Abstract
Frontotemporal dementia (FTD) is a clinical syndrome characterized by progressive decline in social conduct and a focal pattern of frontal and temporal lobe damage. Its biological basis is still poorly understood but the focality of the brain degeneration provides a powerful model to study the cognitive and anatomical basis of social cognition. Here, we present Dr. A, a patient with a rare hereditary bone disease (hereditary multiple exostoses) and FTD (pathologically characterized as Pick’s disease), who presented with a profound behavioral disturbance characterized by acquired sociopathy. We conducted a detailed genetic, pathological, neuroimaging and cognitive study, including a battery of tests designed to investigate Dr. A’s abilities to understand emotional cues and to infer mental states and intentions to others (theory of mind). Dr. A’s genetic profile suggests the possibility that a mutation causing hereditary multiple exostoses, Ext2, may play a role in the pattern of neurodegeneration in frontotemporal dementia since knockout mice deficient in the Ext gene family member, Ext1, show severe CNS defects including loss of olfactory bulbs and abnormally small cerebral cortex. Dr. A showed significant impairment in emotion comprehension, second order theory of mind, attribution of intentions, and empathy despite preserved general cognitive abilities. Voxel-based morphometry on structural MRI images showed significant atrophy in the medial and right orbital frontal and anterior temporal regions with sparing of dorsolateral frontal cortex. This case demonstrates that social and emotional dysfunction in FTD can dissociate from preserved performance on classic executive functioning tasks. The specific pattern of anatomical damage shown by VBM emphasizes the importance of the network including the superior medial frontal gyrus as well as temporal polar areas, in regulation of social cognition and theory of mind. This case provides new evidence regarding the neural basis of social cognition and suggests a possible genetic link between bone disease and FTD.
INTRODUCTION
The frontal lobes have long been judged to organize important aspects of human behavior. Damage in this region affects not only cognitive functions but also social behavior, personality, personal memories and self-awareness (Eslinger, 1985; Stuss, 1986; Dimitrov, 1999). Frontotemporal dementia (FTD) is a powerful model for studying the anatomical substrate of social dysfunction (Hodges, 2001a; Hodges, 2001b; Neary, 1998) and its attendant decline in social conduct, emotional blunting, and loss of insight (Gregory, 1996b; Miller, 1997). Despite the profound impact these behaviors have on social interactions, some patients show relatively preserved cognitive functioning (Rahman, 1999). In FTD the whole frontal lobe is often affected although the orbitofrontal, anterior cingulate, and insular regions on the right side seem particularly vulnerable (Rosen, 2002). The anatomical substrates of behavioral change in FTD are beginning to be studied: disinhibition and impulsivity seem to relate to orbital frontal damage, while apathy correlates with medial frontal damage. Preservation of executive function can be interpreted as evidence for relative sparing of the dorsolateral frontal convexity (Hodges, 2001a; Hodges, 2001b).
Patients with focal non-neurodegenerative prefrontal damage often show altered emotional and social behavior, such as disinhibition and misinterpretation of social situations, especially when the damage involves the ventromedial (VM) prefrontal cortex (PFC). One particularly well-documented example is patient E.V.R (Eslinger, 1985). Subsequent to removal of an orbitofrontal meningioma, E.V.R. became profoundly socially dysfunctional. He was divorced twice and entered into disastrous business ventures that led to bankruptcy. The term “acquired sociopathy” is used to describe social misconduct produced from orbitofrontal injury. In this context, the DSM-III criteria for sociopathic disorder bore striking resemblance to E.V.R., including “reckless regarding others’ personal safety” and “lack of remorse”. The relationship between orbitofrontal cortex and sociopathy has generated debate regarding the cognitive mechanisms underlying social intelligence.
Within the realm of social cognition, novel researchers have focused specifically on the neural correlates of the human ability to model mental states and agency, the socalled Theory of Mind (ToM). Research into the relationship between meta-cognitive tasks and social intelligence has engendered interest in three distinct brain areas as the loci of mentalizing: 1) the superior temporal sulcus 2) the temporal poles, and 3) the superior medial frontal gyrus. Using several different tasks, functional imaging studies investigating brain regions involved in mentalizing find activations in these focal regions (Goel, 1995; Baron-Cohen, 1999b; Brunet, 2000; Castelli, 2000; McCabe, 2001; Vogeley, 2001; Berthoz, 2002; Gallagher, 2002; Wicker, 2003 Similarly, most, but not all lesion-based studies have confirmed similar deficits in the superior frontal gyrus (Stuss, 2001; Shamay-Tsoory, 2005; Bird, 2004).
Here, we present a patient with a rare hereditary bone disease who presented with a profound behavioral disturbance characterized by acquired sociopathy. Genetic, anatomical, pathological and cognitive investigation of this case provides a unique vantage from which to investigate: a) the possibility that the genetic cause of a rare hereditary bone disease may also play a role in the pattern of neurodegeneration in frontotemporal dementia, b) the brain regions necessary and sufficient for social cognition and perspective taking vis a vis novel mentalizing tasks.
CASE REPORT
A 66-year-old, left-handed, retired physician was referred to our clinic due to a multiple-year history of behavioral changes. Approximately five years earlier, Dr. A began to have problems with social function. At the time Dr. A, a practicing surgeon, began to have trouble running his medical practice. He started to see fewer patients and his medical practice partners began to pressure him to increase his billing. Dr. A was unresponsive to their requests, and underwent a forced retirement in April 2002.
Behavioral change became evident four years prior to presentation and gradually worsened. Once described as “Mr. Fun” by his grandchildren, Dr. A. became progressively more aloof. On one occasion, he abandoned his two young grandchildren (approximately 3 years-old) suggesting that they return home on their own in the dark. When asked about this incident, Dr. A. seemed unconcerned, and stated that they were familiar with the neighborhood and should have been able to get home by themselves. Around the same time period, he began to exhibit insensitivity to others. After one of his daughters arranged an elaborate birthday celebration for him, Dr. A declined to participate without an explanation. His social withdrawal continued and he left his son’s wedding party abruptly and returned to his hotel room to sit quietly in the dark. He became more aggressive and stubborn and pushed his wife when she attempted to redirect him.
In the years preceding presentation, there were several incidents of sexually inappropriate behavior. At a wedding rehearsal dinner, his sexual advances toward three different women caused considerable embarrassment. This inappropriate behavior continued to the time of referral to our clinic. Additionally, Dr. A’s eating changed over the previous four years. His family noted that he began to eat voraciously and his manners deteriorated. He began to eat out of boxes before food was unpacked, using his hands instead of utensils. He subsisted on junk food, ice cream sundaes and pizza.
Shortly following his retirement in 2002, he began to drink heavily finishing multiple glasses of wine in rapid succession. Dr. A was sent to rehab more than once, but each time left against medical advice after a few days. On several occasions, he entered his neighbor’s garage and stole several bottles of expensive liquor. He later denied this indiscretion, but was cited by the police and instructed to stay away from the neighbor’s property. Shortly afterwards he was arrested for returning. During this time, Valium and Vicodin misuse commenced and he started taking up to 30 mg of Valium per day. When admitted to a detoxification and rehab unit, caregivers noted Dr. A. showed lack of insight as well as his difficulty with understanding the consequences of his actions. He was started on sertraline for anxiety/agitation. Dr. A was treated with Aricept by a local doctor despite the absence of evidence for an Alzheimer-type dementia for approximately two months, but this medication was discontinued in 2003 due to lack of improvement.
In December 2003, the patient was evaluated by UCSF Memory and Aging center. In March 2004, he underwent follow-up examination. In August 2004, the patient was seen by orthopedic oncologist for a chondrosarcoma and elected to enroll in comfort care with transition to hospice. In December 2004, the patient died of sepsis. An autopsy was performed.
