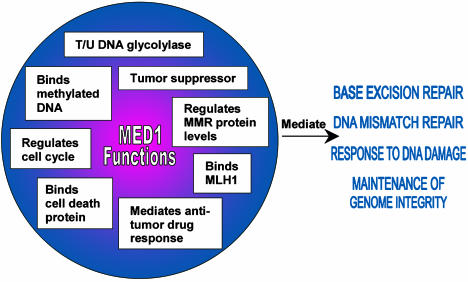
Maintaining genome integrity is a dynamic process that involves many different cellular pathways, including methylation maintenance, homologous recombination, DNA replication, DNA repair, and cell cycle control. A defect in one or more of these pathways can compromise genome integrity and result in cancer. A number of DNA repair proteins have been identified that also impact the regulation of the cell cycle and/or apoptosis (1, 2). Individuals carrying germ-line mutations in such genes, like MLH1 or BRCA1, are predisposed to develop cancer (2). In this issue of PNAS, Cortellino et al. (3) provide evidence that MED1 belongs to this critical group of multifunction proteins. They show that MED1 impacts three processes necessary for the maintenance of genome integrity: base excision repair (BER), DNA mismatch repair (MMR), and the cell cycle response to DNA damage. Their work adds significant information to the growing body of research on the function of MED1 in maintenance of genome integrity (Fig. 1).
Fig. 1.
Summary of known MED1 functions and the cellular processes impacted by MED1 functions.
The multifunctional nature of MED1, also known as MBD4, was apparent from the time of the molecule's discovery. The molecule was identified by using two different screening methods. The human and mouse MBD4 genes were isolated based on DNA sequence homology to the methyl-binding domain of MeCP2, a human 5-methylcytosine (5-meC) binding protein and transcriptional repressor (4). It was shown that the MBD4 gene product could bind 5-meC both in vitro and in vivo. Thus, the first functional description of the molecule was as a mediator of the “biological consequences of the methylation signal” (4). The second identification of MED1 was based on an interaction with MLH1 that was found by using a yeast two-hybrid system (5). Because MLH1 is a MMR protein, this interaction indicated that MED1 might function in MMR. Although subsequent reports refer to either MED1 or MBD4, for convenience only the designation MED1 will be used here.
The MED1 protein has two functional domains. A 5-meC-binding domain in the amino terminus of MED1 directs binding to hemimethylated or fully methylated DNA (5). The carboxyl terminus of the MED1 protein encodes a DNA N-glycosylase activity (6–8). MED1's strongest glycosylase activity is in the removal of a thymine or uracil base from a DNA substrate when either deoxythymidine or deoxyuridine is mispaired with deoxyguanidine (6–8). These specific mispairings occur as a consequence of deamination of 5-meC and cytosine, respectively. The optimal substrates for the MED1 glycosylase are G:T and G:U mispairs in the context of CpG or 5meCpG sites (7). Consequently, the MED1 glycosylase activity appears to function in preserving genome integrity at CpG sites.
Other, albeit weaker, substrates for the MED1 glycosylase have been noted. The MED1 glycosylase can remove 5-meC from 5-meC:G base pairs, with greater activity on hemimethylated than fully methylated DNA (8). The MED1 glycosylase can repair lesions induced by exogenous chemical exposure. Specifically, the MED1 glycosylase can remove 5-fluorouracil (5-FU) from 5-FU:G base pairs (7). MED1 demonstrates a weak glycosylase activity on 3,N4-ethenocytosine DNA adducts when paired with guanine (9). Also, MED1 removes thymidine from O6-methylguanine:T base pairs, a process that can lead to futile cycles of excision and resynthesis after exposure to alkylating agents (3).
Human cells have more than one glycosylase to repair cytosine deamination. Another glycosylase, TDG, has a substrate specificity similar to MED1 (10). However, unlike MED1, TDG does not possess a methyl-binding domain (11). Because the carboxyl-terminal catalytic domain of MED1 has the same substrate specificity as the intact protein, the methyl-binding domain of MED1 may not be necessary for mutation avoidance at CpG sites (9). Thus, the significance of tethering the methyl-binding and glycosylase functions within the MED1 molecule is not understood and remains an important area of investigation.
The isolation of MED1 as an MLH1-binding protein resulted from an attempt to identify a protein that might recognize hemimethylated DNA and provide the strand discrimination signal needed for mammalian MMR (5). Evidence collected to date indicates that MMR operates independently of methylation status (12). Yet, it is clear that MED1 does impact MMR. MED1 and MLH1 physically interact to form an immunoprecipitable complex, with the carboxyl-terminal portion of MED1 being sufficient for this interaction (5). The MED1/MLH1 interaction led Cortellino et al. (3) to investigate MED1 function in MMR by generating mouse embryo fibroblasts in which the MED1 gene is functionally inactive. They discovered that loss of MED1 function causes several MMR proteins to be down-regulated, an effect manifested at the protein level but not the mRNA level. This is a significant result because it shows that the loss of a BER enzyme responsible for mutation avoidance at CpG sites can cause MMR to be compromised. The MED1 protein, therefore, is a functional link between the BER and MMR pathways.
Given the link between MED1 and MMR, evidence was sought for an MED1-mediated effect on microsatellite instability (MSI). MSI was observed when a human cell line was transfected with a MED1 construct possessing glycosylase activity but no methyl-binding domain (5). In contrast, transgenic mice homozygous for a MED1 mutation lacking both the methyl-binding and glycosylase activities did not demonstrate MSI (13). These results prompted the suggestion that the carboxyl-terminal portion of MED1 may direct the inappropriate sequestering of MMR enzymes in the absence of its methyl-binding domain (11).
