Abstract
Purpose: Recent studies have raised concerns about exposure to low-dose ionizing radiation from medical imaging procedures. Little has been published regarding the relative exposure and risks associated with breast imaging techniques such as breast specific gamma imaging (BSGI), molecular breast imaging (MBI), or positron emission mammography (PEM). The purpose of this article was to estimate and compare the risks of radiation-induced cancer from mammography and techniques such as PEM, BSGI, and MBI in a screening environment.
Methods: The authors used a common scheme for all estimates of cancer incidence and mortality based on the excess absolute risk model from the BEIR VII report. The lifetime attributable risk model was used to estimate the lifetime risk of radiation-induced breast cancer incidence and mortality. All estimates of cancer incidence and mortality were based on a population of 100 000 females followed from birth to age 80 and adjusted for the fraction that survives to various ages between 0 and 80. Assuming annual screening from ages 40 to 80 and from ages 50 to 80, the cumulative cancer incidence and mortality attributed to digital mammography, screen-film mammography, MBI, BSGI, and PEM was calculated. The corresponding cancer incidence and mortality from natural background radiation was calculated as a useful reference. Assuming a 15%–32% reduction in mortality from screening, the benefit∕risk ratio for the different imaging modalities was evaluated.
Results: Using conventional doses of 925 MBq Tc-99m sestamibi for MBI and BSGI and 370 MBq F-18 FDG for PEM, the cumulative cancer incidence and mortality were found to be 15–30 times higher than digital mammography. The benefit∕risk ratio for annual digital mammography was >50:1 for both the 40–80 and 50–80 screening groups, but dropped to 3:1 for the 40–49 age group. If the primary use of MBI, BSGI, and PEM is in women with dense breast tissue, then the administered doses need to be in the range 75–150 MBq for Tc-99m sestamibi and 35 MBq–70 MBq for F-18 FDG in order to obtain benefit∕risk ratios comparable to those of mammography in these age groups. These dose ranges should be achievable with enhancements to current technology while maintaining a reasonable examination time.
Conclusions: The results of the dose estimates in this study clearly indicate that if molecular imaging techniques are to be of value in screening for breast cancer, then the administered doses need to be substantially reduced to better match the effective doses of mammography.
Keywords: radiation risk, mammography, PEM, BSGI, MBI
INTRODUCTION
Recent studies have raised concerns about exposure to low-dose ionizing radiation from medical imaging procedures. These studies have focused primarily on the relatively high doses associated with computed tomography and various cardiac imaging procedures.1 Little has been published regarding the relative exposure and risks associated with some of the newer breast imaging techniques such as breast specific gamma imaging (BSGI), molecular breast imaging (MBI), or positron emission mammography (PEM), and how these risks compare to those of mammography. Understanding the risk associated with these procedures is particularly important as unlike many CT or cardiac imaging procedures, women may undergo these procedures on multiple occasions during their lifetime.
The driving force behind the development of these alternative breast imaging techniques has been the limitations of mammography in certain groups of women. For over 30 years, mammography has been the principal screening and diagnostic imaging modality for the detection of breast cancer. Meta-analyses of the major randomized trials on the effectiveness of screening mammography have demonstrated statistically significant reductions of 20%–30% in mortality from breast cancer for women aged 50–69 yr.2 Estimates of the sensitivity of mammography in the general population vary from 75% to 95%; however, this sensitivity is significantly reduced in certain subgroups of women, notably women under the age of 50 yr,3 women with dense breast parenchyma,4 and women at increased risk for breast cancer.5 The reduced sensitivity of mammography in women under age 50 was highlighted by the recent report from the U.S. Preventative Services Task Force recommending against annual screening mammography in this age group.6 The recognition of the limitations of mammography in these subgroups has fueled interest in alternative breast imaging modalities that offer potential improvements in sensitivity and specificity, particularly in the subgroups of women for whom the sensitivity of mammography is impaired.
Alternative techniques to those using ionizing radiation include ultrasound and contrast-enhanced breast MRI. A recent comprehensive review has summarized the benefits of these modalities relative to mammography in screening for breast cancer.7 The American College of Radiology Imaging Network trial (ACRIN 6666) of whole breast ultrasound and MRI in women at elevated risk of breast cancer showed that the supplemental yield for ultrasound was 4.2 cases per 1000 women screened (compared to 7.6 cases per 1000 women screened with mammography).8 However, the addition of ultrasound came with a substantial risk of false-positive results (i.e., biopsy with benign results and∕or short interval follow-up). In addition, it required a significant time commitment from the radiologist (mean time: 20.8 min for a bilateral scan) and hence it may not be cost-effective. A subgroup of ACRIN 6666 subjects were offered a single contrast-enhanced breast MR imaging screening examination. Of 1215 women invited, 42.1% declined to undergo the procedure, with claustrophobia as the most common reason given for declining.9 The limitations of ultrasound and contrast-enhanced breast MRI (false-positive results in the case of ultrasound and claustrophobia∕high cost∕variable specificity in the case of MRI) are likely to limit their widespread adoption as alternative screening techniques.
A number of molecular imaging techniques may demonstrate better sensitivity than mammography in certain subgroups. PEM utilizes two small coincidence detectors to obtain high resolution tomographic images of the breast and appears to have a comparable sensitivity to MRI.10 BSGI utilizes a single multicrystal sodium iodide-based gamma camera to image the breast and has been shown to be of value in the evaluation of women at high risk of breast cancer.11 At the Mayo Clinic, we have been investigating the use of MBI as a potential screening tool for breast cancer. This technique is similar to BSGI, but utilizes a different detector technology (semiconductor-based gamma camera) and uses two opposing detectors optimized for breast imaging.12 Results in a large screening study of ∼1000 women who have both dense breasts and additional risk factors for breast cancer indicate that MBI may be considerably more sensitive than mammography in the detection of breast cancer in this population, while demonstrating comparable specificity.13
Although multiple possible radiotracers can be used for breast imaging with these techniques, F-18 FDG has been the primary radiopharmaceutical used for PEM and Tc-99m sestamibi has been used for both BSGI and MBI. Because these techniques involve intravenous injections of radiotracers, they pose a very different type of radiation risk than x-ray mammography. These tracers distribute throughout the body, exposing many organs and tissues to radiation, in contrast with mammography, in which the only organ affected by radiation is the breast. Even though the radiation dose to breast tissue is low with these molecular imaging techniques, the dose to other organs and the consequential radiation risk can be significantly higher. Hence, to compare the risk of these different imaging modalities, it is necessary to look at the hypothetical incidence of cancer resulting from these procedures, rather than simply comparing radiation dose to the breast.
The purpose of this paper is to estimate and compare the risks of radiation-induced cancer from mammography and techniques such as PEM, BSGI, and MBI. Since all such risk estimates are based on models of radiation risk, we also included estimates of the risks of radiation-induced cancer from natural background radiation, as these provide a useful reference mark against which to compare risk estimates from these imaging techniques. Current established models for estimation of the risk of inducing cancer from ionizing radiation are based on data from epidemiological studies of various cohorts, the largest of which is the survivors of the 1945 atomic bombings in Japan. Biological Effects of Ionizing Radiation (BEIR) VII is the most recent in a series of publications from the National Academy of Sciences that reviews the health risks from exposure to low levels of ionizing radiation.14 This report presents the risks from exposure to low-dose, low-linear energy transfer radiation and was used in this study as the foundation for estimates of radiation risk attributed to various breast imaging techniques. A key premise in evaluating the appropriateness of a screening technique is that the potential benefits of the technique outweigh the potential harms. In light of the recent controversy on the appropriateness of screening mammography for women in their 40s,6 where the majority of women have dense breast tissue, we also calculated the potential risk∕benefit ratio for different modalities of breast cancer screening in both a normal and dense breast population, as well as for different ages of screening initiation.