Neurocognitive Review of Symptoms
At the time of his initial evaluation Dr. A’s family stated that his memory was “excellent” and he did not lose objects such as his wallet or keys. He read well, although he restricted his reading material to model train magazines. He had no problems with fluency, comprehension or naming. His spontaneous conversational repertoire was restricted to certain topics such as model railroads and the careers of his children. He talked to strangers for extended periods of time about these subjects apparently oblivious to the fact that they were uninterested. In the spatial domain, Dr. A had no difficulties and gave excellent directions to his daughter on how to get about in Central California. He traveled between cities by bus and successfully navigated multiple bus lines and transfers. Shortly prior to his evaluation, Dr. A’s family suggested that his decision-making and problem-solving were poor. He bought many model train components, but was unable to put the tracks or the trains together. When he attempted to help with a family move he spent most of his time shuffling boxes around without purpose. When given money he used it to buy alcohol. Alcohol use seemed unrelated to his pain. There was slightly decreased mobility secondary to hip surgery, but he was able to walk well and rode his bike well up to the time that he was institutionalized. There were no tremors or parkinsonian symptoms.
Past Medical History
Past medical history was significant for multiple osteochondromata with multiple surgeries beginning in 1966 with removal of a sarcomatous osteochondroma from the left scapula. His bone tumors required surgery nearly every five years including left shoulder surgery for removal of a left humerus/axilla osteochondroma (2002) and right hip surgery (1988). He used alcohol, benzodiazepines, and opioids. He had well-controlled hypertension and had a remote history of partial thyroidectomy and appendectomy. Medications at the time of the first evaluation included: quetiapine 25 mg b.i.d. for behavior control; lisinopril 20 mg b.i.d.; atenolol 25 mg b.i.d.; hydrochlorothiazide 25 mg q.d.; (all for hypertension) and sertraline 100 mg q.d. for treatment of mood.
Family History
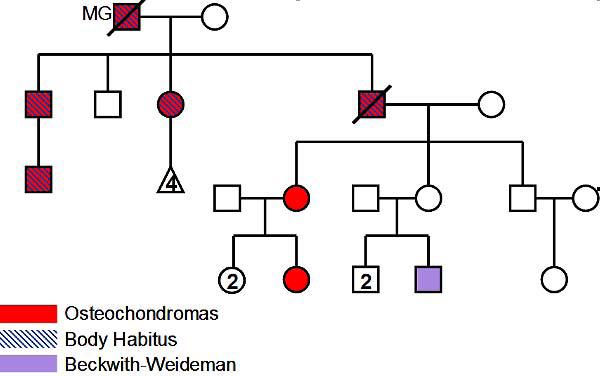
Family history was significant for bone tumors in multiple members of the family (Figure 1). The patient’s father carried a diagnosis of myasthenia gravis and died at the age of 88 of cardiac failure and pneumonia. His mother is alive, independent and well at age 93. He has two younger brothers and one younger sister with no known neurological history, although his younger sister took citalopram following some difficult life events. His two daughters and one son are neurologically well. He has one grandson with Beckwith-Wiedemann syndrome.
Figure 1.
Family history of multiple osteochondromas.
Examination
Basic neurological examination included normal cranial nerves and sensation. Strength examination was somewhat limited due to the patient’s multiple orthopedic issues. Some proximal weakness was noted, particularly of the deltoids and the hip flexors and neck flexor and extensors, which had approximately 4/5 strength. The remainder of his strength exam, including distal strength, was 5 and symmetric, although he had to be encouraged to give full effort. He was unable to supinate his left arm completely secondary to his shoulder problems, but he did not have a pronator drift. His finger and foot taps were rapid, rhythmic, and normal amplitude. Tone was normal in the upper and lower extremities. His reflexes were 2+ at biceps, 2+ at patellae, 2+ at Achilles. His Babinski responses were never tested as he consistently declined to remove his shoes for the examination. His gait was notable for a slightly wide base, with moderate bowing of the legs. He held his left shoulder lower than his right but toe and heel walks were normal. He stumbled with tandem walk. He did not have a Romberg sign, and maintained his stance with retropulsion.
Electromyography study
Nerve conduction studies were normal and EMG studies revealed: 1) an increased incidence of short-duration, low amplitude motor unit action potentials (MUAPs), 2) rapid recruitment of MUAPs in the left extensor digitorum communis, iliopsoas, and biceps brachii muscles, and 2) decrease peak-to-peaks amplitude of the interference pattern of the left biceps brachiii. These abnormal electrodiagnostic studies provide evidence for a proximal myopathy not associated with fibrillation potentials.
Laboratory
Patient had blood drawn in February 2003. The results were unremarkable, with normal CBC, normal Chem 7, normal liver function test, thyroid function test, a CK 72, a CK MB of 184, a Folate of 118.5, B12 of 569, and a negative RPR.
Pathology
Neuropathological examinations were performed with a focus upon FTD-related pathology (Mirra, 1991; Lowe, 1998; Trojanowski, 2001; Munoz, 2003). At autopsy, the brain weighed 1360 grams. Gross inspection revealed asymmetric cerebral cortical atrophy of the frontal lobes – most profoundly atrophic on the ventral aspects of the right side. Additionally, the temporal lobes showed atrophy – more so, anteriorly and superiorly. Hydrocephalus ex vacuo was noted especially at the lateral ventricles. A small 2mm vascular lesion in the basis pontis was observed.
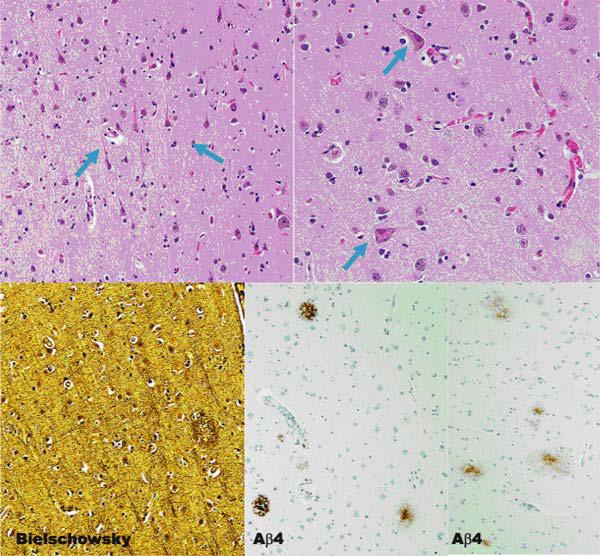
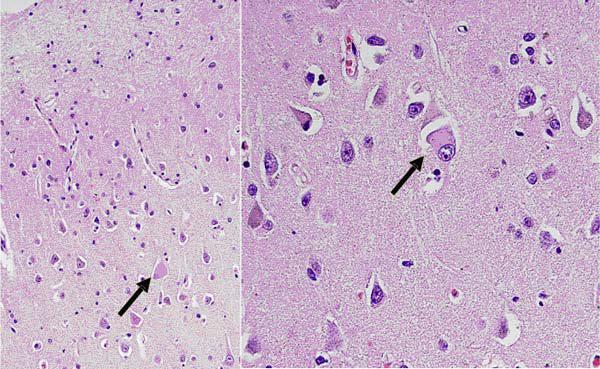
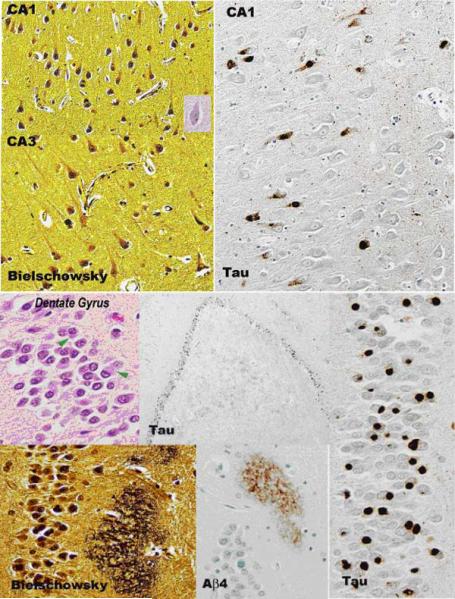
Traditional histopathology staining with hematoxylin and eosin, modified Bielschowsky silver impregnation, and immunostaining with antibodies to β-amyloid revealed multiple foci of neuronal degeneration and loss. Within the frontal lobe, sparse primitive and diffuse neuritic plaques were found. Neurofibrillary tangles were not found in abundance (Figure 2). The cingulate gyrus revealed conspicuous ballooned achromatic neurons and granulovacuolar degeneration (Figure 3). Hippocampal histopathology revealed changes in both the pyramidal layer as well as the dentate gyrus. In the pyramidal layer, silver-impregnated inclusions, Pick’s bodies, were evident. As well, tau immunoreactive inclusions were identified. The dentate gyrus showed numerous Pick body inclusion and neuritic plaques (Figure 4). Both the subiculum and hippocampus revealed immunoreactive astrocytes and “astrocytic plaques”. In addition, mild focal spongiosis was identified in both the frontal and temporal lobes.
