P2-receptor antagonist on EFS-induced contraction
To test whether the P2 receptor may mediate EFS-induced contraction, muscle strips were pretreated to suramine (a nonselective P2 receptor antagonist, 10-6~10-4 M) for 10 min before electrical stimulation. Suramine decreased the amplitude of EFS-induced contractions in a concentration-dependent manner. Contraction was inhibited by 45% at 10-4 M (n=5, p<0.01) (Fig. 1).
Fig. 1.
Effect of the P2 receptor antagonist on the response of EFS-induced contraction. Suramine (a P2 receptor antagonist) inhibited EFS-induced contraction in a concentration-dependent manner, suggesting that P2 receptors participate in muscle contraction in response to EFS. Values are expressed as means±S.E.M. *p<0.05, **p<0.01 versus the control.
Effect of P2X antagonist on EFS-induced contraction
To test the role of the P2X receptor in EFS-induced contraction, muscle strips were pretreated with NF 023, a P2X receptors antagonist, for 10 min before electrical stimulation. NF 023 decreased contraction in a concentration-dependent manner. Contraction was inhibited by 35% at 10-4 M (n=5, p<0.01) (Fig. 2).
Fig. 2.
Effect of the P2X receptor antagonist on the response of EFS-induced contraction. NF023 (a P2X receptor antagonist) inhibited EFS-induced contraction in a concentration-dependent manner, suggesting that P2X receptors participate in muscle contraction in response to EFS. Values are expressed as means±S.E.M. **p<0.01 versus the control.
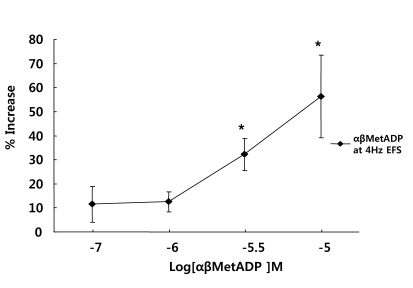
Effects of α,β-meATP on EFS-induced contraction
To test whether the P2X-receptor was involved in EFS-induced muscle contraction, muscle strips were pretreated with α,β-meATP (a P2X agonist of mainly P2X1, 10-7~10-5 M) for 5 min before electrical stimulation. α,β-meATP potentiated EFS-induced contraction in a concentration-dependent manner in the range 10-7~10-5 M. Contraction was increased by 156% at 10-5 M (n=5, p<0.01) (Fig. 3).
Fig. 3.
Effect of the P2X receptor agonist on the response of EFS-induced contraction. α,β-meATP, P2X receptor agonist, potentiated EFS-induced contractions, suggesting that EFS-induced contraction involves P2X receptors, mainly P2X1. Values are expressed as means±S.E.M. *p<0.05 versus the control.
Effects of P2Y antagonist on EFS-induced contraction
To test the role of the P2Y receptor in EFS-induced contraction, muscle strips were pretreated with Reactive blue for 10 min. Reactive blue, a P2Y receptor antagonist, decreased contraction in a concentration-dependent manner. Contraction was inhibited by 57% at 10-4 M (n=4, p<0.01) (Fig. 4).
Fig. 4.
Effect of the P2Y receptor antagonist on the response of EFS-induced contraction. Reactive blue (a P2Y receptor antagonist) inhibited EFS-induced contraction in a concentration-dependent manner, suggesting that P2Y receptors participate in muscle contraction in response to EFS. Values are expressed as means±S.E.M. *p<0.05, **p<0.01 versus the control.
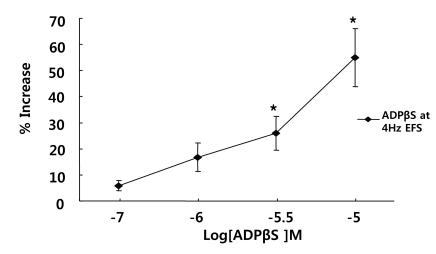
Effects of P2Y receptor agonist on EFS-induced contraction
To test whether the P2Y receptor was involved in the contractions, muscle strips were exposed to ADPβS (10-8~10-5 M) for 5 min before electrical stimulation. ADPβS potentiated EFS-induced contraction in a concentration-dependent manner in the range of 10-8~10-5 M. Contraction was increased by 154% at 10-5 M (n=4, p<0.01) (Fig. 5).
