Abstract
The effect of a period of passive movement training on angiogenic factors and capillarization in skeletal muscle was examined. Seven young males were subjected to passive training for 90 min, four times per week in a motor-driven knee extensor device that extended one knee passively at 80 cycles min−1. The other leg was used as control. Muscle biopsies were obtained from m. v. lateralis of both legs before as well as after 2 and 4 weeks of training. After the training period, passive movement and active exercise were performed with both legs, and muscle interstitial fluid was sampled from microdialysis probes in the thigh. After 2 weeks of training there was a 2-fold higher level of Ki-67 positive cells, co-localized with endothelial cells, in the passively trained leg which was paralleled by an increase in the number of capillaries around a fibre (P < 0.05). Capillary density was higher than pre-training at 4 weeks of training (P < 0.05). The training induced an increase in the mRNA level of endothelial nitric oxide synthase (eNOS), the angiopoietin receptor Tie-2 and matrix metalloproteinase (MMP)-9 in the passively trained leg and MMP-2 and tissue inhibitor of MMP (TIMP)-1 mRNA were elevated in both legs. Acute passive movement increased (P < 0.05) muscle interstitial vascular endothelial growth factor (VEGF) levels 4- to 6-fold above rest and the proliferative effect, determined in vitro, of the muscle interstitial fluid ∼16-fold compared to perfusate. The magnitude of increase was similar for active exercise. The results demonstrate that a period of passive movement promotes endothelial cell proliferation and angiogenic factors and initiates capillarization in skeletal muscle.
Introduction
Capillary growth in skeletal muscle is a dynamic process which primarily is dependent on the degree of physical activity where enhanced activity promotes growth (Andersen & Henriksson, 1977) and inactivity leads to capillary regression (Prior et al. 2009). Regular exercise is therefore beneficial for the maintenance or increase in the level of capillarization in the muscle. The physiological signals that stimulate capillary growth in response to muscular work have been proposed to be: (1) shear stress, (2) passive stretch of the tissue, (3) enhanced muscle metabolism and (4) changes in oxygen levels (Egginton, 2009). To discriminate between the role of muscle metabolism versus enhanced shear stress and muscle stretch, we previously examined the effect of an acute bout of passive movement of the lower leg (Hellsten et al. 2008). Passive movement of the lower leg has been found to result in an approximate three-fold increase in muscle blood flow, and stretch of the muscle tissue without an alteration in either EMG activity or muscle oxygen uptake (Krustrup et al. 2004; Hellsten et al. 2008). The passive movement model induced an enhanced level of muscle interstitial vascular endothelial growth factor (VEGF) protein and an increased endothelial cell proliferative effect of muscle interstitial fluid from the muscle as well as a higher expression of endothelial nitric oxide synthase (eNOS) mRNA in the muscle (Hellsten et al. 2008). Thus, stimuli from shear stress and passive stretch, without a simultaneous increase in metabolism, appear to be sufficient to initiate an angiogenic response. This observation in humans agrees well with studies on laboratory animals where a chronic enhancement in blood flow was achieved by addition of the α-adrenergic antagonist prazosin to the drinking water and resulted in a clear angiogenic response (Dawson & Hudlicka, 1989; Rivilis et al. 2002; Baum et al. 2004). Similarly, chronic over-load in rodents by surgical extirpation of the tibialis anterior, which results in stretch of the EDL muscle, leads to angiogenesis (Egginton et al. 1998; Rivilis et al. 2002). These studies on laboratory animals have also shown an increase in endothelial cell proliferation and increased capillarization. Whether a period of passive movement training provides stimuli to induce a sufficient angiogenic response leading to capillary growth in humans has not been examined. In addition to the usefulness of the passive model for understanding which physiological factors that are of importance for capillary growth in skeletal muscle, the model holds a clear therapeutic potential. For patients with severe peripheral arterial disease that experience limb pain when exercising and for patients with e.g. knee-injuries, the passive model may prove to be a useful tool to improve or maintain capillarization of the limb.