Figure 2.
Multiple foci of neuronal degeneration are shown in H & E staining above (shown by blue arrows). Neuritic plaques in the frontal lobe shown below (middle) with rare NF tangles (right).
Figure 3.
Cingulate gyrus. Balloned neurons with granulovacuolar degeneration.
Figure 4.
Hippocampus. Pyramidal layer reveals Pick Body inclusions. Dentate Gyrus demonstrates neuritic plaques.
In summary, neuropathological investigations identified cerebral cortical atrophy in an asymmetric pattern, most convincingly in the frontal and temporal lobes. Numerous neurons elaborated tau protein, especially within the hippocampus and temporal lobe. Additionally, scattered tau positive astrocytes were found cerebral cortex and subcortical white matter. These patterns are most consistent with a diagnosis of Pick’s disease.
In light of the patient’s proximal muscle weakness, histopathology was performed on his biceps and quadriceps muscles. These investigations revealed rare non-specific myopathic changes. A single muscle fiber showed myophagocytosis, but no inflammatory infiltrates are seen. No evidence of an inflammatory or necrotizing myopathy was present.
Finally, dissection of the anterolateral chest wall revealed the previous diagnosed osteochondroma. Histopathology identified the mass as a grade II chondrosarcoma.
Genetics
As shown in the family tree (Figure 1), the patient was a member of family greatly affected by multiple osteochondromas. The possibility of genetic testing for hereditary multiple exostosis, also called multiple osteochondromatosis, was pursued. Individuals with HME often develop benign cartilage-capped tumor (exostoses) at the ends of the long bones or the surface of flat bones (Zak, 2002). HME usually presents early in life with 80% of patients diagnosed before the age of 10. Bony deformity, bowing of the long bones, limited range of motion, and premature osteoartrosis may be associated with HME. A commonly benign condition, its prevalence is estimated at 1 in 50,000. A rare but severe risk in patients with multiple exostoses is the development of malignant chondrosarcoma, which occurs in 1-2% of patients.
There is genetic heterogeneity in HME, and at least two loci are known to be associated with this condition, called Ext1, which maps to 8q24.1, and Ext2, which maps to 11p12-p11. HME is an autosomal dominant trait regardless of the gene involved. Mutations are found in approximately 80% of individuals, with 70% showing mutations in Ext1.
The patient as well as a sister were both tested and were found to be heterozygous for a deletion of four nucleotides in exon 2 of the EXT2 gene (c416_19delACAG). This mutation causes a frameshift and premature protein truncation 129 codons downstream. This mutation has not been reported previously in HME. Although the genetic correlation exists between mutations in EXT1 and EXT2 and HME, the mechanism by which alterations in heparan sulfate synthesis leads to ectopic bone growth is unknown.
COGNITIVE AND BEHAVIORAL ASSESSMENT
The patient underwent standard neuropsychological testing, as well as assessment with a battery of tests designed to objectively measure his emotional, social, and personality functioning (Table 1). Methods and results are presented consecutively for each task.
Neuropsychological testing
Dr. A showed superior functioning in the visuospatial domain while his language functioning was average. Performance in the domains of both verbal and nonverbal memory was complicated by his extremely poor effort on memory tests, but was consistent with normal encoding.
Dr. A’s executive functioning was extensively tested using portions of the Delis-Kaplan Executive Function System (D-KEFS) battery (Delis DC 2004). These included the D-KEFS Trails, a modification of the standard Trailmaking test, and the D-KEFS Color Naming Test, which is a variation of the traditional Stroop Test.
He performed in the average to high average range on tests of information processing speed, set-shifting and sequencing, working memory, and verbal response inhibition. However, on the most taxing of executive tasks requiring a combination of set-shifting and inhibition (D-KEFS Stroop Set-Shifting), impairments were evident. He also showed dissociation in his performance of verbal and nonverbal generation tasks, with impaired verbal fluency but average design fluency.
Behavioral battery
Despite his normal performance in most classic executive tasks, Dr. A’s ability to function was dramatically impaired by his behavioral deficits. Various novel tests were employed to explore the cognitive mechanism underlying his behavioral and social impairment.
Emotion Recognition
The ability to recognize the affective value of voices and facial expressions is fundamental to normal social interaction. Studies have shown that patients with right hemispheric disease consistently find it difficult to identify the emotion conveyed by voice (Starkstein, 1994). Subtests of the Comprehensive Affective Testing System (CATS) were used to assess Dr. A’s capacity to recognize emotional voice prosody (Emotional Prosody), and to name emotions he heard (Name Prosody) in the categories happy, sad, angry, frightened, or neutral. Parts 1 and 2 of The Awareness of Social Inference Test (TASIT) were used to examine the patient’s emotion comprehension across multiple input modalities, including facial affect, voice prosody, and upper body posture and gestures.
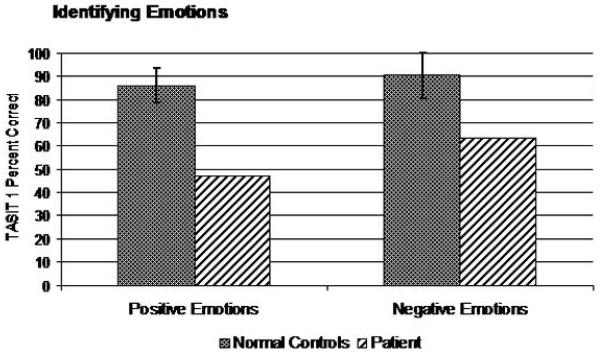
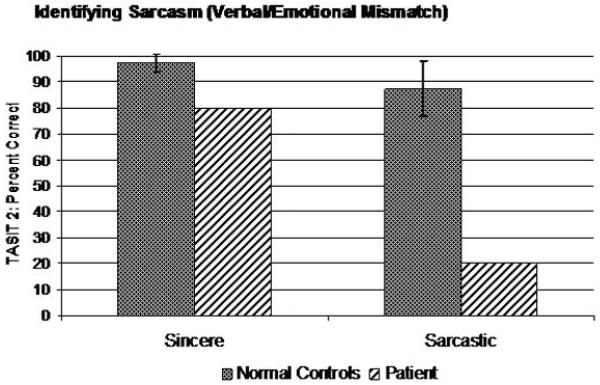
Dr. A did well when asked to discriminate whether phrases with neutral content had the same or different emotional prosody (CATS Emotional Prosody, 22/22), but did poorly when asked to name the emotional prosody he was hearing, even when offered multiple choice options (CATS Name Prosody: 10/22). On Part 1 of the TASIT, Dr. A. had significant impairment identifying both positive and negative emotions (Total Score: 8/14), though he correctly identified happiness and sadness (Figure 5). Similarly, he was entirely unable to recognize a mismatch between a speaker’s statements and their nonverbal emotional expression (i.e., sarcasm) in a similar set of vignettes (TASIT Part 2: Simple Sarcasm: 4/20; Paradoxical Sarcasm: 12/20), though he clearly understood their meaning when they spoke sincerely in a control task (Sincere: 16/20) (Figure 6).