METHODS
Selection of appropriate model for assessing radiation risk
Over the past 20 years, several national and international organizations have developed risk models for the estimation of the incidence of cancer from low levels of ionizing radiation. These include the committee on the BEIR,14 the International Commission on Radiological Protection (ICRP),15 the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR),16 and national organizations such as the Environmental Protection Agency (EPA) and the National Institutes of Health. The most recent report addressing the issue of low levels of ionizing radiation is the BEIR VII report issued in 2006.14 This provides a comprehensive comparison of the various risk models used over the past 20 years. A full review and discussion of the strengths, weaknesses, and assumptions inherent in each model is outside the scope of this paper, but it is generally recognized that the uncertainties in estimates of radiation-induced cancer incidence and mortality are of the order of a factor of 2 for each of these models.17 This is comparable to or greater than the variability in estimation of radiation-induced cancer risk and mortality between models. For example, the estimated number of deaths per 100 000 males of mixed ages exposed to 100 mGy was 480 using the preferred models of BEIR VII. The 95% confidence interval on this estimate was 240–980 deaths. By comparison, the estimated number of deaths from the models employed in the BEIR V, UNSCEAR, ICRP, and EPA reports were 770, 710, 506, and 570, respectively, as reported in BEIR VII.14
Given this variability between the various models∕reports and the known uncertainty in any model, we have opted to use a single scheme for all estimates of cancer incidence and mortality. The BEIR VII report utilizes a combination of two models for calculating cancer incidence and mortality: The excess relative risk (ERR) model and the excess absolute risk (EAR) model. Preston et al.18 developed the ERR and EAR models to estimate breast cancer incidence and mortality from analyses of pooled data on breast cancer incidence in various cohorts exposed to ionizing radiation. A recent review by Law et al.19 discussed the strengths and weaknesses of the EAR and ERR models in predicting the risk of breast cancer. In the ERR model, the excess relative risk of breast cancer due to exposure to radiation is a multiple of the natural incidence of breast cancer in the absence of that exposure. The ERR applies a dose-dependent multiplication factor to the natural incidence of breast cancer. For populations that have a naturally high risk factor for breast cancer, the ERR model results in a higher overall risk per unit dose. In the EAR model, the excess risk due to exposure to radiation does not depend on the background incidence that exists in the absence of that radiation exposure. The EAR model gives an absolute risk and appears to provide a better estimate of risk of breast cancer across populations.19 This opinion was echoed in the BEIR VII report which indicated that the EAR model was the preferred model for the estimation of breast cancer risk (BEIR VII, Chapter 12). Since we were primarily concerned with estimating cancer incidence due to breast imaging techniques, we selected the EAR model in preference to the ERR model for all cancer estimates. We noted that for cancers other than breast cancer, the BEIR VII Committee chose a combination of both the ERR and EAR models. However, in order to maintain a consistent model across cancer estimates attributed to mammography, PEM, MBI, and BSGI, we opted for a single model (EAR) for all calculations of cancer incidence and mortality.
The BEIR VII Committee chose the lifetime attributable risk (LAR) model, devised by Vaeth and Pierce,20 to estimate the lifetime risk of radiation-induced breast cancer incidence and mortality. The LAR estimates are obtained by summing the EAR estimates for each year of life after the exposure. The LAR model assumes a latency period of ∼5 yr from exposure to the first risk of cancer from the exposure. The LAR also includes a presumed dose and dose rate effectiveness factor (DDREF). The DDREF is a correction factor that makes some allowance for the likelihood that low-dose or dose rate exposures of ionizing radiation allow a greater chance for DNA repair than those at higher doses or dose rates. A review of the DDREF factor by Law et al.19 concluded that a value of 2 was appropriate for use in dose calculations at the levels of exposure encountered in screening mammography. The BEIR VII report found a believable range of DDREF values from 1.1 to 2.3 and opted for a median value of 1.5. This value was used in all our calculations for mammography, MBI, BSGI, and PEM.
Survival table
For the purposes of this study, all estimates of cancer incidence and mortality were based on a population of 100 000 females followed from birth to age 80. In order to be able to compare the benefits and risks of radiation from various screening techniques to the naturally occurring cancer incidence and mortality that are available from the National Program of Cancer Registries,21 it was necessary to adjust these incidence and mortality rates for the fraction of the 100 000 females expected to survive to various ages between 0 and 80. Mortality tables as a function of age were obtained from the Human Mortality Database22 for females in the U.S. Table 1 presents an abbreviated version of that database and shows the number of female survivors from birth to age 84, starting with a population of 100 000. Linear interpolation was used to calculate the number of survivors each year between 0 and 80. This table is also an integral part of the radiation risk models described below, as equations for estimates of the LAR include an adjustment for the fraction of the initial 100 000 women that survive to a given age.
Table 1.
Number of female survivors from ages 0 to 84, starting with a population of 100 000. Data taken from The Human Mortality Database (Ref. 21).
| Age | Number of survivors |
|---|---|
| 0 | 100 000 |
| 1–4 | 99 387 |
| 5–9 | 99 285 |
| 10–14 | 99 222 |
| 15–19 | 99 153 |
| 20–24 | 98 969 |
| 25–29 | 98 728 |
| 30–34 | 98 454 |
| 35–39 | 98 095 |
| 40–44 | 97 563 |
| 45–49 | 96 719 |
| 50–54 | 95 449 |
| 55–59 | 93 648 |
| 60–64 | 91 070 |
| 65–69 | 87 160 |
| 70–74 | 81 476 |
| 75–80 | 73 218 |
| 80–84 | 61 666 |
Mammography
Estimates of the mean glandular dose (MGD) from a bilateral two-view mammogram (craniocaudal and mediolateral oblique views) vary in the literature. A recent study by Hendrick et al.23 compared the MGD between digital and screen-film mammography in 4366 patients using data from the American College of Radiology Imaging Network Digital Mammographic Imaging Screening Trial (DMIST). The MDG per subject (two views) was 3.72 mGy for digital mammography and 4.74 mGy for screen-film mammography. This does not include the additional dose from extra views. With the availability today of larger digital detector systems, we assumed that the number of extra views required is no different between digital and film-screen systems and therefore we used the value of 5.2% reported for film-screen systems in the DMIST study.23 After correction for the dose from the extra views, this resulted in an average MDG per subject (two views) of 3.91 mGy for digital mammography and 4.98 mGy for screen-film mammography. For this study we opted to report estimates of cancer incidence and mortality using the values from DMIST as it reflects the most current technology and also was the only report to include the effects of extra views in the overall estimate of MGD. Due to the conflicting recommendations on when to initiate breast cancer screening,6 we have evaluated the effects of radiation based on breast cancer screening programs with annual mammograms from ages 40 to 80 and from ages 50 to 80.
As mentioned above, the preferred model for female breast cancer in the BEIR VII report is the EAR model, as it is based on both the atomic bomb survivors and U.S studies and includes both age at exposure and attained age as modifying factors. The EAR from mammography in females was calculated using the equation for EAR from BEIR VII [Eq. 12-2] shown as Eq. 1.1 below.
| (1.1) |
In this equation, e=age of the woman at the time of exposure to radiation and ranged from either 40 to 80 or from 50 to 80 yr. The parameter a=attained age, i.e., age at which we wish to estimate the cancer risk. The parameter βF represents the EAR per 104 person-year-Sieverts. The parameter γ represents the per-decade increase in age at exposure and
| (1.2) |
Equation 1.2 differs from that described in BEIR VII, in that e* does not go to zero after age 30 as described in BEIR VII (Table 12-2), but continues to decline with age. This was found to provide a better match between the cancer risk estimated in this study and that published by BEIR VII (Table 12D-1). The parameter η is the exponent of attained age. The parameters for the breast, βF, γ, and η were taken from BEIR VII (Table 12-2) and are listed in Table 2. Breast cancer mortality was estimated by taking the breast cancer incidence rate and multiplying it by the ratio of the sex and age-specific mortality and incidence rates for the U.S. population,21 consistent with the method used in the BEIR VII report.