Angiogenesis is a complex process which involves a number of pro- and anti-angiogenic factors with specific functions. Capillary growth in skeletal muscle may also occur either by sprouting or by longitudinal splitting, where passive tension promotes sprouting and shear stress promotes longitudinal splitting. When a new capillary is formed by sprouting from an existing vessel, the basement membrane that stabilizes the capillary has to be degraded, furthermore, the endothelial cells that make up capillaries have to be activated, divide and migrate. Finally the capillary has to be stabilized again. Vascular endothelial growth factor (VEGF) is probably one of the most important factor for endothelial activation, proliferation and migration. VEGF shows a close interaction with nitric oxide (NO) formed from nitric oxide synthase (NOS); NO regulates VEGF expression and VEGF has been shown to regulate NO formation (Tsurumi et al. 1997). Matrix metalloproteinases (MMPs) as well as angiopoietin-2 (Ang-2) are important for the destabilization of the capillary whereas angiopoietin-1 (Ang-1) is involved in the stabilization process of the newly formed capillary. MMPs can be inhibited by the tissue inhibitor of MMPs (TIMP-1), thus limiting the degree of extracellular matrix degradation. When a new capillary is formed by longitudinal splitting, the capillary lumen is divided, a process that requires less matrix remodelling and MMPs are not significantly involved in the process (Rivilis et al. 2002). In the present study we determined increases in the gene expression of angiogenic factors to elucidate whether capillary growth processes are initiated by passive movement training and to understand which angiogenic factors that respond to this training form.
The aim of the present study was to examine whether a period of regular passive movement training would provide a sufficient angiogenic response to initiate capillary growth in skeletal muscle in young healthy males. Subjects performed 4 weeks of passive movement training of the lower leg. Muscle biopsies were obtained from m.v. lateralis at rest for the determination of mRNA content of several angiogenic factors and muscle interstitial fluid was collected via microdialysis probes for determination of interstitial VEGF and the proliferative effect of muscle interstitial fluid.
Methods
Subjects
Seven healthy male subjects participated, with a mean age of 25 (range: 20–38) years, weight of 76.0 (range: 60–97) kg, and maximal pulmonary maximum oxygen uptake 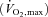 of 3.7 (range: 2.5–4.2) l min−1. The subjects were non-smokers and habitually active but performed no regular training. The subjects were fully informed of the risks and discomfort and all provided written consent. The study was carried out in accordance with the guidelines contained in the Declaration of Helsinki and was approved by the local ethics committee of Copenhagen.
of 3.7 (range: 2.5–4.2) l min−1. The subjects were non-smokers and habitually active but performed no regular training. The subjects were fully informed of the risks and discomfort and all provided written consent. The study was carried out in accordance with the guidelines contained in the Declaration of Helsinki and was approved by the local ethics committee of Copenhagen.
Experimental design
The subjects carried out 4 weeks of passive leg movement training with one leg. The training was performed 4 times per week with, in total, 17 (range 15–20) training sessions, each of 90 min duration. With the subject seated in an upright position, the knee was passively extended from an angle of 90 deg to 35 deg and back to 90 deg again at a rate of 80 cycles min−1 by a machine specifically built for this purpose. The other leg served as control. After two familiarization sessions, prior to the training and after the passive training period, the subjects performed an incremental exercise test to exhaustion with each leg on a one-leg knee-extensor ergometer. The test was initiated by a 10 min warm-up at a power output of 10 W at 60 cycles min−1 followed by 5 min rest. Hereafter the work load was set at 30 W and increased 5 W every min until exhaustion was achieved, when the subject was unable to maintain a kicking frequency of =55 cycles min−1 for 10 s. A biopsy was obtained from m. vastus lateralis from both legs prior to a training session before and after 2 and 4 weeks of passive training. The biopsies at 2 and 4 weeks of training were obtained minimally 48 h after the last training session.
Experimental protocol
At 48–60 h after the last training session an experiment was performed. On the morning of the experimental day the subjects had a light breakfast. The subjects rested in the supine position, and the skin, subcutaneous tissue and fascia of the thigh were anaesthetized by injection with lidocain (Xylocaine; 20 mg ml−1) to prepare for the muscle biopsy and insertion of microdialysis probes. As described above, a biopsy was then obtained from m. vastus lateralis for both legs. Microdialysis probes were placed in the thigh muscle as previously described (Hoffner et al. 2003) and perfused with buffer including a small amount (3.1 nm) of labelled [2-3H] adenosine for calculation of probe recovery for each sampling (Hoffner et al. 2003). Approximately 60 min after probe insertion, the subjects performed 10 min of exercise at a power output of 10 W (Nordsborg et al. 2003). At ∼110 min after insertion of the probes, dialysate was collected at rest for two periods of 20 min. Then, 30 min of passive exercise with the trained leg was carried out, followed by 30 min of one-legged knee-extensor exercise at 10 W, 30 min of passive movement, and 20 min of one-legged knee-extensor exercise at 30 W. Subsequently the same passive movement and exercise protocol was carried out for the untrained leg. Dialysate samples were collected during each of these periods.