Figure 5.
Performance on emotion recognition task.
Figure 6.
Performance on sarcasm identification task.
Theory of Mind/Perspective Taking Tasks
Theory of mind (ToM) is defined as the cluster of abilities necessary to understand the mental processes of others (Baron-Cohen, 1985; Castelli, 2000). A deficit in theory of mind is thought to be a major contributor to the social impairment shown by children with autism. Less is known about ToM functions in acquired brain disease. Dr. A underwent a series of neuropsychological tests in order to evaluate whether ToM deficits could be the basis of his behavioral disturbance:
1) Visual Perspective-Taking
Visual perceptive taking tasks are ToM tasks in which subjects are asked to identify what cartoon or narrative characters know about a situation based on what they can or cannot see.
The 12-item task described by Gregory (2002) was employed. Dr. A was shown a series of still pictures depicting a vignette in which two individuals hide a ball together. The female character moves the ball when the man has left the room. On half of the items, there was a “cheat” condition in which the man peeked in to see the woman move the ball, but did not tell her he had seen her. For each item, the subject was then asked a control question (“where is the ball?), a first order ToM question (“Where does the man think the ball is?), and a second order ToM question (“Where does the woman think the man thinks the ball is?).
Dr. A performed normally on the control questions, suggesting that the memory and executive demands of the task did not affect his scores, but performed poorly on first order ToM questions (8/12), and at chance level on second order ToM questions (5/12).
2) Complex Social Inferences
Another important aspect of modeling other people’s mental states is to infer their goals and intentions. Dr. A was tested on items from the Awkward Moments Test (Heavey, 2000) in order to assess his ability to infer the implicit intentions of characters.
Dr. A was asked to watch two-minute vignettes showing realistic social interactions. Each of the videos requires second-order ToM reasoning to fully understand the characters’ interactions. After watching each of the 4 videos, he was asked a series of questions to determine the degree to which he understood the interaction.
Though he performed well on control questions designed to assess his ability to follow and recall the basic narrative of the vignettes, Dr. A provided very concrete responses that failed to imagine the character’s thoughts or intentions (Intention Score: 8/24). His responses suggested he typically was able to at least partly comprehend what the main character thought or felt (first order ToM), but did not make second-order ToM inferences about what one character thought another character knew, even with maximal prompting from the examiner.
3) Complex Intentional Biological Movement
In a PET functional imaging study, Castelli et al. (2000) used silent animations wherein two triangles were scripted to portray emotive actions such as intention to deceive in order to study brain activation associated with ToM and intentional movement. Here, we used an adaptation of this paradigm to examine whether a deficit in interpreting biologically relevant movement played a role in Dr. A.’s behavioral impairments.
Dr. A was asked to describe the activities of two triangles moving around a computer screen in four brief animated vignettes (Castelli, 2000), (Mental State items only). When normal individuals watch these videos, they are drawn to anthropomorphize the triangles in such a way that they attribute specific emotions and intentions to them based on their movements in relation to one another. To adjust for the exclusion of the Random Movement and Goal Directed videos from the testing, multiple choice control questions were also administered after the free response condition.
Dr. A’s initial free response descriptions attributed goal-directed activity to the triangles, but made inaccurate inferences about what those goals were. His responses failed to attribute social meaning to the triangles’ movements, and did not ascribe any emotional content to their behavior (Intentionality: 10/20; Appropriateness; 4/12; Length; 11/16). On half of the items, Dr. A failed control questions designed to measure his simple attention and memory for the items. On the other items, was able to correctly select the triangles’ emotions on multiple choice testing, but responded to 1st and 2nd order mentalizing questions incorrectly.
4) Empathy
Empathy connotes a complex form of psychological inference in which the use of observation, shared knowledge, and emotional recognition helps to deduce the thoughts and feelings of others.
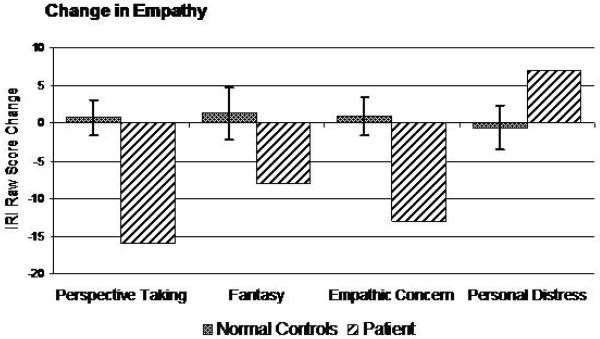
To assess empathic ability in this case, the Interpersonal Reactivity Index (IRI) questionnaire was given to the patient’s wife. The IRI was administered to assess the degree to which his daily behavior demonstrated four aspects of empathy (cognitive perspective taking, cognitive fantasy, emotional concern, and emotional personalized distress). His wife was asked to describe Dr. A’s behavior by filling out the IRI twice, first describing his current behavior, then a retrospective account of his behavior before the onset of his disease. The degree to which his empathy changed was compared to the degree to which empathy changed over a 5-year period in a set of 10 age-matched healthy control subjects
His wife reported that Dr. A was much less likely to spontaneously take other people’s perspective or to project himself into fictional situations such as movies or books. He also showed much less emotional concern for others, while at the same time demonstrating more self-centered emotional reactivity than he had premorbidly. The degree to which he had changed was significantly greater than controls, and his absolute levels of empathy were very low compared to controls (Figure 7).
Figure 7.
Change in empathy.
Summary of Cognitive and Behavioral Testing
In conclusion, Dr. A’s performance on standard neuropsychological testing was consistent with normal, even superior cognition in most domains, with the exception of verbal generation and a demanding task combining verbal response inhibition with set-shifting. Despite his preserved cognitive skills, Dr. A showed significant impairments in emotion recognition, and in various tasks designed to test the ability to infer mental states and intentions to others.
NEUROIMAGING STUDY
A neuroimaging study was performed to investigate the neural correlates of Dr. A’s social impairment in the context of preserved cognitive abilities.
Voxel-based morphometry (VBM, Ashburner and Friston, 2002) was used to demonstrate regional decreases in gray matter volume by compairng the patient’s T1-weighted MRI scan at age 67 to those of 28 male normal controls aged between 57 and 77. Structural MR imaging was accomplished using a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, N.J.), a standard quadrature head coil to obtain T1-weighted (MP-RAGE) images of the entire brain.
Standard VBM technique (Good et al 2001) were applied and implemented in SPM2 (Wellcome Dept. Cogn. Neurol, London; http://www.fil.ion.ucl.ac.uk/spm). Age-matched template and a-priori gray, white, and CSF images were created using the images of 30 age-matched control subjects. Modulated gray matter images were smoothed with a 12 mm FWHM isotropic Gaussian kernel. The intensity of these smoothed images was then proportionally scaled with each subject’s total intracranial volume as a global correction factor. The general linear model was then used to compare the patient’s fully preprocessed gray matter image to those of the controls with each subject’s age entered into the design matrix as a nuisance covariate. A one-tailed t-contrast was used to identify areas of gray matter atrophy in the patient relative to controls, and the final results are presented at p<0.001, uncorrected for multiple comparisons.