Fig. 5.
Effect of the P2Y receptor agonist on the response of EFS-induced contraction. ADPβS, a P2Y receptor agonist, potentiated EFS-induced contractions, suggesting EFS-induced contraction involves P2Y receptors, mainly P2Y1. Values are expressed as means±S.E.M. *p<0.05 versus the control.
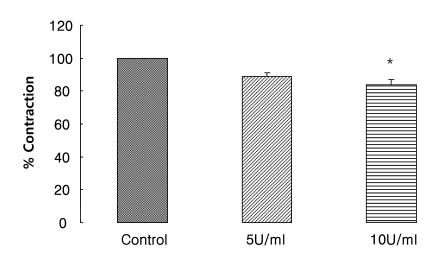
Effects of apyrase on EFS-induced contraction
To investigate the endogenous ATP release from nerve terminals via EFS, muscle strips were pretreated with the ecto-ATPase activator apyrase for 20 min. Apyrase (5 and 10 U/ml) reduced EFS-induced contraction. Contraction was decreased by 17% at 10 U/ml (n=4, p<0.05) (Fig. 6). 1 Unit means 1.0 µmol of inorganic phosphate liberated from ATP or ADP per min at pH 6.5 at 30℃.
Fig. 6.
Effect of apyrase on the response of EFS-induced contraction. Apyrase (an ecto-ATPase activator, n=4) inhibited EFS-induced contractions, suggesting endogenous ATP is released upon EFS. Values are expressed as means±S.E.M. *p<0.05 versus the control.
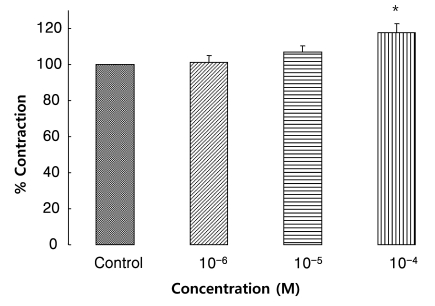
Effects of ARL 67156 on EFS-induced contraction
To confirm endogenous ATP release from nerve terminals via EFS, the ecto-ATPase inhibitor ARL 67156 was used. Muscle strips were pretreated with ARL 67156 (10-6~10-4 M) for 10 min before EFS induction. ARL 67156 increased EFS-induced contraction by 118% at 10-4 M (n=4, p<0.05) (Fig. 7).
Fig. 7.
Effect of ARL67156 on the response of EFS-induced contraction. ARL 67156 (an ecto-ATPase inhibitor, n=4) increased EFS-induced contractions, suggesting endogenous ATP is released upon EFS. Values are expressed as means±S.E.M. *p<0.05 versus the control.
DISCUSSION
In humans and cats, atropine blocks peristalsis in the distal esophagus, indicating a prominent role for muscarinic cholinergic excitation and contraction of esophageal smooth muscle [28]. The activation of muscarinic receptors controls contraction of smooth muscle in part by regulating the concentration of cytosolic free Ca2+ [31]. By EFS, acetylcholine is released and mediated via M2 muscarinic receptors. M2 muscarinic receptors are linked mostly to Gi3 because Ach-induced contraction of permeable cells is inhibited by antibodies against the α-subunit of G13 [32]. In the muscle response to EFS, except acethycholine, purinergic mechanisms are involved in esophageal contraction.