MED1-null transgenic mice provided additional information on MED1 function. MED1-null mice are viable and fertile and are without apparent increase in tumor susceptibility (13, 14). However, in mice heterozygous for an Apc gene mutation that predisposes them to intestinal tumor development, the MED1-null genotype accelerates tumor development (13, 14). MED1-null transgenic mice also have a higher frequency of C to T mutation at CpG sites than mice carrying a wild-type MED1 allele (13, 14). An increase in this mutational specificity in the absence of MED1 provides additional evidence that MED1 normally functions in the mutational surveillance of CpG sites.
Mismatch repair components and MED1 affect the cell cycle in similar manners.
Although it has been known for some time that MMR components can regulate the cell cycle response to DNA damage (15), Cortellino et al. (3) provide the first evidence that MED1 affects the cell cycle in a similar manner. MMR-proficient cells are sensitive to the cytotoxic effects of DNA-damaging agents because the damage triggers cell cycle arrest and apoptosis. MMR-deficient cells, however, fail to signal cell cycle arrest or apoptosis and, consequently, can survive or “tolerate” the cytotoxic effects of DNA-damaging agents. Cortellino et al. (3) show that MED1-deficient cells treated with DNA-damaging agents fail to undergo apoptosis to the extent observed in MED1-proficient cells. Thus, MED1 mediates the cellular response to DNA damage. Because several common chemotherapeutic agents could not induce apoptosis in cells with a MED1 deficiency, other therapies or approaches will be needed to effectively treat MED1-deficient cancers. Finally, an interaction discovered between MED1 and FADD (Fas-associated death domain protein) may provide the mechanism for the MED1-mediated effect on apoptosis (16).
Together, this information shows that MED1 is a central protein linking the pathways of BER, MMR, and response to DNA damage. Additional studies should provide interesting new insights into the benefits and consequences of linking such important processes to a single protein moiety. Furthermore, MED1 may be used to probe some of the central issues in DNA metabolism that remain unresolved, such as the molecular basis of strand discrimination in mammalian MMR and the role of DNA methylation in this process.
See companion article on page 15071.
References
- 1.Bernstein, C., Bernstein, H., Payne, C. M. & Garewal, H. (2002) Mutat. Res. 511, 145–178. [DOI] [PubMed] [Google Scholar]
- 2.Heinen, C. D., Schmutte, C. & Fishel, R. (2002) Cancer Biol. Ther. 1, 477–485. [DOI] [PubMed] [Google Scholar]
- 3.Cortellino, S., Turner, D., Masciullo, V., Schepis, F., Albino, D., Daniel, R., Skalka, A. M., Meropol, N. J., Alberti, C., Larue, L. & Bellacosa, A. (2003) Proc. Natl. Acad. Sci. USA 100, 15071–15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendrich, B. & Bird, A. (1998) Mol. Cell. Biol. 18, 6538–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellacosa, A., Cicchillitti, L., Schepis, F., Riccio, A., Yeung, A. T., Matsumoto, Y., Golemis, E. A., Genuardi, M. & Neri, G. (1999) Proc. Natl. Acad. Sci. USA 96, 3969–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendrich, B., Hardeland, U., Ng, H. H., Jiricny, J. & Bird, A. (1999) Nature 401, 301–304. [DOI] [PubMed] [Google Scholar]
- 7.Petronzelli, F., Riccio, A., Markham, G. D., Seeholzer, S. H., Stoerker, J., Genuardi, M., Yeung, A. T., Matsumoto, Y. & Bellacosa, A. (2000) J. Biol. Chem. 275, 32422–32429. [DOI] [PubMed] [Google Scholar]
- 8.Zhu, B., Zheng, Y., Angliker, H., Schwarz, S., Thiry, S., Siegmann, M. & Jost, J. P. (2000) Nucleic Acids Res. 28, 4157–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petronzelli, F., Riccio, A., Markham, G. D., Seeholzer, S. H., Genuardi, M., Karbowski, M., Yeung, A. T., Matsumoto, Y. & Bellacosa, A. (2000) J. Cell. Physiol. 185, 473–480. [DOI] [PubMed] [Google Scholar]
- 10.Neddermann, P., Gallinari, P., Lettieri, T., Schmid, D., Truong, O., Hsuan, J. J., Wiebauer, K. & Jiricny, J. (1996) J. Biol. Chem. 271, 12767–12774. [DOI] [PubMed] [Google Scholar]
- 11.Bellacosa, A. (2001) Cell Death Differ. 8, 1076–1092. [DOI] [PubMed] [Google Scholar]
- 12.Drummond, J. T. & Bellacosa, A. (2001) Nucleic Acids Res. 29, 2234–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong, E., Yang, K., Kuraguchi, M., Werling, U., Avdievich, E., Fan, K., Fazzari, M., Jin, B., Brown, A. M., Lipkin, M. & Edelmann, W. (2002) Proc. Natl. Acad. Sci. USA 99, 14937–14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millar, C. B., Guy, J., Sansom, O. J., Selfridge, J., MacDougall, E., Hendrich, B., Keightley, P. D., Bishop, S. M., Clarke, A. R. & Bird, A. (2002) Science 297, 403–405. [DOI] [PubMed] [Google Scholar]
- 15.Dobrovolsky, V. N., McKinzie, P. B., Shaddock, J. G., Mittelstaedt, R. A., Hef lich, R. H. & Parsons, B. L. (2003) Mutagenesis 18, 365–370. [DOI] [PubMed] [Google Scholar]
- 16.Screaton, R. A., Kiessling, S., Sansom, O. J., Millar, C. B., Maddison, K., Bird, A., Clarke, A. R. & Frisch, S. M. (2003) Proc. Natl. Acad. Sci. USA 100, 5211–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]