Abstract
When Camillo Golgi invented the black reaction in 1873 and first described the fine anatomical structure of the nervous system, he described a ‘big nerve cell’ that later took his name, the Golgi cell of cerebellum (‘Golgi'schen Zellen’, Gustaf Retzius, 1892). The Golgi cell was then proposed as the prototype of type-II interneurons, which form complex connections and exert their actions exclusively within the local network. Santiago Ramón y Cajal (who received the Nobel Prize with Golgi in 1906) proceeded to a detailed description of Golgi cell morphological characteristics, but functional insight remained very limited for many years. The first rediscovery happened in the 1960s, when neurophysiological analysis in vivo revealed that Golgi cells are inhibitory interneurons. This finding promoted the development of two major cerebellar theories, the ‘beam theory’ of John Eccles and the ‘motor learning theory’ of David Marr, in which the Golgi cells regulate the spatial organisation and the gain of input signals to be processed and learned by the cerebellar circuit. However, the matter was not set and a series of pioneering observations using single unit recordings and electron microscopy raised new issues that could not be fully explored until the 1990s. Then, the advent of new electrophysiological and imaging techniques in vitro and in vivo demonstrated the cellular and network activities of these neurons. Now we know that Golgi cells, through complex systems of chemical and electrical synapses, effectively control the spatio-temporal organisation of cerebellar responses. The Golgi cells regulate the timing and number of spikes emitted by granule cells and coordinate their coherent activity. Moreover, the Golgi cells regulate the induction of long-term synaptic plasticity along the mossy fibre pathway. Eventually, the Golgi cells transform the granular layer of cerebellum into an adaptable spatio-temporal filter capable of performing several kinds of logical operation. After more than a century, Golgi's intuition that the Golgi cell had to generate under a new perspective complex ensemble effects at the network level has finally been demonstrated.
Discovery of Golgi cells
On 16 February 1873 a relatively unknown physician wrote to a friend these words: ‘I spend long hours at the microscope. I am delighted that I have found a new reaction to demonstrate even to the blind the structure of the interstitial stroma of the cerebral cortex. I let the silver nitrate react with the pieces of brain hardened in potassium dichromate. I have obtained magnificent results and hope to do even better.’ (Mazzarello, 2010). This was the first known recording of the invention of the ‘black reaction’, a real breakthrough for brain structure research. The author of this letter was Camillo Golgi (1843–1926; Fig. 1) and for this invention he won the Nobel Prize for Physiology and Medicine in 1906. At the time of this contribution, Golgi was physician in charge in a hospital for chronic patients at Abbiategrasso, 30 km from Pavia, in the north of Italy. He afterwards became professor of General Pathology and Histology at the University of Pavia. Using his extraordinary method, Golgi undertook a systematic scientific exploration of the complex nervous system architecture, starting his ambitious scientific adventure with the study of the cerebellum.
Figure 1.
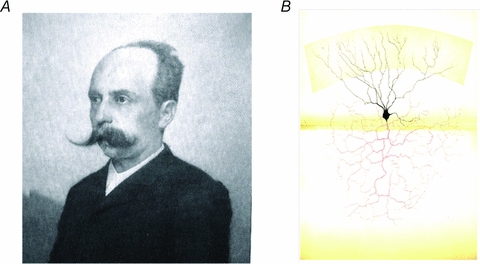
Camillo Golgi, the black reaction and the Golgi cell A, Camillo Golgi, Nobel Prize winner for Phsyiology and Medicine (1906). Camillo Golgi (1843–1926) invented the ‘black reaction’, which allowed him and many other scientists to visualise the fine structure of the nervous system. B, in this picture, Golgi shows a reconstruction of a Golgi cell. Note the precise description of the basal and apical dendrites and of the large axonal plexus, the hallmark of the Golgi cell. (Gangliar cell in the (neonatal) cat cerebellar cortex; Table XIV, Opera Omnia, Golgi, 1903.) ‘Such cells are part of the granular layer and, in the cat and rabbit, when the black reaction succeeds, can be seen in a considerable amount. … Most ramifications of the protoplasmatic prolongations (in black) reach the superior border of the molecular layer. … The nervous prolongation (in red), with repeated subdivisions becoming finer and finer, creates an extremely complicated interlacement of fibres. These, in the vertical plane, spread from one to the other border of the granular layer, and in the width of the granular layer mix up with the interlacements resulting from subdivisions of neighbouring cells of the same type (see Table XVII). This cell is one of the most remarkable specimens among those that are described as second type cells in the text. Regarding the cerebellum, such a cell should be to compared to the one represented in Table XV (a Purkinje cell), representing one of the most remarkable specimens of the first type of cells.’ Translated from Opera Omnia (Golgi, 1903).
The Golgi cell in the first description by Camillo Golgi
In 1874 Camillo Golgi published the work ‘On the fine anatomy of human cerebellum’ (Golgi, 1874), in which he described the Purkinje cells’ dendritic tree and the whole cerebellar cytoarchitecture. Among the various cell types, Golgi described two kinds of ‘big nerve cells’ in the granular layer:
… always in the granular layer, with the silver nitrate method, I verified not only the beautiful characteristics of the nerve cell that lay here (characteristics that somebody denies), but I also underlined their exact dimensions, shapes, dispositions and ramifications …
Speaking about their shape, they are really various, but we can roughly distinguish two main groups, that is: 1. long and narrow cells irregularly fusiform, with the maximum diameter parallel to circumvolution's surface; 2. irregularly round or polygonal cells, with round corners, quite laterally flattened, similar to some others lying in the deep layers of cerebral cortex, with the maximum diameter transversally placed in respect to the direction of granular layer, and so perpendicular to the circumvolution's surface. Both these types have a large number of prolongations (extensions) …
More than once from a single nervous prolongation of one of these cells, I saw the formation, cause of the fine and repeated subdivisions in all directions, of a complicated filament's interlacement, going from the periphery of the granular layer in both lateral directions, widespread for more than 200 μm.
For what concerns their position, we can say that these gangliar cells are present at the same level at the periphery of the granular layer, close to Purkinje cells, in the middle of the layer, and also in the deepest parts; sometime it is possible to see some of them in the medullary rays, where the nervous fibres are still parallel or they have just started diverging.
In this description, it is probable that the first type of cell was the Lugaro cells (named after Ernesto Lugaro, 1870–1940, who provided a detailed description of them). Moreover, there are no doubts that the second cell type is the one that now bears the name of Golgi, i.e. the ‘Golgi cell’ (Figs 1–3). Even today, the most relevant criterion to recognise the Golgi cell is the presence of its impressive axonal plexus (Dieudonné, 1998; Forti et al. 2006), so well highlighted by Golgi himself.
Figure 3.

The relationship between Golgi cells and granule cells Granule cells largely exceed in number the Golgi cells and are much smaller (Purkinje cells are distinctly shown in the background). Therefore a Golgi cell can innervate several granule cells lying within their axonal plexus, capturing another fundamental feature of the granular layer organisation. (Fragment of a rabbit cerebellar convolution (vertical section); Table XIX, Opera Omnia, Golgi, 1903). ‘This drawing was specifically made to illustrate the granular layer. … The so-called granule cells look like nervous cells with a globose shape, really small and equipped with 3, 4, 5 or even 6 prolongations, among which just one has the features of nervous prolongation (the nervous prolongation is just outlined, red thread). Prolongations, which it seems to be correct to name protoplasmatic, even if they appear slightly different from other gangliar cells’ prolongations, end up with a small granulous mass, towards which neighbouring granule cells’ prolongation often converge. In the region in which the granular layer merges into the molecular layer, two large cells are drawn. These are placed laterally and differ from Purkinje cells for the cell body shape, for the way of branching of their protoplasmatic prolongations and, overall, for the very different organisation of the nervous prolongation. … These two large cells are of the same type of the ones already illustrated in Tables XIV and XVII.’ Translated from Opera Omnia (Golgi, 1903).
Golgi's functional hypothesis on the Golgi cell
After this first hint as to the cerebellar structure, Golgi developed a systematic investigation on the whole central nervous system, which he collected in 1885 in a comprehensive work with the title On the Fine Anatomy of the Central Nervous System Organs (Golgi, 1885). In the chapter of this book dedicated to the cerebellum, Golgi resumed his previous detailed morphological description and went further in considering the course of the cytoplasmatic prolongations of the various cerebellar cell types hypothesising their functional role.
It seems obvious to me to consider the cells whose prolongations go directly to form a nervous fibre, as organs with a direct influence on peripheral parts; they would likely be organs connected with motor activity. The other cells, about which I am sure to exclude a direct connection with the fibres that go from the periphery to the centre, seem to me organs connected to sensory activity, or even with automatic actions. In the filaments emanating from the nervous prolongations of this second category of cells, it is easy to recognise a central communication way between the distinct categories of nervous elements.
Golgi noticed that Purkinje cell axons project out of the cerebellum (these fibres form indeed the sole cerebellar cortex output), so he hypothesised for these cells a motor function. He did not find a similar extra-cerebellum projection from granule cells, so he ascribed them to the sensory or ‘automatic’ function: modern evidence has backed up his deduction, because it is in the granular layer that mossy fibres present inputs to the cerebellar circuit, while Purkinje cells provide the output and can influence movement (e.g. see Bower, 1997). Moreover Golgi tried to give a role even to the big nervous cells: thinking about the fact that their impressive axonal plexuses do not go out of the cerebellum, he guessed that these cells (actually the Golgi cells) are connectional elements in the network. Camillo Golgi also hinted at a possible relationship of the Golgi cells with a structure (‘nucleo granuloso’) later named glomerulus (see below). In his Nobel Prize lecture, while classifying the first and second nerve cell types, Golgi presented a picture of the Golgi cell considering it as ‘one of the most characteristic examples of the way in which the nerve process of the second type of cells [i.e. those with short local prolongations] behaves’ (Golgi, 1967).