This study shows that cat esophageal smooth muscle involved P2X and P2Y receptors, and endogenous ATP released by EFS or ATP analogue in cat esophagus caused smooth muscle contraction mediated via P2X and P2Y receptors. EFS-induced contraction was frequency-dependent and tetrodotoxin-sensitive. Contractile response induced by EFS was observed in the presence of guanethidine (10-6 M), while EFS-induced contraction was inhibited by the non-selective P2 receptor antagonist suramine. These data suggest that EFS stimulates the release of ATP from nerve terminals, probably sympathetic nerve terminals. This result seems to be supported by the finding that the ecto-ATPase activator apyrase reduced EFS-induced contraction. Furthermore, the ecto-ATPase inhibitor ARL 67156 increased EFS-induced contraction.
There is evidence that mammals have purinoceptors in the gastrointestinal tract. The mouse stomach fundus, duodenum, ileum, and colon have purinoceptors. The predominant effect of stimulation of these receptors is relaxation with the exception of colon longitudinal muscle which also possesses a contractile P2 receptor [33]. In the rabbit, gastric fundus co-expresses P2X and P2Y receptors in dispersed smooth muscle cells. In the rabbit dispersed gastric smooth cell, ATP preferentially activates P2Y receptors to elicit Ca2+ mobilization and muscle contraction [34]. In the guinea-pig, gastric antral circular muscles have P2 receptors which cause contraction via P2Y by ATP and its analogues [35].
Our data suggest that in cat esophageal smooth muscle, the purinergic mechanism involves P2X receptors. α,β-metATP potentiated EFS-induced contraction in a concentration-dependent manner. α,β-metATP was the most useful agonist acting on P2X receptors (specifically at P2X1, P2X3) and generally inactivated P2Y receptors. Also, the P2X antagonist NF 023 inhibited EFS-induced contractions in a concentration-dependent manner. NF 023 is a suramin-based compound which is moderately selective as an antagonist of P2X receptors. NF 023 is about 30-fold selective for P2X1-like receptors in the rat deferens versus P2Y1-like receptors in the guinea-pig taenia coli [36].
ADPβS, a potent P2Y receptor agonist, potentiated EFS-induced contractions in a concentration-dependent manner. Reactive blue, selective P2Y antagonist, inhibited EFS-induced contraction in a concentration-dependent manner. These data suggest that cat esophageal smooth muscle has P2Y receptors and the predominant effect of stimulation is contraction.
Generally, P2X receptors mediate the rapid non-selective passage of cation (Na+, K+, Ca2+) across the cell membrane resulting in an increase in intracellular Ca2+ and depolarization [37]. P2X receptors selectively activated by α,β-metATP mediate Ca2+ influx via dihydropyridine-sensitive, voltage-gated Ca2+ channels; the Ca2+ channels are activated by depolarization of the plasma membrane that result from the opening of ligand-gated cation P2X receptors/channel [12]. P2Y receptors, which promote phospholipase C-catalyzed generation of inositol phosphate and subsequent mobilization of intracellular calcium, are receptors for endogenous ADP and ATP, with ADP being the most potent natural agonist [38].
In summary, these studies suggested that P2 purinoceptors are involved in EFS-induced contraction. Upon EFS, endogenous ATP or ATP-related purine analogues are released which stimulate both P2X receptors and P2Y receptors. However, the subtype of the receptors that mainly mediate contraction is not known, because there are presently few selective agonists and antagonists that clearly discriminate between families of P2X and P2Y receptors, or between subtypes of receptors within each family. In addition, immunohistochemical and biochemical assays are needed to confirm that P2X and P2Y receptors exist.
ACKNOWLEDGEMENTS
This research was supported by Chung-Ang University Research Scholarship Grants in 2009-2010.