Significant gray matter reductions were found in frontal cortex, especially ventrally and medially, as well as insular cortex. Of note, consistent with the patient’s relatively normal executive functions, the dorsolateral prefrontal cortex was comparatively spared (Figure 8).
Figure 8.
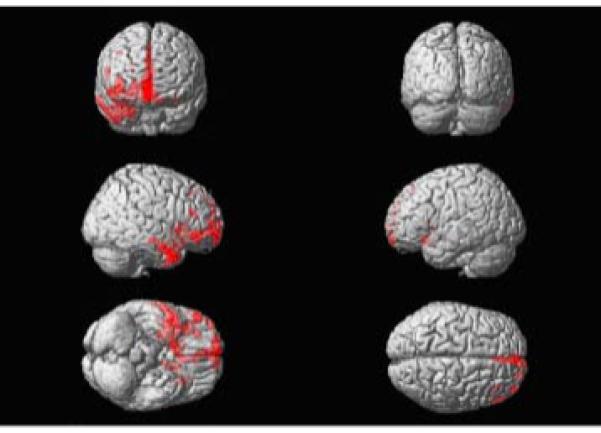
Voxel-based morphometry reveals that the areas of most significant atrophy are in the orbital and medial frontal and right greater than left orbitofrontal regions. The insula was involved as well.
DISCUSSION
We report a patient who presented with an unusual combination of pathology. Dr. A suffered from a rare hereditary bone disease and autopsy-proven FTD/Pick’s disease. The association between bone disease and FTD is thought provoking and suggests a possible role of genes that regulate normal development in FTD pathogenesis. Moreover, Dr. A showed right medial and orbital frontal and anterior temporal atrophy in association with severely defective theory of mind and emotional cognition with preserved executive functioning. These findings provide new insights into the discrete neural basis of social functioning in FTD.
Multiple Hereditary Osteochondromas and the EXT Gene
The patient and his sister were both tested and were found to be heterozygous for a deletion of four nucleotides in the EXT2 gene. This mutation causes a frameshift and premature protein truncation. Clinically, he suffered from multiple osteochondromas associated with this mutation.
Some authors have noted that presymptomatic tau mutation carriers in one large FTD pedigree showed focal frontal deficits decades prior to the expected age of onset for dementia (Geschwind, 2001). This finding suggests a potential developmental component related to mutant tau that has altered frontal lobe functioning in early life. Here, we suggest the possibility that Ext1 and Ext2 may play a role in the pattern of degeneration in FTD.
Secreted signaling molecules of the Wnt/Wingless (Wg), Hedgehog (Hh) and transforming growth factor ß (TGF-ß)/Decapentaplegic (Dpp) function as organizers to control growth and pattern formation of tissues during animal development. In the past few years, genetic studies in Drosophila have uncovered the crucial roles of heparan sulfate proteoglycans (HSPG) in signaling events controlled by secreted Wg, Hh and Dpp morphogens (Lander, 2000; Lin 2000; Nybakken 2002). The biochemistry of HSPG polymer formation has also been elucidated. HSPG are catalyzed by members of the hereditary multiple exostoses (EXT) gene family (Esko, 2002; Zak, 2002). In vertebrates, the EXT gene family consists of EXT1, EXT2, EXT3 on chormosomes 8, 11, and 8 respectively (Zak, 2002). Human mutations in EXT1 and EXT2 are associated with the disease hereditary multiple exostoses (HME), a syndrome of benign bone tumors characterized by multiple cartilage-capped outgrowths of various bones (Ahn, 1995; Stickens, 1996). The Drosophila genome contains three EXT family members as well. Previous studies have shown that Tout-velu (Ttv) and Sister of Tout-velu (Sotv), the Drosophila homologues of mammalian EXT1 and EXT2, respectively, are required for Hh signalling (Bellaiche, 1998; The, 1999; Han, 2004). In the anteroposterior axis, Hh functions to organize the patterning of the wing. Hh protein is exclusively expressed in the posterior compartment and moves into a stripe of anterior cells adjacent to the anteroposterior border to induce expression of its target genes (Basler, 1994; Capdevila, 1994; Posakony, 1990; Tabata, 1994).
Of note, HSPGs are widely expressed in the embryonic brain, particularly in developing axon tracts, where the major cell surface carriers of HS are the transmembrane syndecans and the GPI-anchored glypicans (Yamaguchi, 2001). Recently, HS has been removed entirely from the developing brain (Inatani 2003). In this context, Inatani et. al. generated a conditional knockout of the mouse Ext1 gene, which encodes the major HS glycosyltransferase, and crossed these mice to nestin-Cre mice to obtain CNS-specific disruption of Ext1 (Inatani, 2003). These mice, referred to as Nes-Ext1 mice, die on the first day of life and biochemical, in situ, and immunocytochemical analyses confirm that the entire CNS lacks HS. These Nes-Ext1 mice have several severe CNS defects, the most conspicuous being the loss of olfactory bulbs, an abnormally small cerebral cortex, malformation of the caudal midbrain-cerebellum region (loss of inferior colliculus and cerebellum), and an absence of commissural tracts.
Of particular interest is an emerging association between rare hereditary bone disease and FTD. In this context, inclusion body myopathy associated with Paget’s disease and frontotemporal dementia (IBMPFD), a rare and lethal autosomal dominant disorder, is caused by a mutant valosin-containing protein (Watts et. al). Furthermore, Nasu-Hakola disease, also known as PLOSL (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy) is marked by a combination of progressive presenile dementia associated with sclerosing leukoencephalopathy, and systemic bone cysts (Paloneva, 2001.). The disease is characterized by genetic heterogeneity: mutations in two genes (TYROBP and TREM2) encoding different subunits of a membrane receptor complex in natural killer and myeloid cells have been associated with the disease.
To our knowledge, no association between mutations in Ext genes and dementia has been reported in the literature. Further studies are needed to evaluate whether HME families have increased prevalence of FTD and whether neural HSPGs participate in the atrophic changes found in frontotemporal dementia.
Social Cognition and Perspective-Taking
Dr. A presented with a clear pattern of personality change, notably in the realm of social interaction. Cognitive testing confirmed deficits in interpersonal conduct, finding impairment in the identification of emotions, nonverbal cues, empathy, and ToM tasks. On the other hand, classically described frontal executive skills were preserved. VBM results on Dr. A’s MRI scan showed atrophy in the right medial and orbital frontal and temporal polar cortices (Figure 8) with sparing of dorsolateral prefrontal regions. This case demonstrates the focality of the cognitive and anatomical involvement in FTD. Dr.A’s pattern of anatomical deficits confirms the idea that a network of medial frontal and temporal polar regions is necessary for normal social functioning.
Recently, the so-called metacognitive faculties - awareness of one’s own mental states, and the interplay between these states and the mental states of others - have generated burgeoning scientific interest and investigation (Frith, 2003; Blakemore, 2004; Stuss, 2001). ToM denotes the ability to make inferences regarding other individuals’ mental states in order to understand and predict their behavior. Deficits in ToM are characteristic of autistic children, thus raising the possibility that ToM has a specific brain basis (Baron-Cohen, 1985), which more recent studies have directly investigated (Frith, 1999). However, the neural basis of ToM is still debated.
