Abstract
Background
During surgery, ischaemic pre- (IPC) and post-conditioning (IPO) protects the liver against ischaemia/reperfusion injuries (I/R-injuries). The impact of ischaemic conditioning on liver regeneration has been less well studied. Angiogenesis is an important part of liver regeneration after hepatectomy. The aim of the present study was to investigate the effect of ischaemia/reperfusion and ischaemic conditioning on the expression of genes with angiogenic potential in a model of rat liver ischaemia.
Methods
A model of total liver ischaemia (30 min) and reperfusion (30 min) was employed using Wistar rats. Rats were randomized into five groups: (C) control (IRI) ischaemic, IPC, IPO and IPC + IPO. Liver enzymes were sampled at the end of reperfusion. Liver biopsies were analysed using cDNA microarrays.
Results
Alanine aminotransferase (ALT) increased significantly in all the ischaemic groups compared with controls (P = 0.000). Searching databases 99 genes involved in rat liver angiogenesis were identified. Compared with group (C) the number of genes significantly up-regulated was as follows: IRI (n = 5), IPC (n = 24), IPO (n = 33) and IPC + IPO (n = 18). No genes were down-regulated in the four groups compared with controls.
Conclusion
Ischaemic conditioning, as demonstrated in the present study, seems to be potent activators of angiogenic genes. This might be favourable to the regenerating liver.
Keywords: resection < liver, ischaemia re – perfusion < transplant
Introduction
During hepatic resection of malignancies it is of great importance to perform the procedure safely, without transfusion demanding blood loss. Blood transfusion in this setting is known to have a negative impact on both peri- and post-operative morbidity and mortality, and on the long-term oncological outcome.1–3 Vascular clamping is a frequently used method to control blood loss.4 However, this causes varying degrees of ischaemia/reperfusion injuries (I/R-injuries) in the liver tissue, depending on the length of the ischaemic period, the presence of an underlying hepatic disease and the usage of hepato-protective methods such as ischaemic condition.
It has been demonstrated, that ischaemic pre- and post-conditioning (IPC or IPO), defined as brief periods of ischaemia and reperfusion before or after sustained ischaemia, reduces the extent of I/R-injuries in the liver.5–7 This hepatoprotection against I/R-injuries seems to be mediated by a widespread activation of basic cellular mechanisms, resulting in an increased resistance to oxidative stress and inflammation.8
Angiogenesis is an important part of liver regeneration after hepatectomy.9 The effect of I/R and ischaemic conditioning on the expression of genes with angiogenic potential is virtually unknown. The aim of the present study was to profile and compare expression levels of genes, involved in rat liver angiogenesis, subjected to different ischaemic protocols.
Methods
Experimental design
The surgical and experimental protocols were approved by the Danish Research Animal Committee, Copenhagen, Denmark, according to license number 2007/561–1311 and followed the Guide for the Care and Use of Laboratory Animals, published by the National Board of Health.
Forty-eight male Wistar rats, weighing 300–350 g (M & B Taconic, Eiby, Denmark), were used for the experiment. Animals were housed in standard animal laboratories with a constant temperature of 23°C and artificial 12-h light/dark cycles. They had free access to food and water until the start of the experiment. The rats were divided into five groups: (C) control (n = 8) (IRI) ischaemia (n = 10) (IPC) (n = 10) (IPO) (n = 10) and (IPC + IPO) (n = 10). All animals were anaesthetized using 0.75 mL/kg Hypnorm subcutaneously (s.c.) (fentanyl/fluanisone; Jansen Pharma, Birkeroed, Denmark) and 4 mg/kg Midazolam s.c. (dormicum; La Roche, Basel, Switzerland), and then placed on a heating pad. A midline laparotomy was performed, and total hepatic ischaemia was accomplished using a microvascular clip, placed on the hepatoduodenal ligament, i.e. the Pringle manoeuvre. Reflow was initiated after 30 min of ischaemia by removal of the clamp. Discoloration of the liver was used as a positive marker for hepatic ischaemia. Reperfusion was ascertained by the return of the normal reddish colour of the liver.