Golgi cells and the cerebellar network according to Santiago Ramón y Cajal
Following the introduction of the black reaction other histologists – namely the Italian Romeo Fusari, the Swiss Albrecht von Kölliker, the Belgian Arthur van Gehuchten and especially the Swede Gustaf Retzius – observed the Golgi cells of the cerebellum (Fusari, 1883; Van Gehuchten, 1891; Retzius, 1892). Retzius, in particular, published very clear and detailed drawings of these cells and described them in a paragraph of a work devoted to the histology of the nervous system. Retzius in 1892 introduced the terminology ‘Golgi'schen Zellen’ (Golgi cells) to indicate this type of neuron. But the most extended description of the Golgi cells came with the great Spanish histologist Santiago Ramón y Cajal, who shared the Nobel Prize with Camillo Golgi in 1906.
Ramón y Cajal was one of the first and main users of the black reaction. By applying this new method, he proceeded through a systematic investigation of nervous elements. This work resulted in a fascinating and exhaustive treatment of the ‘fine anatomy’ of central nervous structures: the cerebellum, of course, was included. Ramón y Cajal dedicated some works (Ramón y Cajal, 1888, 1889a,b) and an entire chapter of his Histology of the Nervous System of Man and Vertebrates (first edition in Spanish 1899, 1904; French edition 1909, 1911; English edition, 1995) to the cerebellar granular layer, in which he identified four main components: granule cells (small and abundant), cytoplasmatic eosinophil islands (glomeruli), poor glial cells, and large neurons different from granule cells. Among the ‘large cells’ group, he identified subgroups: stellate cells (ordinary or with a long axon) that he named Golgi cells following Retzius's terminology, horizontal fusiform cells (which are quite certainly Lugaro cells), and displaced stellate cells (a heterogeneous group still today not well characterised, and identified as ‘non-conventional large interneurons’; in this group we can include unipolar brush cells, synarmotic neurons, candelabrum neurons and perivascular neurons, besides Lugaro cells; Ambrosi et al. 2007).
Ramón y Cajal proceeded with a detailed description of Golgi cells’ morphological characteristics, looking at soma, dendrites and axon, and confirming what Camillo Golgi had observed before. By considering localisation and soma, he identified them as big stellate or polygonal neurons, present everywhere in the granular layer but more abundant in the region close to Purkinje cells (compared to which they are smaller and more stellate). Golgi cells have a big nucleus, pale and eccentric, with a big spherical nucleolus; their cytoplasm is abundant and contains scarce and small Nissl bodies. Dendrites depart from the soma in any direction (determining the stellate shape) and some, as Retzius pointed out, reach the plexiform or molecular layer with spines that contact granule cells’ axons. The dendritic tree is not placed on a unique plane as for Purkinje cells but, as precisely described by Golgi (Golgi, 1874, 1885, 1903) and then confirmed by Kölliker, van Gehuchten, Retzius and Ramón y Cajal (Kölliker, 1890, 1891, 1896; van Gehuchten, 1891, 1893; Retzius, 1892; Ramón y Cajal, 1888, 1889a,b, 1899, 1904, 1909, 1911, 1995), it is displaced, ascending and disorganised. The axon of these cells is peculiar: thick and almost similar to a dendrite, it has an initial bifurcation and a large number of collaterals, forming a real axonal plexus, with varicose and hook-like endings contacting an enormous number of granule cells inside glomeruli. Depending on the position of the Golgi cell in the granular layer, its axon can be descending (cell close to Purkinje cells), tangential (cell close to white matter) or going in any direction (cell in the middle of the granular layer); the axon length and the arborisation degree of the axonal plexus are markers used to distinguish different subtypes of Golgi cells.
It is very interesting to focus on Ramón y Cajal's description of cerebellar glomeruli, because it is almost the same as what we are now able to produce with much more powerful histological techniques. He described the glomeruli as vacuolar dendritic cytoplasmatic islands, without nuclei, underlining that they are not cells. Glomeruli, Ramón y Cajal continued, contain granule cells’ dendrites, Golgi cells’ axonal collaterals and mossy fibres’ rosettes. In summary, the cerebellar glomerulus is a structure in which mossy fibres and Golgi cells are able to contact and influence a huge number of granule cells. Ramón y Cajal's account, extraordinarily detailed, continues with the description of the other large neurons present in the granular layer and with the comparative and developmental histology of the cerebellum. What emerges from his work is a clear interest in cerebellar neurons, particularly for the small granule cells and for the large stellate cells discovered by Camillo Golgi.
From the sixties to the nineties: first functional descriptions of Golgi cells
In the course of the first 60 years of the 20th century the Golgi cells were usually mentioned only in the anatomical handbooks, without adding new inferences about their putative physiological function. Their first rediscovery happened in the 1960s, thanks to improved histological and functional measurements leading to new hypotheses on the cerebellar circuit. The basic design of the cerebellar cortex and, inside it, of Golgi cell anatomical and functional connectivity was defined (Palay & Chan-Palay, 1974). Golgi cells were shown to receive excitatory inputs from mossy fibres and parallel fibres (the granule cell axons) and to give inhibitory outputs to granule cells into the glomeruli. Each granule cell receives three to four inhibitory synapses on different dendrites (Hámori & Somogyi, 1983; Jakab & Hàmori, 1988). The Golgi cell–granule cell synapses consist of small boutons located proximally to the granule cell dendritic endings, which, in turn, receive the excitatory mossy fibre terminals. Both the mossy fibre and Golgi cell terminals, together with several tens of granule cell dendrites (see Palkovits et al. 1971; Ito, 1984), are included in the cerebellar glomerulus. Observations that are not often mentioned are that the glomerulus includes Golgi cell basal dendrites (Hámori & Szentágothai, 1966) and that the climbing fibres send collaterals to the Golgi cell (Scheibel & Scheibel, 1954). Another surprising observation that was predictive of most recent discoveries on temporal coherence in the inhibitory cerebellar network (see below) was that electrotonic coupling occurred among basket cells and Golgi cells (Sotelo & Llinás, 1972).
A breakthrough in Golgi cell function happened in 1964, when R. Llinas and S. Sasaki in Sir J. C. Eccles's laboratory discovered the inhibitory nature of the Golgi cell (Eccles et al. 1964). This was in fact the first example of inhibitory feedback to be assigned to a particular neuronal element. Feedback inhibition of motoneurons, known as Renshaw cell inhibition (Renshaw, 1946), was originally described 25 years before the actual cell type responsible was identified anatomically (Jankowska & Lindstrom, 1971). With the subsequent elaboration of the so called ‘beam theory’, Eccles proposed an interpretation of Golgi cell's function (summarised in Eccles et al. 1967): the Golgi cell was considered to inhibit granule cells through two mechanisms, feedforward and feedback. By causing a strong inhibition in granule cells close to the core of mossy fibre activity, Golgi cells would improve the spatial discrimination of the inputs that reach the cerebellar cortex. However, this theory lacked some critical elements. Firstly, it was uniquely based on anatomy and disregarded circuit dynamics. Secondly, beam formation was uniquely attributed to lateral inhibition generated by basket stellate inhibitory innervations on Purkinje cells and had nothing to do with the Golgi cell–granule cell circuit. Thirdly, it made the assumption that Golgi cells generated a random inhibitory feedback. These are clearly three oversimplifications that have subsequently been reconsidered leading to re-evaluation of the connectivity and functional role of Golgi cells and of the entire cerebellar circuit (see below).
David Marr, one of the fathers of neurocomputation, in 1969 proposed his own theory to explain cerebellar functioning, which is known as the ‘motor-learning theory’. He defined, on a theoretical basis, a computational role for Golgi cells. According to Marr, Purkinje cells are liable for the learning of motor patterns carried by mossy and climbing fibres to the granular layer. The number of patterns that can be learned at the Purkinje cell level decreases with the increase of the amount of active parallel fibres per input. It is necessary for one element of the circuit to act as regulator of the codon size, which represents the number of granule cells activated by a beam of mossy fibres. The Golgi cell is in an ideal position to play this role. Thus, Marr predicted that Golgi cells would be able to regulate granular layer excitability, and so the amount of information that can be elaborated, transmitted and learned.
Although the beam and motor learning theories were quite attractive and seemed to provide a comprehensive explanation for the whole cerebellum and for the Golgi cell inside it, the investigation of cellular dynamics and of detailed connectivity patterns moved ahead leading to new results mining the plausibility of the two theories. The contrast between the simplified Golgi cell connectivity proposed by the two theories and the morphological observations on the cerebellar glomerulus was striking. The very specific gating nature of the inhibitory feedback was investigated (Precht & Llinás, 1969) showing that the activity of a given granule cell–Purkinje cell set was more powerfully inhibited by homonymous activation of mossy fibres (in that case from the vestibular system) than from mossy fibres innervating the same granule cells from the contralateral vestibular nerve. This indicated that Golgi cell inhibition was probably glomerulus specific and not a random inhibitory feedback, as originally supposed. Moreover, as explained, potentially important observations like those on electrotonic coupling and on the fine structure of glomerular organisation were not considered. These observations, which address the real nature of the issue, have found their continuation in the single cell recordings and detailed computational models developed in the last two decades (see below).