Table 2.
Values of βF and η used for estimation of EAR (cancer incidence) due to mammography, MBI, BSGI, and PEM. Values were extracted from BEIR VII (Table 12-2).
| Cancer site | βF | γ | η |
|---|---|---|---|
| Stomach | 4.9 | −0.41 | 2.8 |
| Colon | 1.6 | −0.41 | 2.8 |
| Liver | 1.0 | −0.41 | 4.1 |
| Lung | 3.4 | −0.41 | 5.2 |
| Breast | 9.4 | −0.51 | 3.5 (attained age<50) |
| 1.1 (attained age>50) | |||
| Uterus | 1.2 | −0.41 | 2.8 |
| Ovary | 0.70 | −0.41 | 2.8 |
| Bladder | 0.75 | −0.41 | 6.0 |
| Other cancers | 4.8 | −0.41 | 2.8 |
A matrix was constructed for a range of age of exposure and attained age of either 40–80 or 50–80 yr. To account for the presence of a risk-free latent period between exposure and risk, the EAR was adjusted as shown in Eq. 1.3 below
| (1.3) |
where L is a risk-free latent period from time of exposure to time of the first occurrence of cancer. A period of 5 yr was selected for solid cancers based on the BEIR VII recommendation. To calculate the cumulative lifetime risk from ages 40 to 80 and from 50 to 80, it is necessary to include the probability of survival until age a conditional on survival to agee. Using interpolated values from Table 1, the ratio S(a)∕S(e) (survival at attained agea, conditional on survival to exposed agee) was computed for all attained ages≥exposed age. The LAR (e) was then computed using a slight modification of the equation from BEIR VII [Eq. 12-4], as shown in Eq. 1.4 below
| (1.4) |
where the summation is from e=40 to a or 50 toa, as appropriate. This summation yielded the LAR for an attained age a from all mammograms since ages 40 or 50 up to the attained age. From the LAR at each attained agea, the cumulative risks of cancer from mammography were obtained by summing the LAR from ages 40 to 80 and from 50 to 80.
Molecular imaging techniques
For MBI, BSGI, and PEM, organ dose estimates for Tc-99m sestamibi were taken from Table 4.7.4 of ICRP 80 and those for F-18 FDG were taken from Table 3.2.1 of ICRP 80.24 The MIRD 19 report25 provides updated estimates of some organ doses for F-18 FDG and where these differed from those in ICRP 80, the updated values were used. To match these organ estimates with the cancer sites in the BEIR VII report, the following adjustments were made: (a) The average of the doses to the small intestine, upper large intestine, and lower large intestine was used to calculate incidence of colon cancer and (b) the total body dose was used to calculate the incidence of “other solid cancers” not specifically addressed by the BEIR VII report. The EAR from F-18 FDG and Tc-99m sestamibi in females was calculated using Eq. 1.1, assuming annual exposures from MBI, BSGI, and PEM beginning at age 40 or 50. The values for the coefficients βF and η were taken from BEIR VII (Table 12-2) and are presented in Table 3. However, e*was assumed=0 fore≥30, and Eq. 1.1 was simplified to
| (1.5) |
Table 4.
Estimated lifetime cumulative risk of radiation-induced cancer (incidence and mortality) attributed to a single examination at ages 40, 50, 60, and 70 yr of age using various breast imaging procedures. All numbers are cumulative to age 80 for 100 000 females undergoing a single examination.
| Procedure | Age at exposure (yr) | Cancers induced | Cancer deaths |
|---|---|---|---|
| Digital mammography (3.91 mGy) | 40 | 4.7 | 1.0 |
| 50 | 2.2 | 0.5 | |
| 60 | 0.9 | 0.2 | |
| 70 | 0.2 | 0.0 | |
| Screen-film mammography (4.98 mGy) | 40 | 6.0 | 1.2 |
| 50 | 2.9 | 0.6 | |
| 60 | 1.1 | 0.3 | |
| 70 | 0.2 | 0.1 | |
| Tc-99m sestamibi (925 MBq) | 40 | 34 | 20 |
| 50 | 29 | 17 | |
| 60 | 22 | 12 | |
| 70 | 9.2 | 5.2 | |
| F-18 FDG (370 MBq) | 40 | 36 | 17 |
| 50 | 30 | 15 | |
| 60 | 22 | 12 | |
| 70 | 9.5 | 5.2 |
Table 3.
Values ofβF, γ, and η used for estimation of EAR (incidence and mortality) due to background radiation. Values were extracted from BEIR VII (Table 12-1).
| EAR | βF | γ | η |
|---|---|---|---|
| Incidence | 28 | −0.41 | 2.8 |
| Mortality | 13 | −0.37 | 3.5 |
The LAR attributed to each molecular imaging technique was then calculated using the values of EAR [from Eqs. 1.1, 1.5] and Eq. 1.4 and utilizing the same exposed age ranges. For PEM, we assumed an administered dose of 370 MBq per procedure.10 For MBI and BSGI, we evaluated two different dose ranges. Previous studies from our laboratory reported an administered dose of 740 MBq Tc-99m sestamibi for MBI procedures.12 BSGI studies using a single-detector system have reported doses in the range 925–1110 MBq.11 Hence we assumed a typical dose range of 740–1110 MBq for MBI and BSGI procedures, with an average value of 925 MBq. We have recently implemented a number of hardware and software enhancements to MBI technology, which has resulted in a reduction in the required dose of Tc-99m sestamibi to 296 MBq.26 Initial results indicate that the required dose of Tc-99m sestamibi can be further reduced to 74–148 MBq (Ref. 26) and we have recently commenced clinical studies evaluating this low-dose strategy. We used an average value of 111 MBq and a range of 74–148 MBq as the potential low-dose range for MBI procedures.
Background radiation
As the majority of background radiation exposure is an unavoidable consequence of living on planet earth, it serves as a useful index against which to compare the effects of radiation from mammography and molecular imaging procedures. Background radiation levels are known to vary significantly with location. Excluding exposure due to medical procedures, the average value in the U.S is 3.1 mSv∕yr.27 To match the models used above, the cancer incidence and mortality from background radiation in females were calculated using the equation for EAR from BEIR VII [Eq. 12-2] shown as Eq. 1.1 above and its associated parameters (BEIR VII, Table 12-1) that are presented in Table 3 below. For background radiation, e*was defined as
| (1.6) |
A matrix was constructed for a range of age of exposure and attained age of 0–80 yr. The LAR associated with background radiation was then calculated using the values of EAR [from Eq. 1.1] and Eq. 1.4 described above, but with the summation now going from e=0 to a and assuming annual background exposure of 3.1 mSv until age 80.
U.S. total cancer and breast cancer incidence and mortality
To put the risk of cancer attributed to mammography, MBI and PEM, and background radiation into perspective, we also calculated the cumulative cancer incidence and cancer mortality in the U.S. (all cancers, all races) in women and the cumulative incidence and mortality from breast cancer in women using results from the National Program of Cancer Registries for 2006.21 These provide cancer incidence and mortality in the U.S. by five-year age groups. Linear interpolation was performed to obtain the total cancer and breast cancer incidence and mortality numbers for each year from birth to 80 yr. These values were then multiplied by the fraction of the population surviving at each year (Table 1) to permit direct comparison with estimates of cancer incidence from the various sources of radiation. Cumulative total cancer and breast cancer incidence and mortality were then obtained from ages 0 to 80.