Rats in the control group were operated on in the same way as the other groups using mobilization of the hepatoduodenale ligament, but without placing a microvascular clip. All other rats were subjected to 30 min of total hepatic ischaemia, followed by 30 min of reperfusion before a biopsy was taken from the liver. In the IPC groups, animals were subjected to 10 min of hepatic ischaemia, followed by 10 min of reperfusion prior to the sustained 30 min of ischaemia. In the IPO group, three cycles, each composed of 30 s of reperfusion and 30 s of ischaemia, were applied immediately after onset of reperfusion. The IPC + IPO groups were subjected to a combination of the two interventions before and after ischaemia (Fig. 1).
Figure 1.
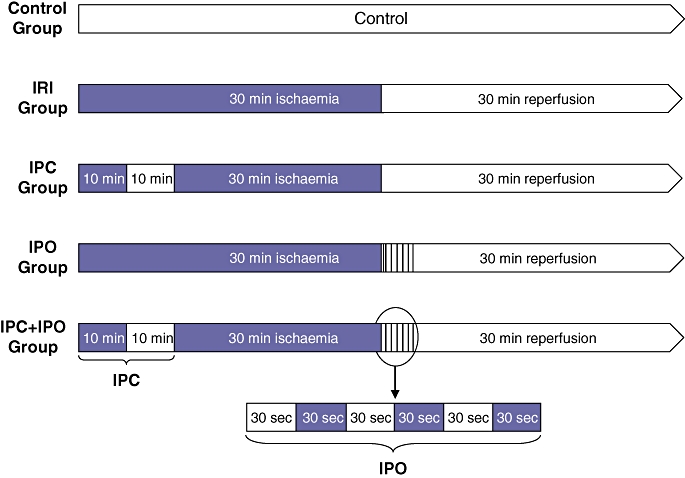
Experimental protocol for the five groups. Black areas represent periods of hepatic ischaemia, white areas represent periods of normal hepatic blood perfusion. Liver biopsies were collected at the end of each experiment. IRI, 30 min of ischaemia. IPC, ischaemic pre-conditioning + 30 min of ischaemia. IPO, 30 min ischaemia + ischaemic post-conditioning. IPC + IPO, ischaemic pre-conditioning + 30 min of ischaemia + ischaemic post-conditioning
At the end of each experiment a biopsy was taken from the right liver lobe. The biopsy was immediately frozen in liquid nitrogen and stored at −80°C for further analysis. From the common iliac artery blood samples were taken for measurement of alanine aminotransferase (ALT), alkaline phosphates and bilirubin. Afterwards all rats were killed with an overdose of pentobarbital.
RNA isolation
Liver samples were thawed and the tissue was homogenized in RLT plus buffer on the Tissuelyser (Qiagen, Hilden, Germany) and total RNA extracted on the QIAsymphony SP (Qiagen) using the QIAsymphony RNA extraction kit.
Microarray analysis
For Gene expression analysis, the following procedures were performed all according to Affymetrix (Santa Clare, CA, USA) standard procedures. Briefly, 150 ng total RNA was used for the target preparation as a starting material with the cDNA Syntesis Kit and WT Terminal Labeling Kit. The target was loaded onto the Affymetrix Rat Exon 1.0 ST array cartridge and hybridized for 16 h. The arrays were washed and stained in the Affymetrix Fluidics Station and scanned using the Affymetrix 7G GeneChip Scanner.
The raw image files from the quantitative scanning were analysed by Affymetrix Gene Expression Analysis Software and data were normalized using the RMA in Affymetrix Expression Console.
Gene expression analysis
Gene expression levels were analysed in the five groups using Affymetrix Rat Exon 1.0 ST array. This standard array, comprising 29.215 probe sets, detects the expression levels of all rat genes. From the probe set we selected all genes known to be involved in angiogenesis in the rat, and compared expression levels of genes between our experimental groups. This selection was based on a search for genes involved in rat angiogenesis in the Ingenuity Pathway database and the data sheet supplied with the microarray. We hereby identified 99 genes, which were the basis of the following data analysis. The 99 genes were divided into three main function areas: inducers, inhibitors and modulators of angiogenesis.
Statistical analyses
Statistical analyses of blood samples were performed by SPSS® 11.0 (SPSS Inc., Chicago, IL, USA). Data analyses were performed by the non-parametric Kruskal–Wallis (anova) test, followed by the Mann–Whitney U-test. A P-value < 0.05 was considered significant.