Golgi cells: the state of the art
A new trend in Golgi cell investigations arrived in the 1990s, when cerebellar neurons began to be recorded in acute slice preparations using the patch-clamp recording technique. Moreover, single unit Golgi cell activities were recorded in vivo, and the connectivity of these neurons has been revisited and their histochemical and functional properties redefined (Figs 4–8) generating the current view of Golgi cell connectivity and function. At the same time, the interest for brain dynamics has risen (Buzsàky, 2006) and the cerebellum has been subjected to intensive investigations aimed at understanding its involvement in timing and sensory expectation (Spencer et al. 2007) involving both the spheres of sensory motor programming and cognition (Ivry & Spencer, 2004).
Figure 4.
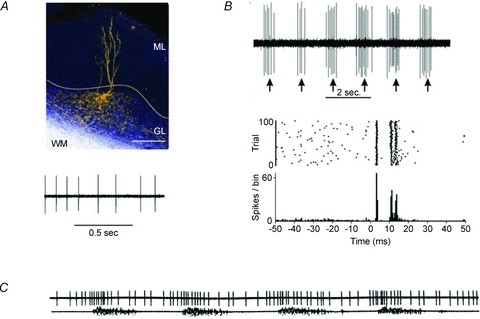
Golgi cell activity in vivo A, the Golgi cell shows rhythmic background activity in vivo (from Holtzman et al. 2006a). B, peripheral sensory stimulation elicits bursts of activity (from Holtzman et al. 2006a). Each burst is usually composed of 2–3 spikes and is followed by a long-lasting inhibitory period (or silent pause in Vos et al. 1999). C, during locomotion, the Golgi cell is entrained into repetitive activity cycles, during which its frequency is modulated.
Figure 8.
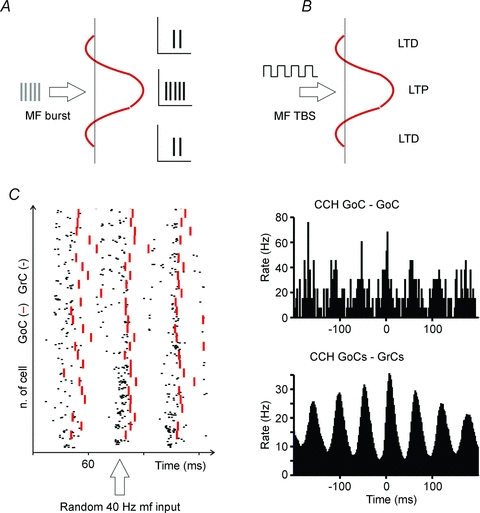
The physiological consequences of Golgi cell activation Golgi cell activation has complex consequences for granular layer responses. A, a burst in a group of mossy fibres (MF) generates stronger and faster granule cell responses in the centre than in the surround of a granular layer field (the red profile represents the excitatory/inhibitory balance in a granular layer field activated by a group of MFs and regulated by Golgi cells; drawn after Mapelli & D’Angelo, 2007). B, a MF theta-burst stimulation (TBS) in a group of MFs generates more effectively LTP in the centre and LTD in the surround of the granular layer field. C, the raster plot shows that a diffused random stimulation of the MFs generates a coherent response of the granule cells (GrC) and of the Golgi cells (GoC). Synchronisation is due to parallel fibre feed-back inhibition. Crosscorrelograms (CCH) are shown for two GoCs and for all the granule cells and the Golgi cells in the network revealing their coherence. Data elaborated from the model of Solinas et al. (2010).
Backing-up Golgi's predictions on local Golgi cell connectivity
Although the basic morphology identified by Camillo Golgi is still accepted (‘… irregularly round or polygonal cells, with round corners, quite laterally flattened …’; Golgi (1874)), several major advances have been made. In a quantitative description recently reported by Barmack & Yakhnitsa (2008), Golgi cells in vivo showed a relatively large (10–20 μm) soma. The axonal plexus extended with a parasagittal organisation, branching within the granule cell layer for ∼650 μm sagittally and for ∼180 μm medio-laterally. The dendrites showed variable morphology and often two to four dendrites emerged as thin processes from a single point in the soma and terminated with several varicosities.
The fact that Golgi cells may be heterogeneous (‘… Speaking about their shape, they are really various … For what concerns their position, we can say that these gangliar cells are present at the same level at the periphery of the granular layer, in the middle of the layer, and also in the deepest parts.’Golgi (1874)) has also been revisited. Five distinct subpopulations of Golgi cells were distinguished by Simat and colleagues (2007) based on neurochemical phenotype, and cell shape, size and location in the granular cell layer. The majority of Golgi cells are in fact both GABAergic and glycinergic (80%), some are specifically GABAergic (15%), and some are specifically glicinergic (5%). Whereas, in general, granule cell inhibition is only GABAergic, in the vestibulo-cerebellum Golgi cells inhibit the granule cells by releasing GABA and the unipolar brush cells (UBCs) by releasing glycine. Moreover, Geurts et al. (2001, 2003) found various expressions of certain biochemical markers (rat-303, calretinin, mGluR2, somatostatin). The Golgi cells may thus contribute to distinct cerebellar subcircuits, although no remarkable differences in their intrinsic excitability have emerged (Forti et al. 2006).
It is important to note that Golgi postulated a local connectivity (Golgi, 1874), which has been subsequently redefined and analysed in detail (Eccles et al. 1967; Palkovits et al. 1971; Palay & Chan-Palay, 1974; Ito, 1984). Functional studies (Figs 4–6) have shown that Golgi cell activity can be influenced both by mossy fibres, parallel fibres and climbing fibres and by molecular layer interneurons (Eccles et al. 1967; Vos et al. 1999a; Holtzman et al. 2006a, b, 2009; Xu and Edgley, 2008; Barmack & Yakhnitsa, 2008; Ros et al. 2009). Tactile punctuate stimulation readily activates the Golgi cells generating a first rapid spike response through sensory mossy fibres; a second spike is then conveyed through cortico-cerebellar pathways and further spikes are possibly conveyed through the parallel fibres. However, while a physiological analysis has been performed for the granule cell → Golgi cell (Dieudonné, 1998; Bureau et al. 2000), stellate/basket cell → Golgi cell (Dumoulin et al. 2001) and Lugaro cell → Golgi cell (Dieudonné & Dumoulin, 2000) connections, the nature of connections from mossy fibres and climbing fibres to Golgi cells remains largely to be determined. Recent results indicate that the mossy fibre → Golgi cell connection is glutamatergic and rapidly and efficiently excites the Golgi cell (Kanichay & Silver, 2008), but several issues remain unanswered. Do all mossy fibre synapses originate from glomerular connections? Do granule cells form their main connections with the Golgi cell through the parallel fibres or are there also en passant synapses along the ascending axon? Are the afferent synapses formed by mossy and parallel fibres functionally equivalent? Concerning the climbing fibre → Golgi cell connection (Scheibel & Scheibel, 1954; Shinoda et al. 2000), despite climbing fibres being excitatory on Purkinje cells they have an inhibitory effect on the Golgi cells. This can occur in at least two different ways (Xu & Edgley, 2008), (i) by activating the stellate cells, which in turn inhibit the Golgi cells, or (ii) by activating mGluR2 glutamate receptors on Golgi cell dendrites, which activate an inward rectifier current preventing depolarisation (Watanabe & Nakanishi, 2003). And what is the impact of the electrical junctions formed by Golgi cells (Sotelo & Llinás, 1972)?
Figure 6.
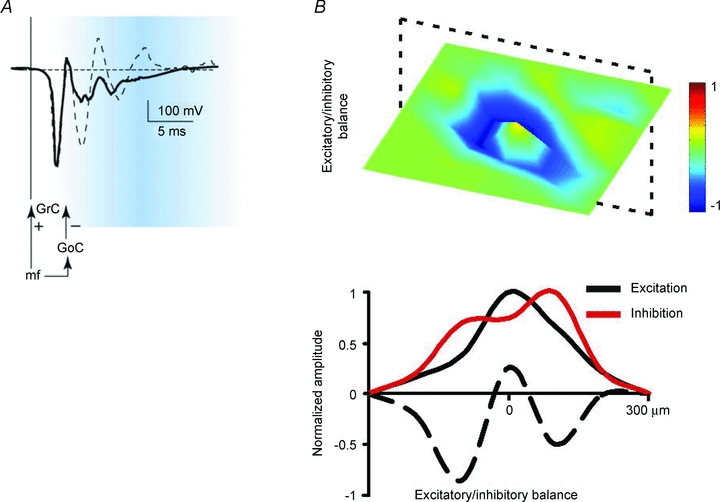
Control of granular layer spatio-temporal dynamics by Golgi cell inhibition A, stimulation of a mossy fibre beam elicits local field potentials in the granular layer, which can be composed by more spikes generated in sequence by granule cells. Activation of the feed-forward inhibitory Golgi cell loop limits spike emission (time-window effect; from Mapelli & D’Angelo, 2007). B, stimulation of a mossy fibre beam elicits local field potentials in the granular layer, which are surrounded by lateral inhibition generated by Golgi cells (centre–surround effect; from Mapelli & D’Angelo, 2007).
Clearly much remains to be done before a complete view of Golgi cell functional connectivity can be drawn. A more detailed explanation on functional synaptic connectivity and a survey on the receptors involved at the different synapses can be found below and in Appendix 2.
How local Golgi cell connectivity may determine function
Camillo Golgi understood that morphology and local connectivity had to reflect the function that a single nerve cell plays in the local network as well as in the economy of the whole nervous system (Golgi, 1967). We consider here six main observations connecting the structure of the Golgi cell to its function.