Functional neuroimaging studies suggest that a distributed neural system is involved in ToM tasks (Ruby, 2004; Berthoz, 2002; Calder, 2002; Gallagher, 2002; McCabe, 2001; Ruby, 2001; Vogeley, 2001; Brunet, 2000; Castelli, 2000; Gallagher, 2000; Baron-Cohen, 1999; Fletcher, 1995; Goel, 1995). Despite the fact that many different tasks have been used, congruent activations have been found in superior frontal gyrus, temporal poles, and STS. The distinct contribution of each of these regions is still unclear. Many but not all studies have shown activations in all three areas of the ToM system (Frith, 2003). Important exceptions (Ruby, 2001, McCabe, 2001, Gallagher, 2002) were associated with tasks lacking semantic and emotional processing that do not elicit temporal lobe activations. In these instances there was no temporal lobe activation suggesting that the temporal poles may contribute to the semantic and emotional aspects of social cognition and perspective-taking.
The few neuropsycholological studies that have investigated ToM in acquired brain lesions have pointed to the ventromedial and orbital frontal lobes as the anatomical basis for ToM (Stone, 1998; Stuss, 2001 and Shamay-Tsoory, 2005). Single case studies have challenged the role of the medial frontal lobe region in ToM by showing preservation of the ability to perform ToM tasks in patients with focal medial and orbital frontal damage. Bird et al.’s patient presented with a rare bilateral infarction of medical frontal cortex and showed no deficits on tasks probing ToM. The authors of this study used tasks that included stories, picture sequences, faux pas, and animations. These tasks may tax temporal polar sub-networks involved in interpretation of narrative, social convention, and emotion. No strict 1st/2nd order visual perspective-taking tasks were described, leaving the possibility that such tasks do necessitate medial frontal structures. In addition, the lesion area in Bird et al.’s case extended only over the internal medial surface of the frontal lobes, but did not include all the dorsomedial areas external to the medial surface. Because there is no consensus yet about the precise neuroanatomy for ToM, this case might actually argue for a more significant contribution from dorsal areas than medial areas. Blair and Cipolotti reported another patient who presented with acquired sociopathy following trauma to the right frontal region, including orbitofrontal cortex (Blair, 2000). Interestingly, J.S. also demonstrated preserved ToM on a metacognitive task, but showed impairment on most other social cognition tasks (attribution of emotion to others, inability to judge inappropriate behaviors). However, the study lacks anatomical description above the level of computed tomography.
In this context, Dr. A underwent a complex of battery of tested that required so-called pure cognitive theory of mind such as 1st and 2nd order tasks and more emotional tasks - sarcasm recognition, intentional movement interpretation, and empathy - in which a consensus regarding their status as ToM tasks has not been reached.
Dr. A was a successful physician and was defined by his family as “Mr. Fun” but, as a result of his disease, he lost his ability to understand sarcasm. Sarcasm requires that one recognize a disparity between verbal content and non-verbal signals, and requires the listener to attend to the nonverbal cues and reject the content of the utterance. These cues can consist of emotional expressions inconsistent with the verbal content, but may also derive from atypically exaggerated nonverbal signals such as range and intensity of facial, voice, and gestural prosody. Consistent with these findings, Dr. A’s deficit in sarcasm recognition implicates right medial superior frontal gyrus and temporal regions including frontal and temporal poles in non-verbal cue processing.
The interpretation of intention also includes processes by which communication occurs via body posture, position, or gesticulation. These computations involve distinguishing intentional movement from random or non-communicative action, and subsequently correctly interpreting the intent as such. This kind of computation has been thought to involve ToM. In a PET functional imaging study, Castelli et al. (2000) used silent animations wherein two triangles were scripted to portray emotive actions such as intention to deceive in order to study brain activation associated with ToM and intentional movement. Castelli et al. showed preferential activation in multiple areas including temporoparietal juction, basal temporal and medial prefrontal areas. Likewise, Dr. A showed little ability to attribute social interaction or belief states to the triangles based on their movements, either spontaneously or after a second viewing of the videos followed by structured questions designed to elicit first- and second-order belief about belief.
Yet another type of social inference is empathy. It connotes a complex form of psychological inference in which the use of observation, shared knowledge, and emotional recognition helps to deduce the thoughts and feelings of others. Two major hypotheses have been offered explain empathic processing: 1) according to simulation theory, empathy may be based on the interplay between witnessing an affective response in another person and projecting one’s own attitudes onto that person’s emotional state (Gallese, 1999; Wicker, 2003) AND 2) “theory” theorists have emphasized the cognitive capacity to take the perspective of the other person (ToM) while keeping self and other differentiated (Adolphs, 1999). In the psychology and behavioral neurology literature, there has not yet been a consensus about how to define the construct of empathy. Depending upon the definition, empathy may or may not require an emotional response.
To assess Dr. A’s empathic ability, the Interpersonal Reactivity Index (IRI) questionnaire was given to the patient’s wife. She was asked to describe his degree of empathy within many domains (perspective taking and fantasy, both considered to be “cognitive” elements of empathy; empathic concern, and personal distress, both considered to be “emotional” elements). Dr. A’s wife suggested that he was much less likely to spontaneously take other people’s perspective or to project himself into fictional situations such as movies or books, and he showed much less emotional responsiveness or “empathic concern” than he had premorbidly. Dr. A’s significant loss of both emotional and cognitive elements of empathy is consistent with the damage to his right dorsomedial frontal, right orbitofrontal, and right temporal polar areas.
In summary, our case, and previous neuropsychological and functional neuroimaging studies suggest that a discrete network of medial superior frontal gyrus and temporal polar brain areas are involved in ToM and related social functions. More studies are necessary to elucidate whether different regions within this network specialize for specific aspect of social cognition. We hypothesize that the anterior temporal lobes are more involved in emotional aspects of social behavior, while the right medial and orbital frontal regions are involved in inferential tasks, such as 1st/2nd order visual perspective-taking. Dr. A. has damage to both anterior temporal and frontal regions and is therefore impaired in all ToM-related tasks.
Conclusion
The case serves to emphasize the complex biology of behavioral disturbance - that the business of social life not only requires neuronal execution of sophisticated programs for empathy, emotional recognition, and theory of mind, but that these processes may only flourish and degenerate in particular neurodevelopmental milieus.