All dialysate samples were immediately frozen and stored at −80°C until time of analysis. Flow rate was calculated to estimate any loss of fluid or abnormal decrease in perfusion rate (Hoffner et al. 2003). The relative loss (RL) for each probe was determined according to the internal reference method (Scheller & Kolb, 1991; Jansson et al. 1994).
Muscle biopsies were divided into two where one was immediately frozen (protein and mRNA analysis) and the other (for histochemistry) was embedded in mounting medium, frozen in isopentane pre-cooled in liquid nitrogen and stored at −80°C.
Immunohistochemistry
Transverse sections 8 μm in thickness were placed onto glass slides, fixed by immersion in acetone of −20°C for 30 s and incubated for 2 min in 2% paraformaldehyde (pH 7.4) at room temperature. The sections were blocked for 1 h with phosphate buffered saline (PBS) with 1% bovine serum albumin (BSA) (pH 7.4). The muscle sections were incubated with either primary antibody CD-31 (M0822, DakoCytomation, Glostrup, Denmark), diluted 1:10 in PBS–1% BSA or primary antibody Ki-67 (556003, BD Pharmingen, San Diego, USA), diluted 1:50 in PBS–1%BSA for 1 h followed by incubation with biotinylated secondary antibody (ABComplex/AP KO376, Dako A/S, Glostrup Denmark or AK-500, Vectastain; Vector Laboratories, Burlingame, CA, USA) for 30 min. Binding was visualized with a Fuchsin+Substrate-Chromogen system (KO625, Dako A/S). Specificity of the staining was assessed by staining without the primary antibody. Number of capillaries and fibres were determined on 189 ± 5 fibres per biopsy using light microscopy (Axiolab, Zeiss) cross. Capillary supply was expressed as capillaries per fibre (C:F ratio), capillary density (CD; cap mm−2) and capillaries around a fibre (CAF). Mean fibre area was assessed by manual drawing of the perimeter of each muscle fibre using the image analysis computer software Tema version 95 (BioRad Scan Beam, Hadsund, Denmark). Number of Ki-67 positive nuclei and fibres were determined using a light microscopy (Axioplan 2, Zeiss) in two Ki-67 stained muscle cross sections in two to five areas corresponding to an average of 443 ± 21 fibres per biopsy. Serial sections stained for CD-31 and Ki-67 were used to assess proliferating cells co-localized with endothelial cells, indicative of capillary growth. Proliferating cells were expressed per fibre (Ki-67/fibre).
Dialysate VEGF protein measurements
Dialysate VEGF levels were determined by enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's protocol (Quantikine Human VEGF; R&D System, Minneapolis, MN, USA). The VEGF concentration was measured in the dialysate and the interstitial concentration was estimated by determination of the RL of tritium-labelled adenosine for each probe (Hoffner et al. 2003).
Measurement of endothelial cell proliferation
Human umbilical vein endothelial cells cultured in medium 200 with low serum supplement containing fetal bovine serum, fibroblast growth factor, heparin and epidermal growth factor, intended for use in the culture of endothelial cells (Cytotech MK-200-2 Medium 200 kit) were grown on 96-well plates for 24 h before the medium was replaced with 50 μl of microdialysate, perfusate, or supplemented medium 200. None of the values exceeded the positive control (addition of medium 200 with growth supplement). After an additional 30 h of incubation, bromodeoxyuridine (BrdU) was added and then incubated for 12 h. Incorporation of BrdU into the DNA was detected using an immunoassay (Roche, Mannheim, Germany) according to the manufacturer's recommendations.