As observed in Figs 1–3 and 4A (see also Dieudonné, 1998; Forti et al. 2006; Barmack & Yakhnitsa, 2008), the Golgi cell axonal plexus extends exclusively in the granular layer and, through thin branches, can form secondary plexuses in the same or even in neighbouring laminae (e.g. see Eccles et al. 1967). The broader extension of the axon than the basal dendrites provides the basis for lateral inhibition, whose functional impact has recently been demonstrated by multi-electrode array recordings and voltage-sensitive dye (VSD) imaging (Mapelli & D’Angelo, 2007; Mapelli et al. 2009; Fig. 6).
In some of Golgi's original drawings the axonal fields of Golgi cells overlap (e.g. see Figs 2 and 3), although in subsequent drawings the Golgi cell axonal fields appear well isolated (e.g. see Eccles et al. 1967; Ramón y Cajal, 1995; Ito, 1984). This overlap would be important to allow the combinatorial inhibition of granule cells and has recently been observed using fluorescence staining in vivo (see Barmack & Yakhnitsa, 2008). Consistently, the analysis of Golgi cell–granule cell neurotransmission has recently shown that each granule cell receives inhibition from different Golgi cells, implying overlap of the axonal fields (Mapelli et al. 2009).
Figure 2.
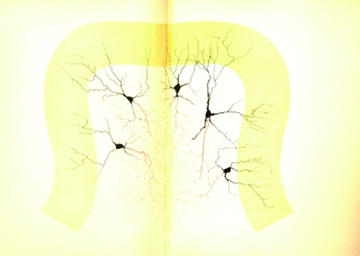
The spatial relationship between multiple Golgi cells Note that no restriction is imposed to axons, which spread and overlap into the granular layer, that apical dendrites ramify in the molecular layer without specific orientation, and that basal dendrites ramify in the granular layer over a surface smaller than that occupied by the axon. In this figure, the fundamental features of the Golgi cell are captured. (Fragment of a (neonatal) cat cerebellar convolution (vertical section); Table XVII, Opera Omnia, Golgi, 1903). ‘The drawing is specifically made to show shape, disposition, ramification laws, localisation and relationships of the large gangliar cells of the granular layer. Protoplasmatic prolongations branch dichotomously in a very different way compared to Pukinje cells. The most distal extensions of the branches often reach the molecular layer peripheral limit. Nervous prolongations, with their fine and repeated subdivisions, form a complicated interlacement with the result that it is impossible to follow the course of single prolongations. Such interlacement does not seem to have borders either toward the inside of the granular layer or toward the molecular layer. Thus, several of these interlacements obviously mix up to form a complicated plexus.’ Translated from Opera Omnia (Golgi, 1903).
In Golgi's drawings, the terminal endings of Golgi cell axons generate hand-like structures matching the size and separation distance of the glomeruli (Figs 1 and 2 and Golgi, 1967), a fact even more evident in subsequent drawings by Ramón y Cajal (1889a). The presence of robust GABA receptor-mediated spillover responses to Golgi cell axon stimulation, which indicates diffusion of GABA onto neighbouring dendrites (Rossi & Hamann, 1998), suggests that Golgi cell terminals can inhibit clusters of granule cells sending their dendrite in the same glomerulus (Mapelli et al. 2009).
A feature that was already evident in Golgi's drawings and clearly described by Ramón y Cajal is that, at variance from Purkinje cells, the structure of Golgi cell dendrites is not rigorously organised in a plane but rather is more irregular and three-dimensional. Thus, Golgi cells may not be suited to detect ordered time sequences transmitted through the parallel fibres (Braitenberg et al. 1997). These observations combined with recent electrophysiological and modelling data support the view that Golgi cells can both precisely respond to topographically organised inputs and perform an extended spatio-temporal integration of parallel fibre information modulating their basal activity state (Vos et al. 2000; De Schutter & Bjaalie, 2001; De Schutter, 2002).
Another fact is that, in Golgi's and Ramón y Cajal's drawings, the axonal plexus of Golgi cells is always shown in parasagittal sections. This implicates that the Golgi cell axon expands preferentially in the parasagittal plane, as subsequently confirmed by confocal imaging (Barmack & Yakhnitsa, 2008). The axonal organisation matches the parasagittal distribution of mossy fibre ramifications (Sultan, 2001). Moreover, the entire Golgi cells, comprising their axon and dendrites, are segregated into parasagittal compartments for zebrin-2, aldolase C, nitric oxide synthase (NOS) and other markers of granular layer neurons and Purkinje cells (Sillitoe et al. 2008). This observation is related to a major issue of cerebellar organisation, in which mossy fibre inputs coherently activate certain granular layer areas, certain sets of Purkinje cells and specific portions of the olivo-nuclear complex, thus forming structural and functional modules (Brown & Bower, 2001; Voogd et al. 2003; Pijpers et al. 2006; Apps & Hawkes, 2009). Therefore, it seems that Golgi cells, through mossy fibre (and potentially climbing fibre) inputs to their dendrites, are wired within microcircuits involving specific cortico-nuclear modules, while through their parallel fibre connections they can be interconnected with multiple modules.
Finally, the presence of gap junctions among the inhibitory interneurons (stellate, basket and Golgi cells) was early observed both within and across classes (Sotelo & Llinás, 1972). The role of this inhibitory syncytium, which may facilitate the synchronisation of vast areas of the cerebellar cortex, has only been recently addressed. The functionality of gap junctions between stellate cells (Mann-Metzer & Yarom, 2000) and between Golgi cells (Duguéet al. 2009) has been demonstrated using double-patch recordings, while low-resistance electrical communication between molecular layer interneurons and Golgi cells remains to be demonstrated. Clearly, low-resistance electrical connections between Golgi cells have the potential, together with other mechanisms, to enhance the local coherence of cerebellar activity.
Golgi cell physiology: dynamic properties and network entrainment
A completely new view on the Golgi cell has been opened by electrophysiological recordings in vitro and in vivo (Figs 4–6).
Electrophysiological patch-clamp recordings in vitro (see Appendix 2) have shown that the Golgi cell is a pacemaker neuron that fires autonomously at 1–10 Hz (Dieudonné, 1998; Forti et al. 2006; Fig. 7A). When perturbed, it shows discharge adaptation, post-inhibitory rebound and afterhyperpolarisation (Fig. 7A–B). In addition, Golgi cells are resonant around their oscillation frequency, making them suitable to enhance responses in the theta-frequency band (Solinas et al. 2007a,b; Fig. 7C). Rhythmic activity is also observed in vivo both in awake (cat: 2 to <50 Hz, Edgley & Lidierth, 1987; monkey: 10–80 Hz, Miles et al. 1980) and anaesthetised animals (rat: 2–30 Hz, Schulman & Bloom, 1981; Vos et al. 1999a; Holtzman et al. 2006a,b;), probably as a reflection of pacemaking modulated by synaptic activity. It has been recently proposed that electrical coupling between Golgi cells could be critical to allow the emergence of low-frequency pacemaking, at the same time synchronising oscillations in neighbouring Golgi cells (Fig. 5A; Duguéet al. 2009). Golgi cells show ‘loose synchrony’ over hundreds of micrometres along the coronal axis (Volny-Luraghi et al. 2002; Tahon et al. 2005), possibly reflecting synchronisation along the parallel fibre beam and feed-back inhibition onto granule cells (Vos et al. 1999b).
Figure 7.
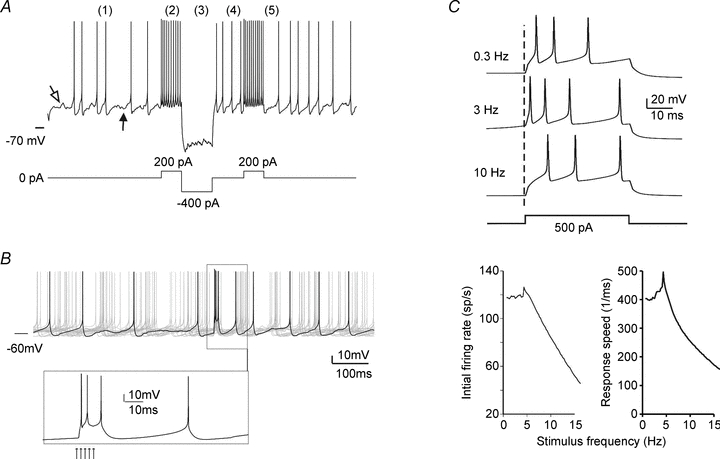
The electrical activity of Golgi cells The Golgi cell shows response dynamics, which can support the cycles of activation and inactivation observed in various functional conditions (see Figs 5 and 6). A, the Golgi cell in vitro shows (1) pacemaker activity at around 7 Hz. In response to depolarisation (2), the Golgi cell shows high-frequency discharge with frequency adaptation. In response to hyperpolarisation (3), the Golgi cell shows sagging inward rectification followed by (4) rebound excitation. Bursts of activity are followed by (5) a silent pause. B, demonstration of the silent pause following a burst response to a mossy fibre stimulus (arrows in the inset). C, when stimulated with pulses repeated at different frequencies, the Golgi cell shows enhanced responses (faster and higher frequency spikes) at the resonant frequency of 6 Hz. The tracings are simulated from the models of Solinas et al. (2007a,b, 2010) and reproduce response behaviours reported in Dieudonné (1988), Forti et al. (2006) and Solinas et al. (2007a,b);.
Figure 5.