Significance analysis of microarray data was performed in open source TM4 software Multiexperiment Viewer v4.5,10 using significance analysis for microarrays (SAM), which is a statistical technique especially developed for microarray data analysis. It assigns a score to each gene on the basis of change in gene expression relative to the standard deviation of repeated measurements. For genes with scores greater than an adjustable threshold, SAM uses permutations of the repeated measurements to estimate the percentage of genes identified by chance, as described by Tusher et al.11
As cutoff levels of significance we used the smallest delta value with a False Discovery Rate = 0.
Results
Blood samples
Blood samples showed a significant increase in ALT in the groups IRI, IPC, IPO, and IPC + IPO compared with the control group (P = 0.000). Further, ALT was significantly elevated in the IPC + IPO group compared with the IRI (P = 0.043) and IPC (P = 0.015) groups. No difference was found between the IPO and IPC + IPO groups (Fig. 2). Alkaline phosphate and bilirubin were comparable between the groups.
Figure 2.
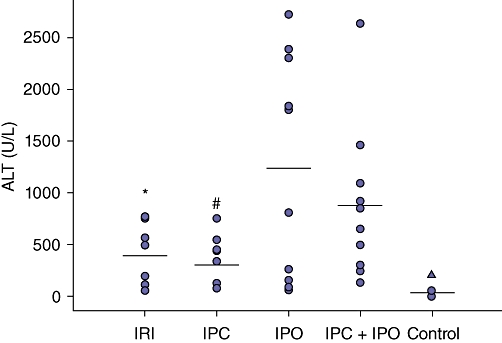
Scatter plot of alanine aminotransferase (ALT) levels in rat serum after 30 min of reperfusion in the five groups. Horizontal lines represents mean of groups. IRI, 30 min of ischaemia (n = 10). IPC, ischaemic pre-conditioning + 30 min of ischaemia (n = 10). IPO, 30 min ischaemia + ischaemic post-conditioning (n = 10). IPC + IPO, ischaemic pre-conditioning + 30 min of ischaemia + ischaemic post-conditioning (n = 10), control (n = 8). *Group IRI compared with IPC + IPO, P = 0.043; †group IPC compared with IPC + IPO, P = 0.015. ▴control group compared with the four intervention groups, P = 0.000
Supervized SAM two class unpaired
The effects of ischaemia and reperfusion and ischaemic conditioning on gene expression levels were compared in a two class unpaired SAM analysis. Here we tested the four intervention groups against the control group. Results are described in Fig. 3. IRI only affects 5 out of 99 genes significantly. A major increase in genes affected was observed in the conditioned groups, with 24 (IPC), 33 (IPO) and 18 (IPC + IPO) genes significantly up-regulated. None of the tested genes were down-regulated after ischaemia or ischaemic conditioning when compared with expression levels in the control group. All affected genes are listed in Table 1.
Figure 3.
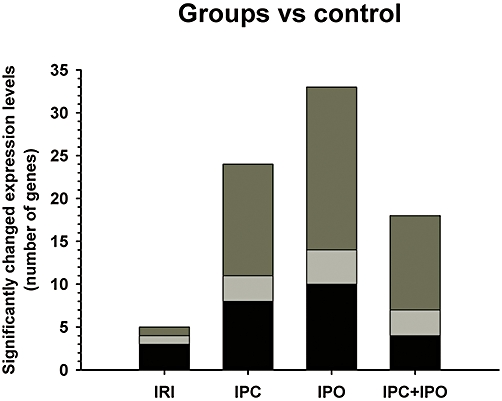
Pair-wise comparison of groups vs. control by unsupervised significance analysis for microarrays (SAM) two class unpaired. Describes the number of genes with significantly up-regulated expression levels in the rat liver. Black areas represent angiogenesis inducing agents, pale grey angiogenesis inhibitors and dark grey angiogenesis modulating agents. All of the 99 genes tested in this comparison analysis are known to be involved in angiogenesis. IRI, 30 min of ischaemia. IPC, ischaemic preconditioning + 30 min of ischaemia. IPO, 30 min ischaemia + ischaemic post-conditioning. IPC + IPO, ischaemic pre-conditioning + 30 min of ischaemia + ischaemic post-conditioning
Table 1.