References
- Abell F, H. F, Frith U. Do triangles play tricks? Attribution of mental states to animated shapes in normal and abnormal development. J. Cogn. Dev. 2000;15:1–20. [Google Scholar]
- Adolphs R. Social cognition and the human brain. Trends in Cognitive Sciences. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:291–299. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Ahn J, L. H, Lindow S, Horton WA, Lee B, Wagner MJ, Horsthemke B, Wells DE. Cloning of the putative tumour suppressor gene for hereditary multiple exostoses (EXT1) Nat. Genet. 1995;11:137–143. doi: 10.1038/ng1095-137. [DOI] [PubMed] [Google Scholar]
- Ashburner J, F. K. Voxel-based morphometry - the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, L. A, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, R. H, Wheelwright S, Bulmore ET, Brammer MJ, Simmons A. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999b;11:1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Basler K, S. G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- Bechara A, D. A, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bellaiche Y, T. I, Perrimon N. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature. 1998;394:85–88. doi: 10.1038/27932. [DOI] [PubMed] [Google Scholar]
- Berthoz S, A. J, Blair RJ, Dolan RJ. An fMRI study of intentional and unintentional (embarassing) violations of social norms. Brain. 2002;125:1696–708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Bird CM, C. F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on ‘Theory of Mind’ and cognition. Brain. 2004;127:914–928. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- Blair RJR, C. L. Impaired social response reversal: a case of ‘acquired sociopathy’. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, W. J, Frith U. Social cognitive neuroscience: where are we heading? Trends Cogn Sci. 2004;8:216–22. doi: 10.1016/j.tics.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Blonder LX, B. S, Heilman KM. The role of the right hemisphere in emotional communication. Brain. 1991;114:1115–27. doi: 10.1093/brain/114.3.1115. [DOI] [PubMed] [Google Scholar]
- Bottini G, C. R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Prackowiak RS, Frith CD. The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain. 1994;117:1241–53. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Brothers . Neurophysiology of the perception of intentions by primates. In: Gassiniga MS, editor. The cognitive neurosciences. MIT Press; Cambridge (MA): 1995. pp. 1107–15. [Google Scholar]
- Brothers . Friday’s footprint: how society shapes the human mind. Oxford University Press; New York: 1997. [Google Scholar]
- Brunet E, S. Y, Hardy-Bayle MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11:157–66. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, D. G, Hofbauer RK, Ha B, Chen J, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc. Natl. Acad. Sci. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J, G. I. Targeted expression of the signalling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 1994;13:4459–4468. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, H. F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Cummings J. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–6. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- Damasio AR, T. D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to repond autonomically to social stimuli. Behav Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Damasio H, G. T, Frank R, Galaburda AM, Damasio AR. The return of Pheneas Gage: clues abou the brain from the skull of a famous patient. Science. 1994;264:1102–5. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Delis DC, K. J, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10:301–3. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Dimberg U, T. M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychological Science. 2000;11:86–89. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- Dimitrov M, P. M, Zahn TP, Grafman J. A thoroughly moden Gage. Neurocase. 1999;5:345–54. [Google Scholar]
- Esko JD, S. S. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, D. A. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–41. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Frith CD, F. U. Interacting Minds - a biological basis. Science. 1999;286:1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, F. C. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, H. F, Brunswick N, Flether PC, Frith U, Frith CD. Reading the mind in cartoons and stories: An fMRI study of Theory of Mind in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, J. A, Roepstorff A, Frith CD. Imaging the intentional stance in a competitive game. Neuroimage. 2002;16:814–21. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- Gallese V, G. A. Mirror neurons and the simulation theory of mind-reading. Rends Cogn Sci. 1999;2:493–500. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, R. J, Alarcon M, et al. Dementia and neurodevelopmental predisposition: cognitive dysfunction in presymptomatic subjects precedes dementia by decades in frontotemporal dementia. Ann Neurol. 2001;50:741–746. doi: 10.1002/ana.10024. [DOI] [PubMed] [Google Scholar]
- Goel V, G. J, Sadato N, Hallett M. Modeling other minds. Neuroreport. 1995;6:1741–6. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Good CD, J. I, Ashburner J, Henson RN, Friston KJ, Frackowiak R. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, D. N, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol. 2004:55. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C, L. S, Stone V, Erzinclioglu S, Martin L, Baron-Cohen S, Hodges JR. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain. 2002;125:752–764. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Gregory CA, H. J. Frontotemporal dementia: use of consensus criteria and prevalence of psychiatric features. Neuropsychiatry Neuropsychol. Behav. Neurol. 1996b;9:145–53. [Google Scholar]
- Grice HP. Further notes on logic and conversation. In: Cole P, editor. Radical Pragmatics. Academic Press; New York: 1978. [Google Scholar]
- Han C, B. T, Khodoun M, Tauchi M, Lin X, Lin X. Distinct and collaborative roles of Drosophila EXT family proteins in morphogen signalling and gradient formation. Development. 2004;131:1563–75. doi: 10.1242/dev.01051. [DOI] [PubMed] [Google Scholar]
- Heavey L, P. W, Baron-Cohen S, Rutter M. The Awkward Moments Test: a naturalistic measure of social understanding in autism. J Autism Dev Disord. 2000;30:225–36. doi: 10.1023/a:1005544518785. [DOI] [PubMed] [Google Scholar]
- Heider F, S. M. An experimental study of apparent behavior. Am. J. Psychol. 1944;57:243–259. [Google Scholar]
- Hodges C. A. G. a. J. R. Dementia of frontal type: use of consensus criteria and prevalence of psychiatric features. Neuropsychiatry Neuropsychol. Behav. Neurol. 1996;9:145–153. [Google Scholar]
- Hodges Jr, M. B. The neuropsychology of frontal variant frontotemporal dementia and semantic dementia. Introduction to the special topic papers: part II. Neurocase. 2001b;7:113–21. doi: 10.1093/neucas/7.2.113. [DOI] [PubMed] [Google Scholar]
- Hodges JR, M. B. The neuropsychology of frontal variant frontotemporal dementia and semantic dementia, introduction to the special topic papers: Part II. Neurocase. 2001;7:113–121. doi: 10.1093/neucas/7.2.113. [DOI] [PubMed] [Google Scholar]
- Hodges JR, M. B. The classification, genetics, and neuropathology of frontotemporal dementia (FTD). Introduction to the special topic papers: part I. Neurocase. 2001a;7:31–5. doi: 10.1093/neucas/7.1.31. [DOI] [PubMed] [Google Scholar]
- Hodges JR, P. K, Oxbury S, Funnell E. Semantic dementia—Progressive fluent aphasia with temporal lobe atrophy. Brain Pathol. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hodges JR, P. K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J Int Neuropsychol Soc. 1996;2:511–524. doi: 10.1017/s1355617700001685. [DOI] [PubMed] [Google Scholar]
- Holt CE, D. B. Sugar codes for axons? Neuron. 2005 Apr 21;46(2):169–72. doi: 10.1016/j.neuron.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M, I. F, Plump AS, Tessier-Lavigne M, Yamaguchi Y. Mammalian brain morphogenesis and midline axon guidance require heparan sulfate. Science. 2003;302:1044–1046. doi: 10.1126/science.1090497. (2003) [DOI] [PubMed] [Google Scholar]
- Jorgenson J, M. G, Sperber D. Test of the mention theory of irony. Journal of Experimental Psychology: General. 1984;113:112–120. [Google Scholar]
- Keane J, C. A, Hodges JR, Young AW. Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia. 2002;40:655–665. doi: 10.1016/s0028-3932(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Kramer JH, J. J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cog Behav Neurol. 2003;16:211–8. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Kruez RJ, G. S. How to be sarcastic: The echoic reminder theory of verbal irony. Journal of Experimental Psychology: General. 1989;118:374–386. [Google Scholar]
- Lalande S, B. C, Charlebois N, Whitaker HA. Effects of right and left hemispheric cerebrovascular lesions on discrimination of prosodic and semantic aspects of affect in sentences. Brain Lang. 1992;42:165–86. doi: 10.1016/0093-934x(92)90123-v. [DOI] [PubMed] [Google Scholar]