Estimation of benefit∕risk ratio for breast screening
Calculation of breast cancer mortality from radiation was combined with the reported reduction in breast cancer mortality from screening mammography and used to provide an estimate of the potential benefit of a breast cancer screening technique based on mammography or an equivalent molecular imaging technique in two populations: (a) A normal population of 100 000 women and (b) a population of 100 000 women with dense breast tissue. The average utilization rate for screening mammography in the U.S. was reported as 76.1% in 2006 (Ref. 28) and a recent meta-analysis of Canadian, European, and Australian screening programs has shown the overall reduction in mortality to be at least 25% (estimates range from 25% to 48%).29 The recent guidelines by the U.S. Preventive Services Task force on screening for breast cancer pooled results from all randomized and estimated the reduction in mortality to be 15% for women aged 40–49, 14% for those aged 50–59, and 32% for those aged 60–69.30
In women who have been diagnosed with breast cancer, the observed breast cancer mortality rate (Mo) reported in the National Program of Cancer Registries is less than the true mortality rate (Mt) that would be observed in the absence of screening. From knowledge of the breast cancer incidence and Mo, the value of Mt can be obtained using the following equation:
| (1.7) |
where U=utilization rate of screening mammography and R=fractional reduction in the risk of dying from breast cancer attributed to screening mammography. Assuming a value of R=0.15 for women aged 40–49, R=0.14for women aged 50–59, and R=0.32 for women aged 60 and older,30 and U=0.761,28 as described above, we obtain
Using the true mortality rate for breast cancer, the potential benefits for various screening scenarios were then estimated for the normal population of 100 000 women. Estimates of the reduction in breast cancer mortality were compared to the increased mortality attributed to mammography to obtain a benefit∕risk ratio.
Approximately 25% of women undergoing mammography are reported to have heterogeneously or extremely dense breast tissue.31 Hence, in a population of 100 000 women, the reported incidence of breast cancer (I) is a combination of the incidence in two groups, the incidence in nondense breasts (In) and the incidence in dense breasts (Id)
| (1.8) |
Women with dense breast tissue have a factor of ∼5 increased risk of breast cancer.4, 31 Hence, we assumed that the incidence of breast cancer is five times higher in the dense breast population compared to the nondense population, i.e.,
| (1.9) |
From these two equations, we can obtain an estimate of the breast cancer incidence in a dense breast population. In this population, the sensitivity of mammography is known to be reduced to∼50%,3, 4, 5, 9 whereas a recent study has shown the sensitivity of MBI to be 90%.13 For new technologies such as MBI, BSGI, and PEM, there are no published data that demonstrate a reduction in breast cancer mortality. Hence, the following hypothesis was used to predict the possible benefits of these technologies in a population of women with dense breast tissue. Bailey et al.,32 using a simulation model, found that lowered mammographic tumor detectability accounted for ∼80% of the reduced sensitivity of mammographic screening in younger women and was the major contributing factor to the failure of mammography to reduce breast cancer mortality in this population. Hence, any alternative imaging modality that has a high sensitivity for the detection of breast cancer in this population might be reasonably expected to likewise lead to a corresponding reduction in mortality. As mammography is known to perform well in older women, we hypothesized that the performance of MBI in dense breast tissue would yield a comparable reduction in mortality to that observed with mammography in older women (i.e., R=0.32).
RESULTS
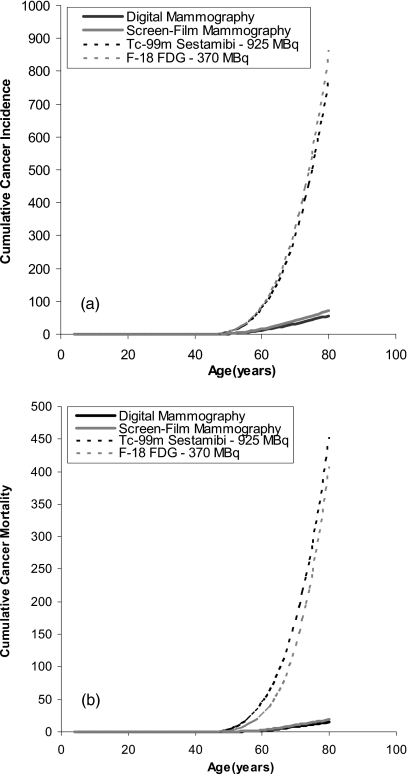
Table 4 shows the estimated lifetime radiation-induced cancer incidence and mortality from a single examination with digital mammography, screen-film mammography, MBI, or BSGI performed at a dose of 925 MBq and PEM performed at a dose of 370 MBq. For a 40 yr old woman, the estimated radiation-induced cancer incidence from molecular imaging techniques using conventional doses of Tc-99m sestamibi or F-18 FDG is approximately five to seven times that of mammography. More problematic, the estimated radiation-induced cancer deaths are more than 20 times higher, due partly to the higher mortality rate for all cancers, compared to breast cancer. Figure 1 shows the estimated cumulative cancer incidence and mortality attributed to annual screening performed from ages 40 to 80 for digital mammography at a MGD of 3.91 mGy, screen-film mammography at a MGD of 4.98 mGy, MBI∕BSGI using 925 MBq Tc-99m sestamibi, and PEM using 370 MBq F-18 FDG. At a dose of 925 MBq Tc-99m sestamibi, the estimated cumulative cancer incidence by age 80 was 782 cancers. The estimated cancer incidence attributed to 370 MBq F-18 FDG was very similar at 800 cancers. By comparison, the estimated cumulative cancer incidence attributed to mammography ranged between 56 and 71 cancers.
Figure 1.
(a) Estimated cumulative cancer incidence attributed to annual screening performed from ages 40 to 80 for digital mammography at a MGD of 3.91 mGy, screen-film mammography at a MGD of 4.98 mGy, MBI∕BSGI using 925 MBq Tc-99m sestamibi, and PEM using 370 MBq F-18 FDG. (b) Estimated cumulative cancer mortality attributed to annual screening performed from ages 40 to 80 for digital mammography at a MGD of 3.91 mGy, screen-film mammography at a MGD of 4.98 mGy, MBI∕BSGI using 925 MBq Tc-99m sestamibi, and PEM using 370 MBq F-18 FDG.
The mortality attributed to a dose of 925 MBq Tc-99m sestamibi was 453 cases∕100 000 females, while that from 370 MBq F-18 FDG was 408 cases∕100 000 females. By comparison, the mortality attributed to digital mammography was estimated at 15 cancers and that attributed to screen-film mammography at 19 cancers. These numbers are more than 20 times lower than those attributed to molecular imaging techniques.
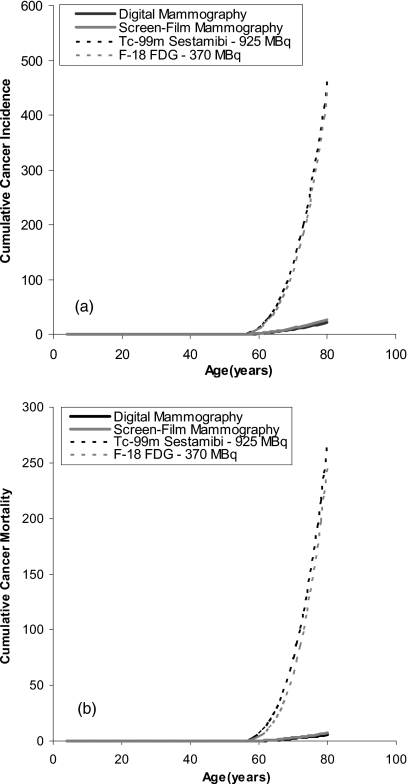
Figure 2 shows the comparable results for estimated cumulative cancer incidence and mortality attributed to annual screening performed from ages 50 to 80 at the same doses as shown in Fig. 1. At a dose of 925 MBq Tc-99m sestamibi, the estimated cumulative cancer incidence dropped from 782 to 460 cancers when the screening range was shortened to ages 50–80. Likewise, the estimated cancer incidence attributed to 370 MBq F-18 FDG dropped from 800 to 442 cancers. Hence changing the screening period from 50 to 80 yr resulted in a ∼40% decrease in estimated cancer incidence for molecular imaging techniques. By comparison, reducing the screening period to ages 50–80 resulted in an even greater reduction in estimated cancer incidence attributed to digital mammography from 56 to 21 cancers and that attributed to screen-film mammography from 71 to 27 cancers (∼62%decrease in cancer incidence). The primary reasons for this greater reduction are the presence of the term e* [Eq. 1.1], which declines with age for breast cancer but remains constant for all other cancers and the reduction in the value of η (Table 2) after age 50 for breast tissue. Even using this shorter screening period, the mortality attributed to 925 MBq Tc-99m sestamibi and 370 MBq F-18 FDG ranged from 248–267 deaths, compared to ∼6 for digital and screen-film mammography, respectively. Hence the development of MBI, BSGI, and PEM as possible screening techniques in breast imaging would require an order of magnitude reduction in the administered doses of the radiopharmaceuticals to justify their application based on radiation risk.