Pair-wise comparison of groups vs. control by significance analysis for microarrays (SAM) two class unpaired
| Gene symbol | IRI | IPC | IPO | IPC + IPO |
|---|---|---|---|---|
| Angiogenesis inhibitors | ||||
| Il1rn | + | + | + | |
| Rnh1 | + | |||
| Scye1 | + | + | + | |
| Serpinf1 | + | + | + | |
| Wars | + | |||
| Angiogenesis modulating agents | ||||
| Anpep | + | + | + | |
| C1galt1 | + | |||
| Ccnd1 | + | + | + | + |
| Ceacam1 | + | |||
| Cyr61 | + | + | + | |
| Elk3_predicted | + | + | ||
| Enpep | + | + | ||
| Mmp14 | + | + | ||
| Pik3r1 | + | + | ||
| Plau | + | + | ||
| Ppap2b | + | |||
| Pten | + | |||
| Ptprj | + | |||
| Rela | + | + | + | |
| Serpine1 | + | + | + | |
| Smad5 | + | + | ||
| Sphk2 | + | + | + | |
| Tnfrsf12a | + | + | ||
| Tnfrsf1a | + | + | + | |
| Zfp36l1 | + | + | + | |
| Angiogenesis inducers | ||||
| Ang2 | + | + | ||
| Angptl2 | + | |||
| Arts1 | + | |||
| Ccl2 | + | + | + | + |
| Epas1 | + | + | ||
| Flt1 | + | |||
| Lgals3 | + | + | + | |
| Myc | + | + | + | + |
| Nrg1 | + | + | ||
| Pold4 | + | |||
| Tgfb1 | + | |||
| Vegfa | + | |||
| Yars | + | + | ||
+ indicates a significant up regulation of expression level in the rat liver compared with the control group. The table categorise genes into three main functions on angiogenesis. All of the 99 genes tested in this comparison analysis are known to be involved in rat angiogenesis. IRI, 30 min of ischaemia. IPC, ischaemic pre-conditioning + 30 min of ischaemia. IPO, 30 min ischaemia + ischaemic post-conditioning. IPC + IPO, ischaemic pre-conditioning + 30 min of ischaemia + ischaemic post-conditioning.
Supervised SAM multiclass
A multiclass analysis of all five groups revealed 20 genes with significant changes across the five groups. These genes are listed in Table 2. Figure 4 is a hierarchical cluster of rat liver samples based on these 20 significant genes. This cluster dendrogram separates the conditioned groups from the IRI and control group.
Table 2.
Significance analysis for microarrays (SAM) multi class analysis of the five experimental groups
| Gene symbol | IRI | IPC | IPO | IPC + IPO |
|---|---|---|---|---|
| Angiogenesis inhibitors | ||||
| Il1rn | 1.09 | 1.69 | 1.36 | 1.71 |
| Scye1 | 1.02 | 1.54 | 2.10 | 1.68 |
| Serpinf1 | 1.00 | 1.27 | 1.31 | 1.24 |
| Angiogenesis modulating agents | ||||
| Anpep | 0.83 | 1.43 | 1.75 | 1.59 |
| Bgn | 0.73 | 1.12 | 1.23 | 1.18 |
| Ccnd1 | 1.53 | 1.74 | 1.51 | 1.46 |
| Ceacam1 | 0.56 | 0.96 | 1.31 | 1.12 |
| Cyr61 | 1.43 | 2.38 | 2.61 | 2.17 |
| Drd2 | 1.10 | 0.83 | 0.77 | 0.91 |
| Enpep | 0.84 | 1.31 | 1.47 | 1.34 |
| Mmp14 | 0.90 | 1.37 | 1.41 | 1.17 |
| Pten | 0.88 | 1.18 | 1.46 | 1.33 |
| Ptprj | 0.72 | 1.03 | 1.29 | 1.22 |
| Serpine1 | 1.27 | 1.79 | 1.32 | 1.49 |
| Tnfrsf12a | 1.14 | 1.46 | 1.22 | 1.15 |
| Tnfrsf1a | 1.23 | 1.61 | 1.69 | 1.69 |
| Zfp36l1 | 1.02 | 2.08 | 2.02 | 2.02 |
| Angiogenesis inducers | ||||
| Myc | 1.56 | 3.13 | 1.66 | 1.99 |
| Sphk1 | 1.01 | 0.91 | 0.79 | 0.86 |
| Yars | 1.02 | 1.27 | 1.38 | 1.20 |
The table categorizes genes into three main functions on angiogenesis. Numbers describes fold change in gene expression levels compared with the control group. All of the 99 genes tested in this comparison analysis are known to be involved in angiogenesis. IRI, 30 min of ischaemia. IPC, ischaemic pre-conditioning + 30 min of ischaemia. IPO, 30 min ischaemia + ischaemic post-conditioning. IPC + IPO, ischaemic pre-conditioning + 30 min of ischaemia + ischaemic post-conditioning.