- Lander AD, S. S. The elusive functions of proteoglycans: in vivo veritas. J. Cell Biol. 2000;148:227–232. doi: 10.1083/jcb.148.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A. a. S., G. Morphogens, compartments, and pattern: lessons from Drosophila? Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Lin X, P. N. Role of heparan sulfate proteoglycans in cell-cell signalling in Drosophila. Matrix Biol. 2000;19:303–307. doi: 10.1016/s0945-053x(00)00073-1. [DOI] [PubMed] [Google Scholar]
- Lowe J. Establishing a pathological diagnosis in degenerative dementias. Brain Pathol. 1998;8:403–406. doi: 10.1111/j.1750-3639.1998.tb00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe K, H. D, Ryan L, Smith V, Trouard T. A functional imaging study of cooperation in two-person reciprocal exchange. Proc. Natl. Acad. Sci. 2001;98:11832–5. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald Exploring the process of inference generation in sarcasm: a review of normal and clinical studies. Brain and Language. 1999;68:486–506. doi: 10.1006/brln.1999.2124. [DOI] [PubMed] [Google Scholar]
- Miller BL, C. L, Mena I, Boone K, Lesser IM. Progressive right frontotemporal degeneration: clinical, neuropsychological and SPECT characteristics. Dementia. 1993;3:204–213. doi: 10.1159/000107324. [DOI] [PubMed] [Google Scholar]
- Miller BL, D. A, Benson DF, Cummings JL, Miller MH. Aggressive, socially disruptive and antisocial behavior associated with fronto-temporal dementia. Br. J. Psychiatry. 1997;170:150–4. doi: 10.1192/bjp.170.2.150. [DOI] [PubMed] [Google Scholar]
- Mirra SS, H. A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mitchell RLC, E. R, Barry M, Cruttenden A, Woodruff PWR. The neural response to emotional prosody as revealed by functional magnetic resonance imaging. Neuropsychologia. 2003;41:1410–21. doi: 10.1016/s0028-3932(03)00017-4. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, P. K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel--based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Munoz DG, D. D, Bergeron C, Mackenzie IR, Delacourte A, Zhukareva V. The neuropathologyand biochemistry of frontotemporal dementia. Ann Neurology. 2003;54:S24–S28. doi: 10.1002/ana.10571. [DOI] [PubMed] [Google Scholar]
- Neary D, S. J, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings JL, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nybakken K, P. N. Heparan sulfate proteoglycan modulation of developmental signalling in Drosophila. Biochim. Biophys. Acta. 2002;1573:280–291. doi: 10.1016/s0304-4165(02)00395-1. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, P. B, Rogers SJ. Executive function deficits in high functioning autistic individuals: relationship to theory of mind. J Child Psychol Psychiatry. 1991;32:1081–105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Peyron R, L. B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Clin. Neurophysiol. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Posakony LG, R. L, Gelbart WM. Wing formation in Drosophila melanogaster requires decapentaplegic gene function along the anterior-posterior compartment boundary. Mech. Dev. 1990;33:69–82. doi: 10.1016/0925-4773(90)90136-a. [DOI] [PubMed] [Google Scholar]
- Preston SD, d. W. F. Empathy: its ultimate and proximate bases. Behav Brain Res. 2002;25:1–20. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- Rahman S, S. B, Hodges JR, Rogers RD, Robbins TW. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain. 1999;122:1469–1493. doi: 10.1093/brain/122.8.1469. [DOI] [PubMed] [Google Scholar]
- Rankin KP, K. J, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol. 2005;18:28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- Ratnavalli E, B. C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, C. L. The Mirror-Neuron System. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rolls ET, C. H, Mason R, Wakeman EA. Orbitofrontal cortes neurons: role in olfactory and visual association learning. J Neurophysiol. 1996;75:1970–81. doi: 10.1152/jn.1996.75.5.1970. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Y. S, Sienkiewicz ZJ. Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. J Neurophysiol. 1990;64:1055–1066. doi: 10.1152/jn.1990.64.4.1055. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, G.-T. M, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, G.-T. M, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rowe AD, B. P, Polkey CE, Morris RG. “Theory of mind” impairments and their relationship to executive functioning following frontal lobe excisions. Brain. 2001;124:600–616. doi: 10.1093/brain/124.3.600. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, T. R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cog Behav Neuro. 2005;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Singer T, S. B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1121. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Snowden JS, G. P, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol. 1989;2:167–182. [Google Scholar]
- Sperber D, W. D. Revevance: Communication and cognition. Basil Blackwell; Oxford: 1986. [Google Scholar]
- Sperber D, W. D. Precis of Relevance: Communication and Cognition. Behavioral and Brain Sciences. 1987;14:697–754. [Google Scholar]
- Starkstein SE, F. J, Price TR, Leiguarda RC, Robinson RG. Neuropsychological and neuroradiologic correlates of emotional prosody comprehension. Neurology. 1994;44:512–22. doi: 10.1212/wnl.44.3_part_1.515. [DOI] [PubMed] [Google Scholar]
- Stickens D, C. G, Burbee D, Ramos P, Thomas S, Hogue D, Hecht JT, Lovett M, Evans GA. The EXT2 multiple exostoses gene defines a family of putative tumour suppressor genes. Nat. Genet. 1996;14:25–32. doi: 10.1038/ng0996-25. [DOI] [PubMed] [Google Scholar]
- Stone VE, B.-C. S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640–56. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Stuss DT, B. D. The frontal lobes. Raven Press; New York: 1986. [Google Scholar]
- Stuss DT, G. G, Alexander MP. The frontal lobes are necessary for ‘theory of mind’. Brain. 2001;124:279–286. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- Swartz JR, M. B, Lesser IM, Booth R, Darby A, Wohl M, Benson DF. Behaviorol phenomenology in Alzheimer’s disease, frontotemporal dementia, and late-life depression: a retrospective analysis. J Geriatr Psychiatry Neurol. 1997;10:67–74. doi: 10.1177/089198879701000206. [DOI] [PubMed] [Google Scholar]
- Tabata T, K. T. Hedgehog is a signalling protein with a key role in patterning Drosophila imaginal discs. Cell. 1994;76:89–102. doi: 10.1016/0092-8674(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Takei Y, O. Y, Sato M, Watanabe A, Tabata T. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development. 2004 Jan;131(1):73–82. doi: 10.1242/dev.00913. [DOI] [PubMed] [Google Scholar]
- The I, B. Y, Perrimon N. Hedgehog movement is regulated through tout velu-dependent synthesis of a heparan sulfate proteoglycan. Mol. Cell. 1999;4:633–639. doi: 10.1016/s1097-2765(00)80214-2. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, D. D. Update on the neuropathological diagnosis of frontotemporal dementias. Exp Neurology. 2001;60:1123–1126. doi: 10.1093/jnen/60.12.1123. [DOI] [PubMed] [Google Scholar]
- Vanhoenacker FM, V. H. W, Wuyts W, Willems PJ, De Schepper AM. Hereditary multiple exostoses: from genetics to clinical syndrome and complications. Eur J Radiol. 2001 Dec;40(3):208–17. doi: 10.1016/s0720-048x(01)00401-6. [DOI] [PubMed] [Google Scholar]
- Vogeley K, B. P, Newen A, Herrmann S, Happe F, Falkai P. Mind reading: neural mechanisms of theory of mind and self-perspective. 2001. [DOI] [PubMed]
- Vujic M, B. A, Romanus B, Wahlstrom J, Martinsson T. Hereditary multiple and isolated sporadic exostoses in the same kindred: identification of the causative gene (EXT2) and detection of a new mutation, nt112delAT, that distinguishes the two phenotypes. Int J Mol Med. 2004 Jan;13(1):47–52. [PubMed] [Google Scholar]
- Wellman HM, W. J. From simple desires to ordinary beliefs: the early development of everyday psychology. Cognition. 1990;35:245–75. doi: 10.1016/0010-0277(90)90024-e. [DOI] [PubMed] [Google Scholar]
- Wicker B, P. D, Baron-Cohen S, Decety J. Being the target of another’s emotion: a PET study. Neuropsychologia. 2003;41:139–46. doi: 10.1016/s0028-3932(02)00144-6. [DOI] [PubMed] [Google Scholar]
- Wimmer H, P. J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deceoption. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Winner E, L. S. Distinguishing irony from deceoption: Understanding the speaker’s second order intention. British Journal of Developmental Psychology. 1991;9(257-270) [Google Scholar]
- Yamaguchi Y. Heparan sulfate proteoglycans in the nervous system: their diverse roles in neurogenesis, axon guidance, and synaptogenesis. Semin. Cell Dev. Biol. 2001;12:99–106. doi: 10.1006/scdb.2000.0238. [DOI] [PubMed] [Google Scholar]
- Zak BM, C. B, Esko JD. Hereditary multiple exostoses and heparan sulfate polymerization. Biochimica et Biophysica Acta. 2002;1573:346–355. doi: 10.1016/s0304-4165(02)00402-6. [DOI] [PubMed] [Google Scholar]