Figure 2.
(a) Estimated cumulative cancer incidence attributed to annual screening performed from ages 50 to 80 for digital mammography at a MGD of 3.91 mGy, screen-film mammography at a MGD of 4.98 mGy, MBI∕BSGI using 925 MBq Tc-99m sestamibi, and PEM using 370 MBq F-18 FDG. (b) Estimated cumulative cancer mortality attributed to annual screening performed from ages 50 to 80 for digital mammography at a MGD of 3.91 mGy, screen-film mammography at a MGD of 4.98 mGy, MBI∕BSGI using 925 MBq Tc-99m sestamibi, and PEM using 370 MBq F-18 FDG.
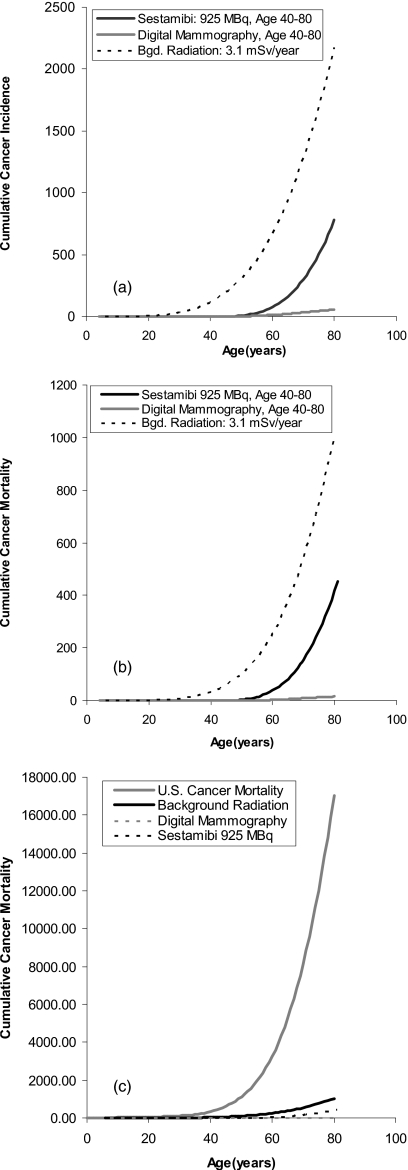
To put into perspective the estimated cancer incidence and mortality attributed to the above techniques, Figs. 3a, 3b show the estimated cumulative cancer incidence and mortality as a function of age from exposure to an annual background radiation level of 3.1 mSv from birth to age 80. By age 80, an estimated 2174 women could have cancers attributable to background radiation and 1011 could die as a result. The comparable estimated cancer incidence attributed to digital mammography (3.91 mGy∕yr from ages 40 to 80) and MBI∕BSGI (925 MBq Tc-99m sestamibi∕yr from ages 40 to 80) are also shown. These numbers are dwarfed by the cumulative cancer incidence and mortality in the U.S. from all cancers [Fig. 3c]. Using the National Program of Cancer Registries and adjusting for a cohort of 100 000 females followed from birth to age 80, there were 37 773 cancers recorded and 10 906 cases of invasive breast cancer. The corresponding cumulative mortality was estimated at 14 400 and 2138 deaths from all cancers and breast cancer, respectively. Figure 3c compares the cumulative mortality from all cancers against that attributed to background radiation, mammography, and current molecular imaging techniques.
Figure 3.
(a) Estimated cumulative cancer incidence from natural background radiation (3.1 mSv∕yr from ages 0 to 80). The comparable cancer incidence attributed to digital mammography (3.91 mGy) and MBI∕BSGI (925 MBq Tc-99m sestamibi) performed annually from ages 40 to 80 are also shown. (b) Estimated cumulative cancer mortality from natural background radiation (3.1 mSv∕yr from ages 0 to 80). The comparable cancer mortality attributed to digital mammography (3.91 mGy) and MBI∕BSGI (925 MBq Tc-99m sestamibi) performed annually from ages 40 to 80 are also shown. (c) Estimated cumulative cancer mortality from all sources (National Program of Cancer Registries) and from natural background radiation (3.1 mSv∕yr from ages 0 to 80). Estimated cancer mortality attributed to digital mammography (3.91 mGy) and MBI∕BSGI (925 MBq Tc-99m sestamibi) performed annually from ages 40 to 80 are also shown.
In 100 000 women, Table 5 shows the breast cancer incidence and mortality expected to occur in a normal population between the ages of 0 and 80, 40 and 49, and 50 and 80 based on data for 2006 from the National Program of Cancer Registries.21 The observed mortality rates were Mo=0.124, 0.166, and 0.232 between the ages of 40 and 49, 50 and 59, and 60–80, respectively. From Eq. 1.7, after correction for the influence of screening mammography, the estimated mortality rate from breast cancer in the absence of a screening mammography program was found to be Mt40–49=0.140, Mt50–59=0.166, and Mt>60=0.307 between the ages of 40 and 49, 50 and 59, and 60 and 80, respectively. Hence, in our population of 100 000 females, a total of1494∗0.140=209, 2444∗0.186=455, and 7643∗0.307–2344 deaths would be anticipated in the absence of a breast screening program for women in the 40–49, 50–59, and 60–80 age brackets. Assuming a 15% (ages 40–49), 14% (ages 50–59), and 32% (ages 60–80) reduction in mortality attributed to screening mammography, then 31, 64, and 583 deaths would be prevented by annual screening mammography from ages 40 to 50 and from ages 50 to 80, respectively.
Table 5.
Using breast cancer incidence and mortality for different age groups and corresponding observed breast cancer mortality rate (Mo) for 2006 [from the National Program of Cancer Registries (Ref. 21)], the table shows estimated cancer mortality rate (Mt) calculated using Eq. 1.5 and the corresponding total mortality that would be observed in the absence of screening mammography. All numbers are cumulative to age 80 for 100 000 females.
| Age range | Breast cancer incidence | Breast cancer mortality (observed) with screening | Mo | Mt | Estimated breast cancer mortality w∕o screening |
|---|---|---|---|---|---|
| 0–80 | 12 520 | 2427 | 0.194 | — | — |
| 40–50 | 1 494 | 185 | 0.124 | 0.140 | 209 |
| 50–60 | 2 444 | 406 | 0.166 | 0.186 | 455 |
| 60–80 | 7 643 | 1773 | 0.232 | 0.307 | 2346 |
In the dense breast population of 100 000 women, the cumulative incidence of breast cancer using Eqs. 1.8, 1.9 was estimated at 31 300 cancers from ages 0 to 80. Similar calculations for women aged 40–49, 50–59, and 60–80 gave 3735, 6110, and 19 108 cancers, respectively (Table 6).
Table 6.