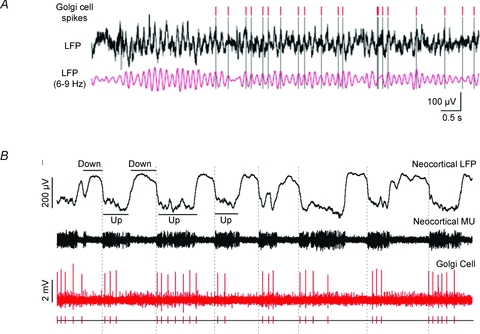
Golgi cell network entrainment A, the Golgi cell spikes are in phase with the local field potential of the granular layer (Duguéet al. 2009). B, Golgi cells can show rhythmic entrainment with the UP–DOWN states characterising neocortical activity (Ros et al. 2009). The different behaviour in A and B may reflect different functional states or simply the fact that the trace in A may be part of an UP state as shown in B.
The mossy fibre inputs occur in two main modalities, namely protracted frequency-modulated discharges and short high-frequency bursts (Chadderton et al. 2004; Kase et al. 1980; Van Kan et al. 1993; Jörntell & Ekerot, 2006; Rancz et al. 2007). Accordingly, the Golgi cells present two well defined response modalities. First, Golgi cells can follow peripheral signals in a continuous fashion modulating their frequency with the intensity of the stimulus (Miles et al. 1980; Edgley & Lidierth, 1987). Secondly, Golgi cells respond to punctuate stimulation with a short burst of spikes. The bursts occur very rapidly (in about 10 ms upon facial stimulation) and consist of one to three well timed spikes in short sequence (Fig. 4B; Vos et al. 1999a; Holtzman et al. 2006a). The first spike corresponds to the trigeminal input (trigemino-cerebellar mossy fibres), the second spike to sensory-motor cortical input (cortico-ponto-cerebellar mossy fibres), and the third one may reflect the parallel fibre input. Following the bursts, the Golgi cell generates a long-lasting inhibitory period (Fig. 4B; Holtzman et al. 2006a,b, 2009; Xu and Edgley 2008) or silent pause lasting for about 100 ms, probably reflecting both intrinsic membrane properties and synaptic inputs (Fig. 7A–B; Solinas et al. 2007a,b;).
The entrainment of Golgi cells into local and extracerebellar networks is observed in different cases (Figs 4 and 5). In states of restive attentiveness and active expectancy (Pellerin & Lamarre, 1997; Hartmann & Bower, 1998; Courtemanche et al. 2009), the granular layer shows rhythmic activity in the theta-frequency band. In the anaesthetised animal, Golgi cells can generate repeated bursts along with granule cells during the ‘up’ phase of the slow delta-frequency cortical oscillations (Ros et al. 2009). Golgi cells can contribute to tune the granular layer toward this slow frequency band through the feed-back inhibitory loop (Maex & De Schutter, 1998), their resonant properties (Figs 7 and 8; Solinas et al. 2007a, 2007b) and electrical synapses (Duguéet al. 2009).
In summary, the Golgi cell, by shifting from a rhythmic discharge to an event-drive state on behavioural demand (D’Angelo, 2008), can both entrain and be entrained in network oscillations demonstrating an intimate relationship with both local and long-range circuits (cf. Buzsaki, 2006).
Multiple inhibition mechanisms contribute to global network computation
The Golgi cells take part in granular layer processing through multiple inhibitory mechanisms as follows.
Golgi cells generate phasic and tonic inhibition (Crowley et al. 2009; Mapelli et al. 2009). This latter occurs through neurotransmitter spillover causing slow and diffused responses within the glomerulus (Brickley et al. 1996; Rossi & Hamann, 1998) and inhibiting numerous granule cells altogether.
Golgi cells organise granular layer responses in centre–surround exploiting lateral inhibition. The centre–surround organisation, once occurring over partially overlapping activation fields, generates combinatorial responses (Mapelli et al. 2009) resembling coincidence detection and spatial pattern separation predicted by the motor learning theory (Marr, 1969; Albus, 1971).
Golgi cells enhance coherent oscillations in the granular layer network. Oscillations occur through feedback inhibition and coherence emerges from the fact that Golgi cell inhibition extends over large granule cell fields. The intrinsic Golgi cell excitable properties, including pacemaking and resonance, may tune oscillations on the theta band complementing the theta-frequency resonance of granule cells (D’Angelo et al. 2001; Lombardo & D’Angelo, unpublished observations).
Golgi cells determine a time window through which granule cell spikes are allowed to pass in response to mossy fibre bursts. This occurs through feed-forward inhibition, which limits the duration and intensity of granule cell excitation (Kanichay & Silver, 2008; D’Angelo & De Zeeuw, 2009).
Golgi cells regulate the gain at the mossy fibre–granule cell relay during prolonged input bursts by exploiting tonic inhibition, which regulates granule input resistance and spike threshold (Mitchell & Silver, 2003).
Golgi cells, by regulating granule cell depolarisation through a shunting inhibition mechanism, control NMDA channel unblock and the induction of mossy fibre–granule cell long-term synaptic plasticity in response to repeated mossy fibre bursts (Mapelli & D’Angelo, 2007).
These properties suggest that Golgi cell inhibition can allow the granular layer to operate as an adaptable spatio-temporal filter (Dean et al. 2010) capable of regulating delay, controlling gain, enhancing contrast and combining multiple afferent input fields (D’Angelo & De Zeeuw, 2009). These mechanisms have been interpreted through mathematical models incorporating the main neurophysiological properties of Golgi cells (Solinas et al. 2010; Figs 7 and 8). And by being embedded into the granular layer, the Golgi cell operates as a hidden neuron in a multi-layered network that could provide extended computational capabilities to the whole cerebellum (Mapelli et al. 2009). How these mechanisms contribute to setting-up cerebellar computation as a whole remains a challenge for future research.
The discovery of Camillo Golgi: a retrospective view and future developments
One of the major issues raised by the Nobel Prize dissertations in Stockholm in 1906 concerned the complexity of brain structural organisation (Ramón y Cajal, 1967; Camillo Golgi, 1967; Appendix 1). Ramón y Cajal was undisputedly the paladin of the neuron theory according to which the nervous tissue is composed, like any other tissues, of single independent cells. On the other hand Camillo Golgi was impressed by the complexity of the brain and thought that it was not possible to account for brain performance by simple point-to-point connectivity. Based on the observations made with the black reaction, 15 years before Ramón y Cajal, he proposed a reticularist theory known as the ‘diffuse nervous net’. This states that the axonal prolongations of nerve cells were fused (or intimately interlaced) in a diffuse web along which the nervous impulse propagated (Kruger, 2007; Kruger & Otis, 2007; Mazzarello, 2007, 2010). The debate between neuronists and reticularists, which was at the origin of modern neuroscience, was a fundamental clash of ideas between the 18th and 19th century. In the end, Ramón y Cajal's neuronal theory of the nervous system triumphed and it is now considered the fundamental paradigm of brain organisation. Golgi, in turn, gave a wrong answer to a real question, i.e. the difficulty of conceptualising complexity with simple point-to-point connectivity. Golgi envisioned multiform dynamical and interacting fields of activity, much as modern neural network theory is predicting even if, of course, in a different way (Rieke et al. 1997; Buzsaki, 2006).
The Golgi cell provides an intriguing example of this duality in that, while maintaining well defined single neuron properties, this neuron can coordinate (and be entrained into) activity of large granular layer fields. The Golgi cell makes extensive connections with the rest of the cerebellar network, receiving inputs from both the mossy fibre and the climbing fibre system (comprising granule cells, stellate cells, basket cells, inferior olivary cells, Lugaro cells) and sending outputs to the granular layer (comprising both granule cells and UBCs). Moreover, the presence of gap-junctions has been proposed to form a Golgi cell electrical syncytium (Sotelo & Llinás, 1972; Duguéet al. 2009), raising a puzzling analogy with the ‘protoplasmic syncytium’ envisaged by Golgi. Eventually, the Golgi cells may be able to coordinate the activity of large granule cell fields with extracerebellar structures (like the thalamo-cortical system) on appropriate frequency bands and to contribute to rhytmicity and to the representation of time in the cerebellum and in cortico-cerebellar loops (Ivry & Spencer, 2004). Therefore, the Golgi cell fully represents a kind of nerve cell which revitalises, under a new light, the discussion on the relationship between single neurons and the networks they are part of.
Acknowledgments
We thank the Institute for Advanced Studies (IUSS) of Pavia, which has partially sponsored E.G., the PRIN2007 project to P.M. and the EU project SENSOPAC to E.D. We thank M. Quattrocelli, P. Bazzigaluppi, F. Hoebeek and Z. Gao for their helpful comments on the manuscript and S. Solinas for contributing to Figs 7 and 8.
Appendix 1 Nets versus nodes: the controversy between Golgi and Cajal
In the mid 1870s, when Golgi began his investigation, the prevailing morphological theory of the brain was that of a reticular dendritic net of ‘connective vessels’ extending and interlinking across the nervous tissue. Instead Camillo Golgi, supported by his observations with the black reaction, believed the axons to be interconnected in a net. However Golgi did not make any definitive statement on the precise mode of that connection: in some of his writings he tended to consider a direct fusion of axon prolongations, and in others he left open the possibility that groups of nerve cells did not form direct anastomoses but only an intimate functional web through the interlacing of the axons. Beside the exact histological intercellular connection, Golgi adhered to the physiological idea of diffuse interactions among nerve centres.