Figure 4.
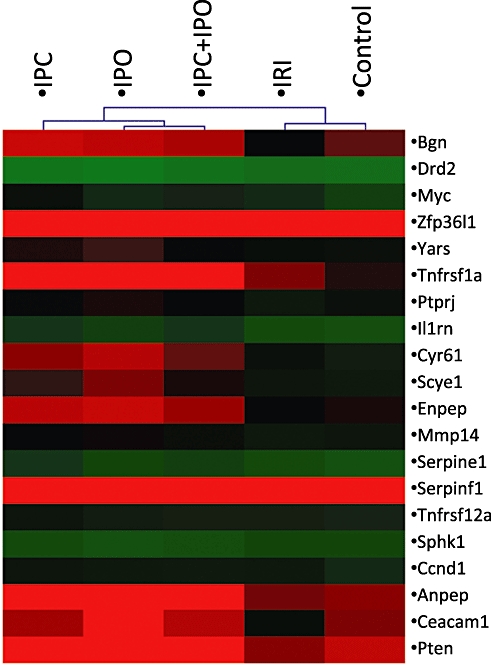
Significance analysis for microarrays (SAM) multiclass analysis identified 20 genes with different expression levels across groups. A hierarchical cluster analysis of these genes is depicted in this figure. Red areas indicate genes with high expression levels and green areas genes with low expression levels. Expression levels are calculated as a mean of groups. The dendrogram on top of the figures describes the association between groups with regard to similarity of expression patterns. IRI, 30 min of ischaemia. IPC, ischaemic pre-conditioning + 30 min of ischaemia. IPO, 30 min ischaemia + ischaemic post-conditioning. IPC + IPO, ischaemic pre-conditioning + 30 min of ischaemia + ischaemic post-conditioning
Discussion
In the present study we tested how I/R and ischaemic conditioning affected the expression levels of genes known to be involved in angiogenesis in the rat liver. We demonstrated that IRI, IPC, IPO and IPC + IPO significantly affected the expression of many angiogenic genes. This has not previously been reported. A comparison analysis with intervention groups and controls showed a pronounced increase in the number of genes involved in angiogenesis in groups subjected to ischaemic conditioning. The number of inhibitors was fairly constant in the different groups, but inducers, and especially modulators, of angiogenesis was affected by ischaemic conditioning. Another interesting observation was that none of the tested genes were down-regulated. A multiclass analysis identified 20 genes with significantly affected expression levels. A subsequent cluster analysis divided these genes (Fig. 4) into two clusters: one containing the control and IRI groups and one containing the conditioned groups. This led us to conclude that IPC, IPO and IPC + IPO affect genes involved in angiogenesis differently than does IRI.