Using breast cancer incidence for 2006 [from the National Program of Cancer Registries (Ref. 21)] and assuming 25% women have dense breast tissue, with fivefold increase in risk of breast cancer, the table shows estimated cancer incidence in a population of 100 000 women with dense breasts. The mortality rate (Mt) was assumed to be the same as in Table 5. All numbers are cumulative to age 80 for 100 000 females.
| Age range | Breast cancer incidence | Estimated breast cancer mortality w∕o screening |
|---|---|---|
| 0–80 | 31 300 | |
| 40–50 | 3 735 | 523 |
| 50–60 | 6 110 | 1138 |
| 60–80 | 19 108 | 5866 |
Table 7 shows the estimated cumulative cancer incidence and mortality attributed to various breast imaging procedures and background radiation for both a normal population and a population of women with dense breast tissue. These estimates are compared to the estimated number of lives saved by screening mammography and MBI. Because of the high incidence of breast cancer in women with dense breasts, the benefit∕risk ratio for both digital and screen-film mammography is actually higher in the dense breast population for all age groups than for the normal population.
Table 7.
Estimated cumulative cancer incidence and mortality attributed to various breast imaging procedures and background radiation, compared to the potential number of lives saved assuming a 15%, 14%, and 32% reduction in mortality from screening mammography for a normal population of women aged 40–49, 50–59, and 60–80, respectively, and assuming a 15% reduction for all women aged 40–80 with dense breast tissue.
| Procedure∕exposure source | Dose | Exposure period (yr) | Cancers induced | Cancer deaths | Reduction in mortality | Benefit∕risk ratio |
|---|---|---|---|---|---|---|
| Digital mammography | 3.91 mGy | 40–80 | 56 | 15 | 845 | 56:1 |
| Normal population | 3.91 mGy | 50–80 | 21 | 6 | 815 | 135:1 |
| 3.91 mGy | 40–49 | 35 | 9 | 31 | ∼3:1 | |
| Screen-film mammography | 4.98 mGy | 40–80 | 71 | 19 | 845 | 44:1 |
| 4.98 mGy | 50–80 | 27 | 7 | 815 | 116:1 | |
| Normal population | 4.98 mGy | 40–49 | 44 | 11 | 31 | ∼3:1 |
| Digital mammography | 3.91 mGy | 40–80 | 56 | 15 | 1129 | 75:1 |
| Dense breast population | 3.91 mGy | 50–80 | 21 | 6 | 1051 | 175:1 |
| 3.91 mGy | 40–49 | 35 | 9 | 78 | 9:1 | |
| Screen-film mammography | 4.98 mGy | 40–80 | 71 | 19 | 1129 | 59:1 |
| 4.98 mGy | 50–80 | 27 | 7 | 1051 | 150:1 | |
| Dense breast population | 4.98 mGy | 40–49 | 44 | 11 | 78 | 7:1 |
| MBIa | 925 MBq | 40–80 | 782 | 453 | 2408 | ∼5:1 |
| Tc-99m sestamibi | 925 MBq | 50–80 | 460 | 267 | 2241 | ∼8:1 |
| Dense breast population | 925 MBq | 40–49 | 322 | 186 | 167 | ∼1:1 |
| Low-dose MBIa | 111 MBq | 40–80 | 94 | 54 | 2408 | 46:1 |
| Tc-99m sestamibi | 111 MBq | 50–80 | 55 | 34 | 2241 | 66:1 |
| Dense breast population | 111 MBq | 40–49 | 39 | 20 | 167 | ∼8:1 |
| PEM | 370 MBq | 40–80 | 800 | 408 | No data | |
| F-18 FDG | 370 MBq | 50–80 | 442 | 248 | No data | |
| 370 MBq | 40–49 | 358 | 160 | No data | ||
| Background radiation | 3.1 mSv | 0–80 | 2174 | 1011 | — |
Estimates for MBI assume a 32% reduction in mortality from MBI for all women ages 40–80 with dense breast tissue. No data on screening is available for BSGI or PEM. All numbers are cumulative to age 80 for 100 000 females.
The potential reduction in mortality from a screening program employing MBI is unknown, but based on the high sensitivity of MBI in women with dense breast tissue,13 it was assumed that MBI would yield a comparable reduction in mortality (∼32%) as seen with screening mammography in women without dense breast tissue. Under this assumption, the benefit∕risk ratio of MBI is less than 10:1 for all groups when a standard dose of 925 MBq is employed, but begins to achieve a comparable benefit∕risk ratio to mammography when the administered dose is reduced to∼111 MBq.
DISCUSSION
Recent articles in the scientific literature have generated considerable debate and controversy about the potential harmful effects of various imaging procedures that utilize ionizing radiation.1 Often overlooked in this debate is the fact that all estimates of radiation-induced cancer are theoretical calculations extrapolated from findings in subjects who received radiation doses 10–100 times greater than what is used in clinical practice. Many recent articles quote the number of deaths caused by CT scans and other imaging procedures as if they were fact.33 In reality, these numbers are “worst case scenario” extrapolations from high-dose studies. To put this type of extrapolation into context, Fig. 3 and Table 7 show the estimated cancer incidence and mortality for natural background radiation. The calculated deaths from background radiation are more than 50 times greater than those from 40 years of screening mammography. It should be noted that currently there is no epidemiological evidence that background radiation induces this number of cancer deaths. Background radiation levels have been recorded in parts of Brazil, India, and China that are three to ten times the U.S. average.15 Despite these higher levels, no increase in the frequency of cancer has been documented in populations residing in these areas of high natural background radiation.15 Brenner et al.34 pointed out that at doses below∼10 mSv, a cohort of 5×106 people would need to be followed-up over their lifetime in order to statistically demonstrate the effects of such low doses. The impracticality of such an experiment implies that we may never know the true effects of very low levels of ionizing radiation. While we are not suggesting that the risks of low levels of ionizing radiation are nonexistent, the lack of evidence for the detrimental effects of background radiation should place in context the predicted cancer incidence and mortality estimated from radiation doses associated with x-ray mammography, MBI, BSGI, and PEM that are discussed below.
A challenge for any study attempting to estimate cancer risk from ionizing radiation is the selection of the appropriate risk model. Different risk models can introduce a factor of 2 in the estimated number of cancer cases. Even within the BEIR VII report, use of different models (EAR vs ERR) can give a twofold difference in the estimate of radiation-induced cancer incidence. For this study, we decided on the EAR model as it is best suited to estimates of radiation-induced breast cancer.15 In addition, the use of this model rather than the generalized risk estimates available in BEIR VII (Tables 12-D1, 12-D2, and 12-D3) allowed the incorporation of corrections for factors such as survival to a given age, LAR to 80 rather than end of life, and use of individual organ dose rather than effective dose for radiopharmaceutical dosimetry. One interesting consequence of the EAR model is the significant difference in how the model treats breast cancer relative to all other cancers. The term e* in Eq. 1.1 results in the risk of breast cancer from radiation to the breast declining with age, whereas for all other cancers the risk of cancer remains relatively constant with age. This is evident in Table 4 where the expected cancer incidence from a single exam performed at ages 40 and 70 differ by a factor of 20–30 for mammography but only a factor of 3–4 for molecular imaging techniques.
One factor not included in our dose estimates was the relative biological effectiveness (RBE) of different energy x rays and gamma rays. There is increasing evidence from radiobiology studies35 that the low-energy x rays used in screening mammography are more effective in inducing biological damage than the higher energy gamma rays used in molecular imaging techniques, i.e., they have an increased RBE value. Risk estimates for radiation-induced cancer, derived principally from the atomic bomb survivor studies, are based on the effects of high energy gamma rays and neutrons and thus the implication is that the risks of radiation-induced breast cancer arising from mammography may be higher than that assumed based on standard risks estimates. Based on a review of existing data from in vitro studies, Brenner et al.36 reported that low doses of low-energy x rays may produce an increased risk per unit dose by RBE factors of 1.2 to 4.8 relative to the higher energy gamma rays used in MBI, BSGI, and PEM. The BEIR VII report only briefly addressed this issue and stated that “it may be desirable to increase risk estimates by… a factor of 2 or 3 for the purpose of estimating risk from low-dose x-ray exposure.” This is still a controversial issue with studies recommending for and against a modification of the risk estimates for mammography.36, 37 While this factor is mentioned in the BEIR VII report, the final tables do not incorporate RBE into their estimates. Law et al.37 have recently argued against any modification of the current models for risk estimation in mammography and indicated that many models already accounted for this effect. All the calculations presented in this study are based on assumption that low-energy x rays have a RBE factor of 1. Increasing the RBE values by a factor of 2 or 4 for mammography would significantly alter the benefits of mammography relative to molecular imaging techniques in a screening environment.