Analysis of skeletal muscle mRNA content: RNA isolation, reverse transcription and PCR
Total RNA was isolated from the muscle biopsies using TRIzol reagent according to the guidelines of the manufacturer (Invitrogen, Carlsbad, CA, USA). First strand cDNA was synthesized from 3 μg total RNA by SuperScript II Reverse Transcriptase (Invitrogen) as previously described (Pilegaard et al. 2000). The mRNA content of VEGF, eNOS, MMP-2, MMP-9, TIMP-1, TIMP-2, Tie-2, Ang-1, Ang-2 and GAPDH was determined by real time PCR (ABI PRISM 7900 Sequence Detection System, Applied Biosystems, Foster City, CA, USA). The cDNAs were amplified using TaqMan Gene expression assays from Applied Biosystems. Prior optimization was performed as previously described (Pilegaard et al. 2003). For each sample, the amount of target gene mRNA was normalized to the GAPDH mRNA content. The effect of the experimental conditions on the level of GAPDH mRNA was statistically determined and no significant effect was found with the passive training.
Thigh blood flow and thigh oxygen uptake during passive movement
Catheters were placed in the femoral artery and the femoral vein of six subjects. Thigh blood flow (TBF) and thigh oxygen uptake 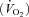 were determined during rest and passive movement on the one-leg knee-extension ergometer. Femoral venous blood flow was determined by the thermodilution technique originally described by (Andersen & Saltin, 1985) and modified by (Gonzalez-Alonso et al. 2000). Measurements of blood flow and blood oxygen content were determined in the supine position at rest and after 30 s, 5 min and 10 min of passive movement at 80 cycles min−1. Oxygen saturation and haemoglobin were determined in femoral arterial and femoral venous blood samples with an ABL 510, Radiometer, Copenhagen, Denmark.
were determined during rest and passive movement on the one-leg knee-extension ergometer. Femoral venous blood flow was determined by the thermodilution technique originally described by (Andersen & Saltin, 1985) and modified by (Gonzalez-Alonso et al. 2000). Measurements of blood flow and blood oxygen content were determined in the supine position at rest and after 30 s, 5 min and 10 min of passive movement at 80 cycles min−1. Oxygen saturation and haemoglobin were determined in femoral arterial and femoral venous blood samples with an ABL 510, Radiometer, Copenhagen, Denmark.
Muscle stretch
The magnitude of muscle stretch was determined on three subjects during passive knee movements. The subjects were placed in a seated position while the leg was passively moved from 35 to 95 deg (knee joint fully extended = 0 deg) as during passive training. An ultrasound transducer (7.5 MHz linear array B-mode probe, length 70 mm, Sonoline Sienna Siemens, Erlangen, Germany) was positioned over the middle aspect of the m. vastus lateralis such that fascicle insertion points on the profound aponeurosis were clearly visible (Bojsen-Moller et al. 2003) and a goniometer (Penny & Giles Biometrics Ltd, Gwent, UK) was situated laterally over the knee joint. During the passive knee flexion (∼3 deg s−1) goniometer and ultrasonography (US) video sequences were sampled synchronously into a PC. Displacement of the fascicle insertion points in the distal direction during knee flexion (represents fascicle lengthening) was determined by automated software tracking as reported previously (Bojsen-Moller et al. 2003) and the relationship between knee joint angle and fascicle lengthening was established.
Electromyographic activity
Electromyographic activity was determined on three subjects during maximal voluntary isometric contraction (MVC) and during 90 min of passive movement of the trained leg at 80 cycles min−1. Electrodes (Neuroline, 720-01-J) were placed 1 cm apart on the belly of the m. vasuts lateralis after palpation, shaving and cleaning the skin with ethanol. The signal was amplified (IP511 AC Amplifier, Atro-Med Inc./Grass, West Warwick, RI, USA), using a 30 Hz and 3 kHz high and low pass filter, respectively, and recorded by an A/D converter system (PowerLab 16/30, ADInstruments, Bellavista, NSW, Australia). Electromyogram (EMG) signals were analysed after applying a digital high and low pass filter of 10 and 500 Hz, respectively, followed by determination of the integrated EMG (iEMG) via root mean square calculation of 1 s segments (ChartPro, ADInstruments, Bellavista NSW, Australia). All subsequent iEMG-values were normalized to the highest obtained iEMG of the three MVCs. Since no visible EMG signal was apparent during passive exercise, iEMG was determined for 1 s blocks after 5, 15, 60 and 89 min of exercise.