The 99 genes involved in angiogenesis have very different effects on this complicated process, which involves degradation of the basement membrane of the capillaries, migration of endothelia cells and organization into new capillary tubes.12 Angiogenesis is controlled locally by changes in the equilibrium of inducers, inhibitors and modulators. We divided genes into one of these three groups, knowing that some of the genes might fit into more than one group. Vascular endothelia growth factor (VEGF) is one of the most potent and well-characterized inducers of angiogenesis. VEGF expression levels seem to be directly correlated with the angiogenic activity.13 VEGFa expression was increased in all conditioned groups, but only significantly in the IPO group. Another group of inducers are angiopoietins (ANG2, ANGPTL2 and others), which are growth factors with largely specific effect on the vascular endothelium.14 ANG2 expression was increased in the IPO and IPC+IPO group, whereas ANGPTL2 expression was up-regulated in the IPC group. Transforming growths factor beta 1 (TGF-β1) stimulates the extracellular matrix production in the angiogenic process,12 but it also plays an important role in the process of liver regeneration.15 TGF-β1 was up-regulated significantly in the IPC group. Several genes are classified as modulators as they are known to affect angiogenesis, without a clear inducing or inhibitory effect. The matrix metallopeptidases (MMPs) are some of the best characterized. They are involved in the remodelling of extracellular matrix during angiogenesis.12 In the IPC and IPO group the expression of MMP14 gene was up-regulated. Only a few inhibitors of angiogenesis were affected, one of these was IL1RN, which is an interleukin-(IL)1α and 1β antagonist. IL-1α and 1β are stimulators of VEGF production. IL1RN was significantly up-regulated in groups IPC, IPO and IPC+IPO. All genes affected can be seen in Table 1.
Circulating ALT levels increased in the four groups subjected to sustained ischaemia compared with the control group. This indicates that our model did induce hepatocellular damage, as ALT is a reliable marker for hepatocellular injuries.16 In the present study IPC did not, as shown by other authors,5,6 affect ALT levels significantly. This could be explained by the short reperfusion period as hepatocellular damage develops during the first hours of reperfusion. Most other studies using ALT as marker for cellular damage have used longer reperfusion periods.6 We chose 30 min of reperfusion, as the early moments of reperfusion seem to be essential in the pathogenesis of I/R-injuries.17 Another explanation why we could not demonstrate a protective effect of conditioning on ALT levels might be that 30 min of ischaemia resulted in only minor I/R-injuries. Surprisingly, we found higher ALT levels in animals subjected to IPO and IPC+IPO which indicate a higher degree of hepatocellular damage. ALT, however, showed large variations in groups subjected to IPO (see Fig. 2), but the reasons for this are unclear. Despite this, in our model of I/R-injuries, we found a pronounced impact of ischaemic conditioning on genes involved in angiogenesis and many of the genes activated after the different conditioning protocols were identical.
Microarray analysis enables us to simultaneously investigate the parallel expression levels of multiple genes, which is the major strength of this method. A downside of this method is that when we measure RNA copy numbers in the different groups, we cannot be sure that this leads to an increase in protein concentrations, these being the main effecter molecules. Our study focuses on the general expression levels of all genes known to affect angiogenesis. We identified angiogenic gene clusters within the experimental groups. The expression levels of single genes were of minor interest in this study and it would therefore be of little value to validate single genes using other methods suh as, e.g. RT-PCR. In future studies, when identifying key mediators of angiogenic activation after ischaemic conditioning, a RT- PCR validation of findings are of course mandatory.
Transfusion demanding blood loss during liver surgery is known to have a negative impact on both peri- and post-operative morbidity and mortality, and on the long-term oncological outcome.2,3 Vascular clamping is a frequently used method to control blood loss.4 However, this causes varying degrees of ischaemia/reperfusion injuries ranging from a light elevation of liver enzymes to liver failure. Many studies have demonstrated a protective effect of ischaemic pre- or post-conditioning5–7 on I/R-injuries which seems to be mediated by a widespread activation of basic cellular mechanisms.8 Despite efforts to reduce blood loss, around 60–70% of patients treated with R0 liver resections for colorectal metastases will experience a relapse of the disease within 2 years, mainly in the liver.18,19 Intrahepatic recurrence may originate from pre-existing micro-metastases in the liver and these dormant micro-lesions are known to be present in 26–70% of liver specimens from patients with colorectal liver metastases,20,21 and they are highly associated with a negative oncological outcome. For tumour growth beyond 1–2 mm3 angiogenesis is required.12 The shift towards angiogenesis can be triggered by an increased production of angiogenic factors, by the liver or the tumour itself. I/R-injuries are known to stimulate the outgrowth of micro-metastases in rodent models.22,23 In a recently published study by Nijkamp et al. an association between prolonged portal triad clamping and decreased time to liver recurrence was ascertained.24 A strong angiogenic stimulus is suggested to be one of the most likely explanations for this unfortunate side effect of I/R-injuries.