Figure 1 and Table 7 show the number of cancers attributed to mammography. The results show that with both screening mammography regimes, the benefit∕risk ratio remains above 50:1 for digital mammography. Looking at the effects of screening between ages 40 and 49, the benefit∕risk ratio drops to 3:1. However, in the dense breast population, despite the lowered sensitivity of mammography, the benefit∕risk ratio increases to 9:1 due to the fivefold higher prevalence of cancer in this population. The recent U.S. Preventative Services Task Force report on screening mammography recommended annual screening at age 50 rather than age 40, as was previously recommended.6 While numerous other factors contributed to this decision, on the basis of radiation risk alone, the numbers in Table 7 would argue in favor of continuing screening mammography for women in their 40s.
Prior discussions on the radiation dose from molecular imaging techniques have focused on the radiation dose to the breast alone. When looked at in isolation, the radiation dose to breast tissue is less than that from mammography, and some reports have focused on this fact to minimize the impact of radiation effects from molecular imaging techniques. However, because these radiopharmaceuticals are administered systemically, the radiation doses to other organs are considerably higher than that to the breast and the doses to these organs dictate the overall radiation burden to the patient. As a consequence, as shown in Table 4, the relative number of cancers induced by a single study at age 40 using a 925 MBq dose of Tc-99m sestamibi or a 370 MBq dose of F-18 FDG is ∼7 times higher than that from digital mammography.
If we assume that the primary application for any molecular imaging technique in a screening environment is in the population of women where the performance of mammography is limited by breast density, then Table 7 shows that even in this case, the benefit∕risk ratio is less than 8:1 for all groups except for women over 50 and would argue against the use of technologies such as MBI, BSGI, and PEM at their current administered dose levels in a screening environment. At our institution, a recent work on dose reduction methods for MBI has indicated that the administered dose of Tc-99m sestamibi can be reduced to 148 MBq with no degradation in image quality and that it may be possible to achieve further reductions to 74 MBq.26 At these dose levels, Table 7 shows that in women with dense breast tissue, MBI can achieve an acceptable benefit∕risk ratio to warrant further evaluation in this population. Dedicated molecular imaging technologies for the breast are still in their infancy and we believe that future refinements to these technologies will enable them to be performed with significantly lower doses than what have been used to date.
Recent studies have shown that both MBI and BSGI are very comparable to contrast-enhanced breast MRI in terms of their ability to detect breast cancer.38, 39 Breast MRI has already been shown to be superior to mammography as a screening technique in women at high risk of breast cancer.5, 7 However, there are no data to support contrast-enhanced breast MRI as a screening tool in average risk women or women with dense breasts and the high cost of MRI relative to mammography (factor of∼10) may prohibit its evaluation in this population. A recent study using MBI as a screening tool in a population of women at increased risk of breast cancer showed that MBI had two to three times the sensitivity of mammography.13 As low-dose molecular imaging techniques are developed, the added advantage of increased sensitivity should eventually be reflected in a further reduction in the mortality from breast cancer and should make these techniques attractive as adjunct screening techniques to mammography.
One disadvantage of molecular imaging techniques compared to mammography is the fact that even if both techniques induce comparable numbers of cancers, the estimated mortality rate from mammography is less than that of molecular imaging techniques due to the low mortality rate for breast cancer (∼20%) relative to that for all solid tumors (∼50%). However, a counterargument to that is the fact that the mortality from breast cancer in women with dense breast tissue is approximately twice that of women with nondense breast tissue.40 We have not incorporated this effect into the above calculations, but it would further strengthen the case for low-dose molecular imaging techniques in younger women with dense breast tissue.
The BEIR VII EAR model indicates that the detrimental effects of radiation to the breast tissue decline far faster with age than those from radiation to the whole body. The implications of this are that the detrimental effects of radiation in women ages 40–49 are significantly greater than in women 50 and older. Figure 1 and Table 7 show that the number of cancers induced by 30 yr of mammography from ages 50 to 80 is a factor of 1.5 times less than that induced by only 10 yr of mammography from ages 40 to 49. This makes mammography a more attractive option in older women where it is also known to yield a significant reduction in mortality.
If the results from the MBI screening study13 are borne out in larger multicenter studies, then there may be a valuable role for molecular imaging techniques in a screening environment for women in their 40s–50s who are disadvantaged by mammography due to its lower sensitivity and for women with dense breast tissue who are further disadvantaged by their higher risk factor for breast cancer and the greater mortality rate from cancers in these women.40
The results of the dose estimates in this study clearly indicate that if molecular imaging techniques are to be of value in screening for breast cancer, then the administered doses need to be substantially reduced to better match the effective doses of mammography. From our calculations, it appears that doses of Tc-99m sestamibi need to be in the range 75–150 MBq and doses of F-18 FDG should be in the range 35–70 MBq. We believe that these dose ranges can be achieved with enhancements to current technology while maintaining a reasonable examination time. PEM technology uses two small opposing scanning arrays of crystals. The use of a full field array system should yield a factor of 5 or more increase in sensitivity. Likewise, in MBI, a recent work has shown a variety of improvements that permit the administered dose to be reduced to 150 MBq.26 We believe that molecular imaging technologies will yield a significant improvement in sensitivity relative to mammography, and with enhancements to the technology, may play an important role as adjunct screening techniques in the future.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institute of Health (Grant No. CA128407) and from the Mayo Foundation.
Conflict of interest: The Mayo Foundation and two of the authors of this work (M.K. O’Connor and C.B. Hruska) obtain royalties from licensing arrangements between the Mayo Foundation and Gamma Medica-Ideas.