In 1886 there was a turning point in the conceptualisation of nervous system structure. The Swiss anatomist Wilhelm His theorised that the nerve cell body and its processes formed independent units. Another Swiss scholar, August Forel, reached similar conclusions in a paper published in 1887. The same year a Spanish psychiatrist, Luis Simarro Lacabra, returned to Madrid from Paris equipped with the latest literature and histological preparations, and there he was visited by Santiago Ramòn y Cajal, a young professor at the University of Valencia: Cajal observed the preparations made with the black reaction and they took his breath away. He soon began an extensive investigation of the nervous system using the Golgi method and, looking at the preparations with the new neuronistic ideas in mind, he soon agreed with the Swiss hypothesis and became ‘the champion of neurons’.
The emergence of ‘neuronism’ drove Golgi to identify even more deeply with ‘reticularism’, in favour of which he wrote a paper in 1891. On the other side, Cajal continued to produce evidence in favour of neuronism. This was the situation when, in 1906, the Karolinska Institute of Stockholm announced the Nobel Prize award to Golgi and Cajal. This seemed the great opportunity to end the scientific rivalries of the previous 16 years. Unfortunately, the confrontation between the two scientists became even worse after the Nobel Prize lecture, in which Golgi strongly attacked the neuronist theory. The Nobel lecture was a disaster for Golgi and, consequently, a triumph for Cajal.
Adapted from Mazzarello et al. (2006).
Appendix 2 The molecular level of organisation: Golgi cell channels and receptors
Far from what Golgi could imagine, the intricate connectivity and the excitable dynamics of the Golgi cell are supported by an even further complexity of ionic channels and receptors (for previous reviews see Farrant & Nusser, 2005; Geurts et al. 2003) providing mechanisms suitable for regulating circuit dynamics and homeostasis. Most relevant factors are the expression of specific receptor subtypes coupled to neurotransmitter spillover in the cerebellar glomerulus.
The main excitatory inputs to Golgi cells are glutamatergic, with AMPA and NMDA receptors at the mossy fibre–Golgi cell relay (Kanichay & Silver, 2006; L. Forti et al. unpublished observation) and AMPA, NMDA and kainate receptors at the parallel fibre–Golgi cell relay (Dieudonné, 1998; Bureau et al. 2000; Misra et al. 2000). Whether NMDA receptors contribute to regulate Golgi cell excitability or are involved in some forms of plasticity remains to be determined. The inhibitory inputs to Golgi cells are both GABAergic and glycinergic. GABAergic inputs are provided by stellate and basket cells (Dumoulin et al. 2001), while mixed GABAergic–glycinergic inputs are formed by the Lugaro cells (Dieudonné & Dumoulin, 2000; Dumoulin et al. 2001).
The main output from Golgi cells is GABAergic and inhibits the granule cells in the cerebellar glomeruli. The IPSCs consist of a fast and a slow component (Rossi et al. 2003) determined by differential receptor subtypes and localisation (Farrant & Nusser, 2005). Different combinations of α1 and α6 subunit-containing receptors with ancillary subunits regulate synaptic response kinetics conferring specific sensitivity to ambient GABA concentration and spillover in the glomerulus (Brickley et al. 1999, 2001; Tia et al. 1996; Nusser et al. 1998; Rossi & Hamann, 1998; Hadley & Amin, 2007). Thus, in addition to determining phasic inhibition, Golgi cells contribute to regulate the basal granule cell input conductance by maintaining a tonic GABA concentration level inside the glomerulus (Brickley et al. 1996; Chadderton et al. 2004). The tonic level of GABA, which is also regulated by non-vesicular release and by the rate of GABA reuptake in glial cells (Rossi et al. 2003) by binding high-affinity receptors (Tia et al. 1996), can control the gain of the mossy fibre–granule cell relay (Mitchell & Silver, 2003). Acetylcholine can increase non-vesicular GABA release from the Golgi cells contributing to set the ambient GABA level in the glomerulus and tonic inhibition of granule cells (Rossi et al. 2003; see below).
Although the main effects of Golgi cells on granule cells are mediated by GABA, Golgi cells also co-release glycine at their synaptic terminals (Duguéet al. 2005). Granule cells do not express glycine receptors, but it is attractive to speculate that glycine plays a role in regulating activation of granule cell NMDA receptors on their glycine binding site. Conversely, both GABA and glycine receptors are expressed in UBCs, in which Golgi cell activity generates mixed GABAergic–glycinergic responses (Duguéet al. 2005). Interestingly, serotonin activates the Lugaro cells, thereby regulating Golgi cell inhibition (Dieudonné & Dumoulin, 2000).
Metabotropic receptors play also an important role in the Golgi cell circuit. The mGluR2 receptors on Golgi cell dendrites enhance an inward rectifier K+ current reducing the response following intense granule cell–Golgi cell transmission (Watanabe & Nakanishi, 2003). Metabotropic receptors also sustain cross-talk between mossy fibre and Golgi cell terminals in the glomerulus: mGluR2 receptor activation on Golgi cell presynaptic terminals inhibits GABA release (Mitchell & Silver, 2000b), while GABAB receptor activation on mossy fibre terminals inhibits glutamate release (Mitchell & Silver, 2000b). Golgi cell functions are therefore deeply integrated with those of the cerebellar glomeruli, allowing fine tuning of their response dynamics depending on functional demand.
The mechanisms of Golgi cell electroresponsiveness have been summarised in a previous review (D’Angelo, 2008). A combination of electrophysiological, pharmacological and modelling experiments (Forti et al. 2006; Solinas et al. 2007a,b;) has revealed the basis of pacemaking, bursting, adaptation and rebound excitation and phase reset. A set of ionic channels, including the h-current, raises membrane potential in a critical region. Here, near-threshold oscillations are generated involving a persistent Na+ current, the M-current and an apamine-sensitive AHP (afterhyperpolarisation) K+ current. The same ionic mechanism allows fast phase reset after a perturbation. In response to a stimulus, the resurgent Na+ current favours generation of doublets, and then adaptation is generated by the M-current and AHP current. Finally, following a hyperpolarisation, the h-current and a low-threshold Ca2+ current cause rebound excitation. Although the investigation of specific ionic currents in Golgi cells is far from complete, present knowledge provides a coherent mechanism through which the Golgi cell simultaneously controls pacemaking and response elicited by depolarisation and hyperpolarisation.
References
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- Ambrosi G, Flace P, Lorusso L, Girolamo F, Rizzi A, Bosco L, Errede M, Virgintino D, Roncali L, Benagiano V. Non-traditional large neurons in the granular layer of the cerebellar cortex. Eur J Histochem. 2007;51(Suppl 1):59–64. [PubMed] [Google Scholar]
- Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–681. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J Neurosci. 2008;28:1140–1152. doi: 10.1523/JNEUROSCI.3942-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JM. Is the cerebellum sensory for motor's sake, or motor for sensory's sake: the view from the whiskers of a rat? Prog Brain Res. 1997;114:463–96. doi: 10.1016/s0079-6123(08)63381-6. [DOI] [PubMed] [Google Scholar]
- Braitenberg V, Heck D, Sultan F. The detection and generation of sequences as a key to cerebellar function. Experiments and theory. Behav Brain Sci. 1997;20:229–245. [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brown IE, Bower JM. Congruence of mossy fiber and climbing fiber tactile projections in the lateral hemispheres of the rat cerebellum. J Comp Neurol. 2001;429:59–70. doi: 10.1002/1096-9861(20000101)429:1<59::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bureau I, Dieudonné S, Coussen F, Mulle C. Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. Proc Natl Acad Sci U S A. 2000;97:6838–6843. doi: 10.1073/pnas.97.12.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsàky G. Rhythms of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- Chadderton P, Margrie TW, Häusser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Chabaud P, Lamarre Y. Synchronization in primate cerebellar granule cell layer local field potentials: basic anisotropy and dynamic changes during active expectancy. Front Cell Neurosci. 2009;3:6 doi: 10.3389/neuro.03.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Fioravante D, Regehr WG. Dynamics of fast and slow inhibition from cerebellar Golgi cells allow flexible control of synaptic integration. Neuron. 2009;63:843–53. doi: 10.1016/j.neuron.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo E, Nieus T, Maffei A, Armano S, Rossi P, Taglietti V, Fontana A, Naldi G. Theta-frequency bursting and resonance in cerebellar granule cells: experimental evidence and modeling of a slow K+-dependent mechanism. J Neurosci. 2001;21:759–770. doi: 10.1523/JNEUROSCI.21-03-00759.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo E. The critical role of Golgi cells in regulating spatio-temporal integration and plasticity at the cerebellum input stage. Front Neurosci. 2008;2:35–46. doi: 10.3389/neuro.01.008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo E, De Zeeuw CI. Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci. 2009;32:30–40. doi: 10.1016/j.tins.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Dean P, Porrill J, Ekerot CF, Jörntell H. The cerebellar microcircuit as anadaptive filter: experimental and computational evidence. Nat Rev Neurosci. 2010;11:30–43. doi: 10.1038/nrn2756. [DOI] [PubMed] [Google Scholar]