ABBREVIATIONS
- EFS
electrical field stimulation
- CGRP
calcitonin gene-related peptide
- αβMeATP
αβ-methylene 5'-adenosine triphosphate
- a ADPβS
denosine 5'-[β-thio]diphosphate trilithium salt
- NANC
non-adrenergic, non-cholinergic
- NO
nitric oxide
References
- 1.Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundberg JM. Pharmacology of cotransmission in the autonomic nervous system: Integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- 3.Park JH, Kim HS, Park SY, Im C, Jeong JH, Kim IK, Sohn UD. The influences of G proteins, Ca, and K channels on electrical field stimulation in cat esophageal smooth muscle. Korean J Physiol Pharmacol. 2009;13:393–400. doi: 10.4196/kjpp.2009.13.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredholm BB, Abbracchio MP, Burnstock G, Daly JW, Harden TK, Jacobson KA, Leff P, Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- 5.Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, Schwabe U, Williams M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. Xxiv. Current status of the nomenclature and properties of P2x receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 7.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (p2x receptors) determined by immunocytochemistry. Proc Natl Acad Sci USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of p2x5 and p2x6 receptors and the distribution and properties of an extended family of atp-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surprenant A, Buell G, North RA. P2x receptors bring new structure to ligand-gated ion channels. Trends Neurosci. 1995;18:224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- 10.Werner P, Seward EP, Buell GN, North RA. Domains of p2x receptors involved in desensitization. Proc Natl Acad Sci USA. 1996;93:15485–15490. doi: 10.1073/pnas.93.26.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2x purinoceptor cdna conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- 12.Buell G, Collo G, Rassendren F. P2x receptors: An emerging channel family. Eur J Neurosci. 1996;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 13.Schachter JB, Li Q, Boyer JL, Nicholas RA, Harden TK. Second messenger cascade specificity and pharmacological selectivity of the human p2y1-purinoceptor. Br J Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an atp receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden K. Uridine nucleotide selectivity of three phospholipase c-activating p2 receptors: Identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- 16.Webb TE, Kaplan MG, Barnard EA. Identification of 6h1 as a p2y purinoceptor: P2y5. Biochem Biophys Res Commun. 1996;219:105–110. doi: 10.1006/bbrc.1996.0189. [DOI] [PubMed] [Google Scholar]
- 17.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A g-protein-coupled receptor for leukotriene b4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 18.Xie MS, Dubyak GR. Guanine-nucleotide- and adenine-nucleotide-dependent regulation of phospholipase d in electropermeabilized hl-60 granulocytes. Biochem J. 1991;278:81–89. doi: 10.1042/bj2780081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zee RY, Michaud SE, Diehl KA, Chasman DI, Emmerich J, Gaussem P, Aiach M, Ridker PM. Purinergic receptor P2y, g-protein coupled, 12 gene variants and risk of incident ischemic stroke, myocardial infarction, and venous thromboembolism. Atherosclerosis. 2008;197:694–699. doi: 10.1016/j.atherosclerosis.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Lazarowski ER, Boucher RC, Harden TK. Calcium-dependent release of arachidonic acid in response to purinergic receptor activation in airway epithelium. Am J Physiol. 1994;266:C406–C415. doi: 10.1152/ajpcell.1994.266.2.C406. [DOI] [PubMed] [Google Scholar]