References
- Smith-Bindman R. et al. , “Radiation dose associated with common computed tomography examinations and the associated attributable risk of cancer,” Arch. Intern Med. 169, 2078–2086 (2009). 10.1001/archinternmed.2009.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. H. et al. , “Breast cancer screening with imaging: Recommendations from the society of breast imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer,” J. Am. Coll. Radiol. 7, 18–27 (2010). 10.1016/j.jacr.2009.09.022 [DOI] [PubMed] [Google Scholar]
- Carney P. A. et al. , “Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography,” Ann. Intern Med. 138, 168–175 (2003). [DOI] [PubMed] [Google Scholar]
- Buist D. S. M. et al. , “Factors contributing to mammography failure in women aged 40–49 years,” J. Natl. Cancer Inst. 96, 1432–1440 (2004). 10.1093/jnci/djh269 [DOI] [PubMed] [Google Scholar]
- Kuhl C. K. et al. , “Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer,” J. Clin. Oncol. 23, 8469–8476 (2005). 10.1200/JCO.2004.00.4960 [DOI] [PubMed] [Google Scholar]
- Calonge N., Petitti D. B., and the U.S. Preventive Services Task Force, “Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement,” Ann. Intern Med. 151, 716–726 (2009). [DOI] [PubMed] [Google Scholar]
- Elmore J. G., Amstrong K., Lehman C. D., and Fletcher S. W., “Screening for breast cancer,” JAMA, J. Am. Med. Assoc. 293, 1245–1256 (2005). 10.1001/jama.293.10.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg W. A., Blume J. D., Cormack J. B., and ACRIN 6666 Investigators, “Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer,” JAMA, J. Am. Med. Assoc. 299, 2151–2163 (2008). 10.1001/jama.299.18.2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg W. A., Blume J. D., and Adams A. M., “Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666,” Radiology 254, 79–87 (2010). 10.1148/radiol.2541090953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg W. A. et al. , “High resolution fluorodeoxyglucose positron emission tomography with compression (‘positron emission mammography’) is highly accurate in depicting primary breast cancer,” Breast J. 12, 309–323 (2006). 10.1111/j.1075-122X.2006.00269.x [DOI] [PubMed] [Google Scholar]
- Brem R. F. et al. , “Occult breast cancer: Scintimammography with high-resolution breast-specific gamma camera in women at high risk for breast cancer,” Radiology 237, 274–280 (2005). 10.1148/radiol.2371040758 [DOI] [PubMed] [Google Scholar]
- O’Connor M. K., Rhodes D., and Hruska C., “Molecular breast imaging,” Expert Rev. Anticancer Ther. 9, 1073–1080 (2009). 10.1586/era.09.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. J., Hruska C. B., Phillips S. W., Whaley D. H., and O’Connor M. K., “Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts,” Radiology (in press). [DOI] [PubMed]
- National Research Council, BEIR VII: Health Risks from Exposure to Low Levels of Ionizing Radiation (National Academies Press, Washington, DC, 2006). [PubMed] [Google Scholar]
- International Commission on Radiation Protection, ICRP Publication 99: Low-Dose Extrapolation of Radiation Related Cancer Risk (Elsevier, Amsterdam, 2006). [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation, Sources and Effects of Ionizing Radiation: UNSCEAR 2006 Report to the General Assembly, with Scientific Annexes (United Nations, New York, 2006). [Google Scholar]
- Law J. and Faulkner K., “Cancers detected and induced, and associated risk and benefit, in a breast screening program,” Br. J. Radiol. 74, 1121–1127 (2001). [DOI] [PubMed] [Google Scholar]
- Preston D. L., Mattsson A., Holmberg E., Shore R. E., Hildreth N. G., and Boice J. D., “Radiation effects on breast cancer risk: A pooled analysis of eight cohorts,” Radiat. Res. 158, 220–235 (2002). 10.1667/0033-7587(2002)158[0220:REOBCR]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Law J., Faulkner K., and Young K. C., “Risk factors for induction of breast cancer by x-rays and their implications for breast screening,” Br. J. Radiol. 80, 261–266 (2007). 10.1259/bjr/20496795 [DOI] [PubMed] [Google Scholar]
- Vaeth M. and Pierce D. A., “Calculating excess lifetime risk in relative risk models,” Environ. Health Perspect. 87, 83–94 (1990). 10.2307/3431010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Program of Cancer Registries, United States Cancer Statistics: 1999–2006 Cancer Incidence and Mortality Data. See http://apps.nccd.cdc.gov/uscs/.
- Human Mortality Database, University of California, Berkeley, USA and Max Planck Institute for Demographic Research, Germany. Available at www.mortality.org or www.humanmortality.de.
- Hendrick R. E. et al. , “Comparison of acquisition parameters and breast dose in digital mammography and screen-film mammography in the American College of Radiology imaging network digital mammographic imaging screening trial,” AJR, Am. J. Roentgenol. 194, 362–369 (2010). 10.2214/AJR.08.2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin D. J., “Recalculated dose data for 19 frequently used radiopharmaceuticals from ICRP Publication 53,” Ann. ICRP 28, 47–83 (1998). 10.1016/S0146-6453(99)00009-3 [DOI] [Google Scholar]
- Hays M. T., Watson E. E., Thomas S. R., and Stabin M., “MIRD Dose Estimate Report No. 19: Radiation absorbed dose estimates from (18)F-FDG,” J. Nucl. Med. 43, 210–214 (2002). [PubMed] [Google Scholar]
- O’Connor M. K., Hruska C. B., Weinmann A. L., Manduca A., and Rhodes D. J., “Development of radiation dose reduction techniques for cadmium zinc telluride detectors in molecular breast imaging,” Proc. SPIE (in press).
- NCRP, “Ionizing radiation exposure of the population of the United States National Council on Radiation Protection and Measurements,” NCRP Report No. 160 (Bethesda, MD, 2009).
- Miller J. W., King J. B., Ryerson A. B., Eheman C. R., and White M. C., “Mammography use from 2000 to 2006: State-level trends with corresponding breast cancer incidence rates,” AJR, Am. J. Roentgenol. 192, 352–360 (2009). 10.2214/AJR.08.1757 [DOI] [PubMed] [Google Scholar]
- Schopper D. and de Wolf C., “How effective are breast cancer screening programmes by mammography? Review of the current evidence,” Eur. J. Cancer 45, 1916–1923 (2009). 10.1016/j.ejca.2009.03.022 [DOI] [PubMed] [Google Scholar]
- Nelson H. D., Tyne K., Nalk A., Bougatsos K., Chan B. K., and Humphrey L., “Screening for breast cancer: An update for the U.S. Preventive Services Task Force,” Ann. Intern Med. 151, 727–737 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon C. M. et al. , “Mammographic density, breast cancer risk and risk prediction,” Breast Cancer Res. 9, 217–226 (2007). 10.1186/bcr1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S. L., Sigal B. M., and Plevritis S. K., “A simulation model investigating the impact of tumor volume doubling time and mammographic tumor detectability on screening outcomes in women aged 40–49 years,” J. Natl. Cancer Inst. 102, 1263–1271 (2010). 10.1093/jnci/djq271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redberg R. F., “Cancer risks and radiation exposure from computed tomographic scans. How can we be sure that the benefits outweigh the risks?,” Arch. Intern Med. 169, 2049–2050 (2009). 10.1001/archinternmed.2009.453 [DOI] [PubMed] [Google Scholar]
- Brenner D. J. et al. , “Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know,” Proc. Natl. Acad. Sci. U.S.A. 100, 13761–13766 (2003). 10.1073/pnas.2235592100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N. and Muirhead C. R., “Review of relative biological effectiveness dependence on linear energy transfer for low-LET radiations,” J. Radiol. Prot. 29, 5–21 (2009). 10.1088/0952-4746/29/1/R01 [DOI] [PubMed] [Google Scholar]
- Brenner D. J. et al. , “Routine screening mammography: How important is the radiation-risk side of the benefit-risk equation?,” Int. J. Radiat. Biol. 78, 1065–1067 (2002). 10.1080/0955300021000016576 [DOI] [PubMed] [Google Scholar]
- Law J., Faulkner K., and Young K. C., “RBE for mammographic x-ray energies,” Br. J. Radiol. 79, 851–852 (2006). 10.1259/bjr/55332466 [DOI] [PubMed] [Google Scholar]
- Hruska C. B., Phillips S. W., Rhodes D. J., and O’Connor M. K., “Concordance of molecular breast imaging and breast MRI findings: A retrospective review,” Eur. J. Nucl. Med. Mol. Imaging 35, S199–S200 (2008). 10.1007/s00259-008-0906-y [DOI] [Google Scholar]
- Brem R. F., Petrovitch I., Rapelyea J. A., Young H., Teal C., and Kelly T., “Breast-specific gamma imaging with 99mTc-sestamibi and magnetic resonance imaging in the diagnosis of breast cancer—A comparative study,” Breast J. 13, 465–469 (2007). 10.1111/j.1524-4741.2007.00466.x [DOI] [PubMed] [Google Scholar]
- Chiu S. Y., Duffy S., Yen A. M., Tabar L., Smith R. A., and Chen H. H., “Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening,” Cancer Epidemiol. Biomarkers Prev. 19, 1219–1228 (2010). 10.1158/1055-9965.EPI-09-1028 [DOI] [PubMed] [Google Scholar]