- De Schutter E, Bjaalie JG. Coding in the granular layer of the cerebellum. Prog Brain Res. 2001;130:279–296. doi: 10.1016/s0079-6123(01)30019-5. [DOI] [PubMed] [Google Scholar]
- De Schutter E. Cerebellar cortex: computation by extrasynaptic inhibition. Curr Biol. 2002;12:363–365. doi: 10.1016/s0960-9822(02)00861-8. [DOI] [PubMed] [Google Scholar]
- Dieudonné S. Submillisecond kinetics and low efficacy of parallel fibre-Golgi cell synaptic currents in the rat cerebellum. J Physiol. 1998;510:845–866. doi: 10.1111/j.1469-7793.1998.845bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonné S, Dumoulin A. Serotonin-driven long-range inhibitory connections in the cerebellar cortex. J Neurosci. 2000;20:1837–1848. doi: 10.1523/JNEUROSCI.20-05-01837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Dieudonné S. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci. 2001;21:6045–6057. doi: 10.1523/JNEUROSCI.21-16-06045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué GP, Dumoulin A, Triller A, Dieudonné S. Target-dependent use of co-released inhibitory transmitters at central synapses. J Neurosci. 2005;25:6490–6498. doi: 10.1523/JNEUROSCI.1500-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué GP, Brunel N, Hakim V, Schwartz E, Chat M, Lévesque M, Courtemanche R, Léna C, Dieudonné S. Electrical coupling mediates tunable low-frequency oscillations and resonance in the cerebellar Golgi cell network. Neuron. 2009;61:126–139. doi: 10.1016/j.neuron.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Llinas R, Sasaki K. Golgi cell inhibition in the cerebellar cortex. Nature. 1964;204:1265–1266. doi: 10.1038/2041265a0. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Berlin: Springer; 1967. [Google Scholar]
- Edgley SA, Lidierth M. The discharges of cerebellar Golgi cells during locomotion in the cat. J Physiol. 1987;392:315–332. doi: 10.1113/jphysiol.1987.sp016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Forti L, Cesana E, Mapelli J, D’Angelo E. Ionic mechanisms of autorhythmic firing in rat cerebellar Golgi cells. J Physiol. 2006;574:711–729. doi: 10.1113/jphysiol.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusari R. Sull’origine delle fibre nervosa dello strato molecolare delle circonvoluzioni cerebellari dell’omo. Atti della Reale Accademia delle Scienze di Torino. 1883;19:47–51. [Google Scholar]
- Geurts FJ, Timmermans J, Shigemoto R, De Schutter E. Morphological and neurochemical differentiation of large granular layer interneurons in the adult rat cerebellum. Neurosci. 2001;104:499–512. doi: 10.1016/s0306-4522(01)00058-6. [DOI] [PubMed] [Google Scholar]
- Geurts FJ, De Schutter E, Dieudonné S. Unraveling the cerebellar cortex: cytology and cellular physiology of large-sized interneurons in the granular layer. Cerebellum. 2003;2:290–229. doi: 10.1080/14734220310011948. [DOI] [PubMed] [Google Scholar]
- Golgi C. Sulla fina anatomia del cervelletto umano. Archivio Italiano per le Malattie Nervose. 1874;2:90–107. [Google Scholar]
- Golgi C. Sulla fina anatomia degli organi centrali del sistema nervoso. Reggio Emilia: Calderini e Figlio; 1885. [Google Scholar]
- Golgi C. Opera Omnia. Milano: Hoepli; 1903. [Google Scholar]
- Golgi C. Nobel Lectures, Physiology or Medicine 1901–1921. New York: Elsevier; 1967. The neuron doctrine. Theory and facts; pp. 189–217. [Google Scholar]
- Hadley SH, Amin J. Rat α6β2δ GABAA receptors exhibit two distinct and separable agonist affinities. J Physiol. 2007;581:1001–1018. doi: 10.1113/jphysiol.2007.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hámori J, Szentágothai J. Participation of Golgi neuron processes in the cerebellar glomeruli: an electron microscope study. Exp Brain Res. 1966;2:35–48. doi: 10.1007/BF00234359. [DOI] [PubMed] [Google Scholar]
- Hámori J, Somogyi J. Differentiation of cerebellar mossy fiber synapses in the rat: a quantitative electron microscope study. J Comp Neurol. 1983;220:365–377. doi: 10.1002/cne.902200402. [DOI] [PubMed] [Google Scholar]
- Hartmann MJ, Bower JM. Oscillatory activity in the cerebellar hemispheres of unrestrained rats. J Neurophysiol. 1998;80:1598–1604. doi: 10.1152/jn.1998.80.3.1598. [DOI] [PubMed] [Google Scholar]
- Holtzman T, Mostofi A, Phuah CL, Edgley SA. Cerebellar Golgi cells in the rat receive multimodal convergent peripheral inputs via the lateral funiculus of the spinal cord. J Physiol. 2006a;577:69–80. doi: 10.1113/jphysiol.2006.117218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman T, Rajapaksa T, Mostofi A, Edgley SA. Different responses of rat cerebellar Purkinje cells and Golgi cells evoked by widespread convergent sensory inputs. J Physiol. 2006b;574:491–507. doi: 10.1113/jphysiol.2006.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman T, Cerminara NL, Edgley SA, Apps R. Characterization in vivo of bilaterally branching pontocerebellar mossy fibre to Golgi cell inputs in the rat cerebellum. Eur J Neurosci. 2009;29:328–339. doi: 10.1111/j.1460-9568.2008.06572.x. [DOI] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. New York: Raven Press; 1984. [Google Scholar]
- Ivry R, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Hàmori J. Quantitative morphology and synaptology of cerebellar glomeruli in the rat. Anat Embryol. 1988;179:81–88. doi: 10.1007/BF00305102. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Lindstrom S. Morphological identification of Renshaw cells. Acta Physiol Scand. 1971;81:428–430. doi: 10.1111/j.1748-1716.1971.tb04918.x. [DOI] [PubMed] [Google Scholar]
- Jörntell H, Ekerot CF. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J Neurosci. 2006;26:11786–11797. doi: 10.1523/JNEUROSCI.2939-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanichay RT, Silver RA. Putative mossy fibre input resets spontaneous Golgi cell firing with high efficacy. FENS Abstr. 2006;3:A191.8. [Google Scholar]
- Kanichay RT, Silver RA. Synaptic and cellular properties of the feedforward inhibitory circuit within the input layer of the cerebellar cortex. J Neurosci. 2008;28:8955–8967. doi: 10.1523/JNEUROSCI.5469-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase M, Miller DC, Noda H. Discharges of Purkinje cells and mossy fibres in the cerebellar vermis of the monkey during saccadic eye movements and fixation. J Physiol. 1980;300:539–555. doi: 10.1113/jphysiol.1980.sp013178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölliker A. Zur feineren Anatomie des centralen Nervensystems. I. Das Kleinhirn. Z Wissenschaft Zool. 1890;49:663–689. [Google Scholar]
- Kölliker A. The minute anatomy of the spinal cord and cerebellum demonstrated by Golgi's method. J Anat Physiol. 1891;25:443–460. [PMC free article] [PubMed] [Google Scholar]
- Kölliker A. Handbuch der Gewebelehre des Menschen. Zweiter Band: Nervensystem des Menschen und Thiere. Leipzig: W. Engelmann; 1896. [Google Scholar]
- Kruger L. The sensory neuron and the triumph of Camillo Golgi. Brain Res Rev. 2007;55:406–410. doi: 10.1016/j.brainresrev.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Kruger L, Otis TS. Whither withered Golgi? A retrospective evaluation of reticularist and synaptic constructs. Brain Res Bull. 2007;30:201–207. doi: 10.1016/j.brainresbull.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Maex R, De Schutter E. Synchronization of Golgi and granule cell firing in a detailed network model of the cerebellar granule cell layer. J Neurophysiol. 1998;80:2521–2537. doi: 10.1152/jn.1998.80.5.2521. [DOI] [PubMed] [Google Scholar]
- Mann-Metzer P, Yarom Y. Electrotonic coupling synchronizes interneuron activity in the cerebellar cortex. Prog Brain Res. 2000;124:115–22. doi: 10.1016/s0079-6123(00)24012-0. [DOI] [PubMed] [Google Scholar]
- Mapelli J, D’Angelo E. The spatial organization of long-term synaptic plasticity at the input stage of cerebellum. J Neurosci. 2007;27:1285–1296. doi: 10.1523/JNEUROSCI.4873-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli L, Rossi P, Nieus T, D’Angelo E. Tonic activation of GABAB receptors reduces release probability at inhibitory connections in the cerebellar glomerulus. J Neurophysiol. 2009;101:3089–99. doi: 10.1152/jn.91190.2008. [DOI] [PubMed] [Google Scholar]
- Mapelli J, Gandolfi D, D’Angelo E. High-Pass Filtering and Dynamic Gain Regulation Enhance Vertical Bursts Transmission along the Mossy Fiber Pathway of Cerebellum. Front Cell Neurosci. 2010;28:4–14. doi: 10.3389/fncel.2010.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarello P. Net without nodes and vice versa, the paradoxical Golgi-Cajal story: a reconciliation. Brain Res Bull. 2007;71:344–346. doi: 10.1016/j.brainresbull.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Mazzarello P. Golgi, a Biography of the Founder of Modern Neuroscience (transl. Badiani A and Buchtel H) New York: Oxford University Press; 2010. [Google Scholar]
- Mazzarello P, Calligaro A, Garbarino C, Vannini V. Golgi, Brain Architect. Milan: Skirà; 2006. [Google Scholar]