- 21.Dubyak GR, el-Moatassim C. Signal transduction via p2-purinergic receptors for extracellular atp and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 22.Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: Subtype-associated signaling responses and structure. Annu Rev Pharmacol Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- 23.Nagaoka M, Nara M, Tamada T, Kume H, Oguma T, Kikuchi T, Zaini J, Moriya T, Ichinose M, Tamura G, Hattori T. Regulation of adenosine 5'-triphosphate (atp)-gated p2x(4) receptors on tracheal smooth muscle cells. Respir Physiol Neurobiol. 2009;166:61–67. doi: 10.1016/j.resp.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Li RW, Seto SW, Au AL, Kwan YW, Chan SW, Lee SM, Tse CM, Leung GP. Inhibitory effect of nonsteroidal anti-inflammatory drugs on adenosine transport in vascular smooth muscle cells. Eur J Pharmacol. 2009;612:15–20. doi: 10.1016/j.ejphar.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Kadowaki M, Takeda M, Tokita K, Hanaoka K, Tomoi M. Molecular identification and pharmacological characterization of adenosine receptors in the guinea-pig colon. Br J Pharmacol. 2000;129:871–876. doi: 10.1038/sj.bjp.0703123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moneta NA, McDonald TJ, Cook MA. Endogenous adenosine inhibits evoked substance P release from perifused networks of myenteric ganglia. Am J Physiol. 1997;272:G38–G45. doi: 10.1152/ajpgi.1997.272.1.G38. [DOI] [PubMed] [Google Scholar]
- 27.Burnstock G, Knight GE. Cellular distribution and functions of p2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 28.Preiksaitis HG, Krysiak PS, Chrones T, Rajgopal V, Laurier LG. Pharmacological and molecular characterization of muscarinic receptor subtypes in human esophageal smooth muscle. J Pharmacol Exp Ther. 2000;295:879–888. [PubMed] [Google Scholar]
- 29.Wang J, Krysiak PS, Laurier LG, Sims SM, Preiksaitis HG. Human esophageal smooth muscle cells express muscarinic receptor subtypes m(1) through m(5) Am J Physiol Gastrointest Liver Physiol. 2000;279:G1059–G1069. doi: 10.1152/ajpgi.2000.279.5.G1059. [DOI] [PubMed] [Google Scholar]
- 30.Park SY, Shim JH, Kim M, Sun YH, Kwak HS, Yan X, Choi BC, Im C, Sim SS, Jeong JH, Kim IK, Min YS, Sohn UD. Mlck and PKC involvements via gi and rho a protein in contraction by the electrical field stimulation in feline esophageal smooth muscle. Korean J Physiol Pharmacol. 2010;14:29–35. doi: 10.4196/kjpp.2010.14.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heppner TJ, Werner ME, Nausch B, Vial C, Evans RJ, Nelson MT. Nerve-evoked purinergic signalling suppresses action potentials, Ca2+ flashes and contractility evoked by muscarinic receptor activation in mouse urinary bladder smooth muscle. J Physiol. 2009;587:5275–5288. doi: 10.1113/jphysiol.2009.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohn UD, Harnett KM, De Petris G, Behar J, Biancani P. Distinct muscarinic receptors, G proteins and phospholipases in esophageal and lower esophageal sphincter circular muscle. J Pharmacol Exp Ther. 1993;267:1205–1214. [PubMed] [Google Scholar]
- 33.Giaroni C, Knight GE, Ruan HZ, Glass R, Bardini M, Lecchini S, Frigo G, Burnstock G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002;43:1313–1323. doi: 10.1016/s0028-3908(02)00294-0. [DOI] [PubMed] [Google Scholar]
- 34.Murthy KS, Makhlouf GM. Coexpression of ligand-gated p2x and g protein-coupled p2y receptors in smooth muscle. Preferential activation of p2y receptors coupled to phospholipase c (plc)-beta1 via galphaq/11 and to plc-beta3 via gbetagammai3. J Biol Chem. 1998;273:4695–4704. doi: 10.1074/jbc.273.8.4695. [DOI] [PubMed] [Google Scholar]
- 35.Jin Z, Guo HS, Xu DY, Hong MY, Li XL, Xu WX. Effects of purinergic analogues on spontaneous contraction and electrical activities of gastric antral circular muscle in guinea-pig. Sheng Li Xue Bao. 2004;56:678–684. [PubMed] [Google Scholar]
- 36.Bultmann R, Wittenburg H, Pause B, Kurz G, Nickel P, Starke K. P2-purinoceptor antagonists: Iii. Blockade of P2-purinoceptor subtypes and ecto-nucleotidases by compounds related to suramin. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:498–504. doi: 10.1007/BF00168442. [DOI] [PubMed] [Google Scholar]
- 37.Bean BP. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992;13:87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- 38.Bogdanov Y, Rubino A, Burnstock G. Characterisation of subtypes of the P2x and P2y families of ATP receptors in the foetal human heart. Life Sci. 1998;62:697–703. doi: 10.1016/s0024-3205(97)01168-5. [DOI] [PubMed] [Google Scholar]