In conclusion ischaemic conditioning, as demonstrated in the present study, seems to be a potent activator of angiogenic genes. In this sense conditioning may prove to be a double-edged sword, i.e. it might be favourable to the regenerating liver, but may stimulate the growth of micrometastases.
Acknowledgments
The work was supported by the Health Research Fund of Central Denmark Region.
Conflicts of interest
None declared.
References
- 1.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, DeMatteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan ST, Ng IO, Poon RT, Lo CM, Liu CL, Wong J. Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. 1999;134:1124–1130. doi: 10.1001/archsurg.134.10.1124. [DOI] [PubMed] [Google Scholar]
- 4.van der Bilt JD, Livestro DP, Borren A, van Hillegersberg R, Borel Rinkes I. European survey on the application of vascular clamping in liver surgery. Dig Surg. 2007;24:423–435. doi: 10.1159/000108325. [DOI] [PubMed] [Google Scholar]
- 5.Clavien PA, Selzner M, Rudiger HA, Graf R, Kadry Z, Rousson V, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–850. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koti RS, Seifalian AM, Davidson BR. Protection of the liver by ischemic preconditioning: a review of mechanisms and clinical applications. Dig Surg. 2003;20:383–396. doi: 10.1159/000072064. [DOI] [PubMed] [Google Scholar]
- 7.de Rougemont O, Lehmann K, Clavien PA. Preconditioning, organ preservation, and postconditioning to prevent ischemia-reperfusion injury to the liver. Liver Transpl. 2009;15:1172–1182. doi: 10.1002/lt.21876. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 9.Drixler TA, Vogten MJ, Ritchie ED, van Vroonhoven TJ, Gebbink MF, Voest EE, et al. Liver regeneration is an angiogenesis- associated phenomenon. Ann Surg. 2002;236:703–711. doi: 10.1097/00000658-200212000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 11.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 14.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 15.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt E, Schmidt FW. Enzyme diagnosis of liver diseases. Clin Biochem. 1993;26:241–251. doi: 10.1016/0009-9120(93)90123-n. [DOI] [PubMed] [Google Scholar]
- 17.Granfeldt A, Lefer DJ, Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovasc Res. 2009;83:234–246. doi: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoyama N, Shirai Y, Ajioka Y, Nagakura S, Suda T, Hatakeyama K. Immunohistochemically detected hepatic micrometastases predict a high risk of intrahepatic recurrence after resection of colorectal carcinoma liver metastases. Cancer. 2002;94:1642–1647. doi: 10.1002/cncr.10422. [DOI] [PubMed] [Google Scholar]
- 21.Linnemann U, Schimanski CC, Gebhardt C, Berger MR. Prognostic value of disseminated colorectal tumor cells in the liver: results of follow-up examinations. Int J Colorectal Dis. 2004;19:380–386. doi: 10.1007/s00384-003-0555-3. [DOI] [PubMed] [Google Scholar]
- 22.Nicoud IB, Jones CM, Pierce JM, Earl TM, Matrisian LM, Chari RS, et al. Warm hepatic ischemia-reperfusion promotes growth of colorectal carcinoma micrometastases in mouse liver via matrix metalloproteinase-9 induction. Cancer Res. 2007;67:2720–2728. doi: 10.1158/0008-5472.CAN-06-3923. [DOI] [PubMed] [Google Scholar]
- 23.van der Bilt JD, Kranenburg O, Nijkamp MW, Smakman N, Veenendaal LM, Te Velde EA, et al. Ischemia/reperfusion accelerates the outgrowth of hepatic micrometastases in a highly standardized murine model. Hepatology. 2005;42:165–175. doi: 10.1002/hep.20739. [DOI] [PubMed] [Google Scholar]
- 24.Nijkamp MW, van der Bilt JD, Snoeren N, Hoogwater FJ, van Houdt WJ, Molenaar IQ, et al. Prolonged portal triad clamping during liver surgery for colorectal liver metastases is associated with decreased time to hepatic tumour recurrence. Eur J Surg Oncol. 2010;36:182–188. doi: 10.1016/j.ejso.2009.10.016. [DOI] [PubMed] [Google Scholar]