- Miles FA, Fuller JH, Braitman DJ, Dow BM. Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol. 1980;43:1437–1476. doi: 10.1152/jn.1980.43.5.1437. [DOI] [PubMed] [Google Scholar]
- Misra C, Brickley SG, Farrant M, Cull-Candy SG. Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum. J Physiol. 2000;524:147–162. doi: 10.1111/j.1469-7793.2000.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. J Neurosci. 2000b;20:8651–8658. doi: 10.1523/JNEUROSCI.20-23-08651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar Cortex. New York: Springer-Verlag; 1974. [Google Scholar]
- Palkovits M, Magyar P, Szentàgothai J. Quantitative histological analysis of the cerebellar cortex in the cat. II. Cell numbers and densities in the granular layer. Brain Res. 1971;32:15–30. doi: 10.1016/0006-8993(71)90152-1. [DOI] [PubMed] [Google Scholar]
- Pellerin JP, Lamarre Y. Local field potential oscillations in primate cerebellar cortex during voluntary movement. J Neurophysiol. 1997;78:3502–3507. doi: 10.1152/jn.1997.78.6.3502. [DOI] [PubMed] [Google Scholar]
- Pijpers A, Apps R, Pardoe J, Voogd J, Ruigrok TJH. Precise spatial relationships between mossy fibers and climbing fibers in rat cerebellar cortical zones. J Neurosci. 2006;26:12067–12080. doi: 10.1523/JNEUROSCI.2905-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht W, Llinás R. Functional organization of the vestibular afferents to the cerebellar cortex of frog and cat. Exp Brain Res. 1969;9:30–52. doi: 10.1007/BF00235450. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Sobre las fibras nerviosas de la capa molecular del cerebelo. Rev Trim Histol Norm Patol. 1888;1:65–78. [Google Scholar]
- Ramón y Cajal S. Sobre las fibras nerviosas de la capa granulose del cerebelo. Rev Trim Histol Norm Patol. 1889a;1:107–118. [Google Scholar]
- Ramón y Cajal S. Sur l’origine et la direction des prolongations nerveuses de la couche moléculaire du cervelet. Internat Mschr Anat Physiol. 1889b;7:12–31. [Google Scholar]
- Ramón y Cajal S. Textura del sistema nervioso del hombre y de los vertebrado: estudios sobre el plan estructural y composición histológical de los centros nerviosos adicionados de consideraciones fisiológicas fundadas en los nuevos descubrimientos. Vol. 1. Madrid: Moya; 1899. [Google Scholar]
- Ramón y Cajal S. Textura del sistema nervioso del hombre y de los vertebrado: estudios sobre el plan estructural y composición histológical de los centros nerviosos adicionados de consideraciones fisiológicas fundadas en los nuevos descubrimientos. 2/3. Madrid: Moya; 1904. [Google Scholar]
- Ramón y Cajal S. Histologie du sistéme nerveux de l’homme et des vertebras (transl, Azoulay L) Vol. 1. Paris: Maloine; 1909. [Google Scholar]
- Ramón y Cajal S. Histologie du sistéme nerveux de l’homme et des vertebras (transl, Azoulay L) Vol. 2. Paris: Maloine; 1911. [Google Scholar]
- Ramón y Cajal S. Nobel Lectures Physiology or Medicine 1901–1921. New York: Elsevier; 1967. The structure and connections of neurons. [Google Scholar]
- Ramón y Cajal S. Histology of the Nervous System of Man and Vertebrates (transl Swanson N and Swanson L) New York: Oxford University Press; 1995. [Google Scholar]
- Rancz EA, Ishikawa T, Duguid I, Chadderton P, Mahon S, Häusser M. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature. 2007;450:1245–1248. doi: 10.1038/nature05995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw B. Central effects of centripetal impulses in axons of spinal ventral roots. J Neurophysiol. 1946;9:191–204. doi: 10.1152/jn.1946.9.3.191. [DOI] [PubMed] [Google Scholar]
- Retzius G. Kleinere Mittheilungen von den Gebiete der Nervenhistologie. Biologische Untersuchungen (Neue Folge) 1892;IV:57–66. [Google Scholar]
- Rieke F, Warland D, de Ruyter van Stevenink R, Bialek W. Spikes. London: MIT Press; 1997. [Google Scholar]
- Ros H, Sachdev RNS, Yu Y, Sestan N, McCormick DA. Neocortical networks entrain neuronal circuits in cerebellar cortex. J Neurosci. 2009;29:10309–10320. doi: 10.1523/JNEUROSCI.2327-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAA receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol. 2003;548:97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Observations on the intracortical relations of the climbing fibers of the cerebellum: a Golgi study. J Comp Neurol. 1954;101:733–763. doi: 10.1002/cne.901010305. [DOI] [PubMed] [Google Scholar]
- Schulman JA, Bloom FE. Golgi cells of the cerebellum are inhibited by inferior olive activity. Brain Res. 1981;210:350–355. doi: 10.1016/0006-8993(81)90908-2. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sugihara I, Wu HS, Sugiuchi Y. The entire trajectory of single climbing and mossy fibers in the cerebellar nuclei and cortex. Prog Brain Res. 2000;124:173–186. doi: 10.1016/S0079-6123(00)24015-6. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Chung SH, Fritschy JM, Hoy M, Hawkes R. Golgi cell dendrites are restricted by purkinje cell stripe boundaries in the adult mouse cerebellar cortex. J Neurosci. 2008;28:2820–2826. doi: 10.1523/JNEUROSCI.4145-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simat M, Parpan F, Fritschy JM. Heterogeneity of glycinergic and GABAergic interneurons in the granule cell layer of mouse cerebellum. J Comp Neurol. 2007;500:71–83. doi: 10.1002/cne.21142. [DOI] [PubMed] [Google Scholar]
- Solinas S, Forti L, Cesana E, Mapelli J, De Schutter E, D’Angelo E. Computational reconstruction of pacemaking and intrinsic electroresponsiveness in cerebellar Golgi cells. Frontiers Neurosci. 2007a;1:2. doi: 10.3389/neuro.03.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas S, Forti L, Cesana E, Mapelli J, De Schutter E, D’Angelo E. Fast-reset of pacemaking and theta-frequency resonance patterns in cerebellar Golgi cells: simulations of their impact in vivo. Frontiers Neurosci. 2007b;1:4. doi: 10.3389/neuro.03.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas S, Nieus T, D’Angelo E. A realistic large-scale model of the cerebellum granular layer predicts circuit spatio-temporal dynamics. Frontiers Neurosci. 2010 doi: 10.3389/fncel.2010.00012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C, Llinás R. Specialized membrane junctions between neurons in the vertebrate cerebellar cortex. J Cell Biol. 1972;53:271–289. doi: 10.1083/jcb.53.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RM, Verstynen T, Brett M, Ivry R. Cerebellar activation during dicrete and not continuous timed movements: and fMRI study. Neuroimage. 2007;36:378–387. doi: 10.1016/j.neuroimage.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan F. Distribution of mossy fiber rosettes in the cerebellum of cat and mice: evidence for a parasagittal organization at the single fiber level. Eur J Neurosci. 2001;13:2123–2130. doi: 10.1046/j.0953-816x.2001.01593.x. [DOI] [PubMed] [Google Scholar]
- Tahon K, Volny-Luraghi A, De Schutter E. Temporal characteristics of tactile stimuli influence the response profile of cerebellar Golgi cells. Neurosci Lett. 2005;390:156–161. doi: 10.1016/j.neulet.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology. 1996;35:1375–1382. doi: 10.1016/s0028-3908(96)00018-4. [DOI] [PubMed] [Google Scholar]
- Van Gehuchten A. La structure des centres nerveus. La moelle épinière et le cervelet. La Cellule. 1891;7:79–122. [Google Scholar]
- Van Gehuchten A. Le système nerveux de l’homme. Lierre: J. van In; 1893. [Google Scholar]
- Van Kan PL, Gibson AR, Houk JC. Movement-related inputs to intermediate cerebellum of the monkey. J Neurophysiol. 1993;69:74–94. doi: 10.1152/jn.1993.69.1.74. [DOI] [PubMed] [Google Scholar]
- Volny-Luraghi A, Maex R, Vos BP, De Schutter E. Peripheral stimuli excite coronal beams of Golgi cells in rat cerebellar cortex. Neuroscience. 2002;113:363–373. doi: 10.1016/s0306-4522(02)00196-3. [DOI] [PubMed] [Google Scholar]
- Voogd J, Pardoe J, Ruigrok TJH, Apps R. The distribution of climbing and mossy fiber collateral branches from the copula pyramidis and the paramedian lobule: Congruence of climbing fiber cortical zones and thepattern of zebrin banding within the rat cerebellum. J Neurosci. 2003;23:4645–4656. doi: 10.1523/JNEUROSCI.23-11-04645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos BP, Volny-Luraghi A, De Schutter E. Cerebellar Golgi cells in the rat: receptive fields and timing of responses to facial stimulation. Eur J Neurosci. 1999a;11:2621–2634. doi: 10.1046/j.1460-9568.1999.00678.x. [DOI] [PubMed] [Google Scholar]
- Vos BP, Maex R, Volny-Luraghi A, De Schutter E. Parallel fibers synchronize spontaneous activity in cerebellar Golgi cells. J Neurosci. 1999b;19:RC6. doi: 10.1523/JNEUROSCI.19-11-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos BP, Volny-Luraghi A, Maex R, De Schutter E. Precise spike timing of tactile-evoked cerebellar Golgi cell responses: a reflection of combined mossy fiber and parallel fiber activation. Prog Brain Res. 2000;124:95–106. doi: 10.1016/s0079-6123(00)24010-7. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Nakanishi S. mGluR2 Postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron. 2003;39:821–829. doi: 10.1016/s0896-6273(03)00530-0. [DOI] [PubMed] [Google Scholar]
- Xu W, Edgley SA. Climbing fiber-dependent changes in Golgi cell responses to peripheral stimulation. J Physiol. 2008;586:4951–4959. doi: 10.1113/jphysiol.2008.160879. [DOI] [PMC free article] [PubMed] [Google Scholar]