Statistics
All data are expressed as means ±s.e.m. A two-way ANOVA with repeated measures was performed in order to evaluate the effect of time and passive movement training on VEGF protein and mRNA in the muscle biopsies, in the VEGF dialysate concentrations and on measurements of the proliferative effect of muscle dialysate on endothelial cells. A Student–Newman–Keuls method for multiple comparisons was used to determine where the significant changes occurred. A level of P < 0.05 was considered statistically significant.
Results
Incremental one-leg knee-extensor exercise test
Peak power output was the same before and after the passive training period for both the untrained (55.7 ± 2.8 and 57.5 ± 4.6 W) and trained leg (57.1 ± 1.0 and 60.0 ± 2.9 W) with no difference between the two legs.
Capillarization
The capillary to fibre ratio (C:F) in the untrained and the passively trained leg (2.48 ± 0.14 vs. 2.41 ± 0.13 C:F) was similar before the training period and remained unaltered (P= 0.136) during the training period (Fig. 1A). The number of capillaries around a fibre (CAF) was similar in the untrained and the passively trained leg (4.69 ± 0.23 vs. 4.50 ± 0.15 CAF) before the training period (Fig. 1B). At 2 weeks of passive training CAF was higher (P < 0.05) than before training in the trained leg. The capillary densities (CD) in the two legs were similar (563 ± 34 vs. 570 ± 36 cap mm−2) prior to the training period. After 2 weeks no change in CD was observed in either leg, but the level was higher in both legs (P < 0.05) after 4 weeks compared to before the training (Fig. 1C). Mean fibre areas (μm2) were not significantly altered neither in the trained nor in the untrained leg (Fig. 1D).
Figure 1. Capillarization and presence of proliferating endothelial cells in skeletal muscle before and after passive training of the leg.
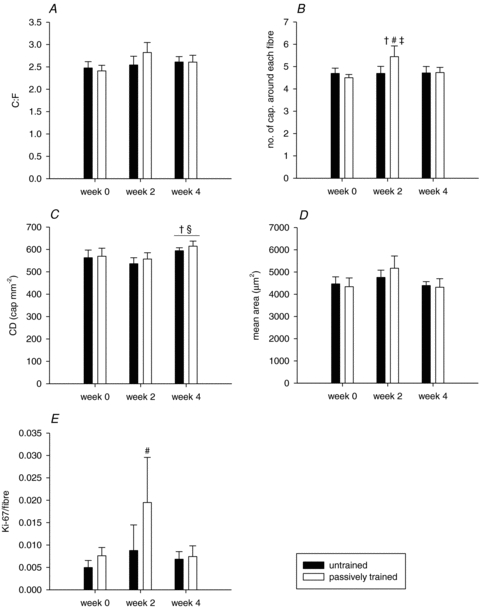
Capillary-to-fibre ratio (C:F, A), no. of capillaries around each fibre (B), capillary density (cap mm−2, C), mean fibre area (μm2, D), and Ki-67 positive cells per fibre (Ki-67/fibre, E) before and after 2 weeks and 4 weeks of passive training in the untrained (filled bars) and passively trained (open bars) leg. Values are means ±s.e.m. (n= 7). †P < 0.05 vs. week 0; #P < 0.05 vs. untrained leg week 2; ‡P < 0.05 vs. passively trained leg week 4; §P < 0.05 vs. week 2.
After 2 weeks of training, the Ki-67/fibre ratios were 3-fold higher (P < 0.05) in the passively trained leg compared to the untrained leg (Fig. 1E). After 4 weeks of training, the Ki-67/fibre ratios were similar to pre-training values and there was no difference between the untrained and the passively trained leg.
Muscle interstitial VEGF protein
The first bout of passive movement and exercise at 10 and 30 W enhanced (P < 0.05) the muscle interstitial VEGF level 4- to 6-fold above that at rest in both legs (Fig. 2). The interstitial VEGF level was not different between passive movement and active exercise at either 10 or 30 W and there was no difference in the interstitial VEGF level between the untrained and passively trained leg.
Figure 2. Interstitial concentration of VEGF protein in skeletal muscle at rest, and during passive movement and exercise.
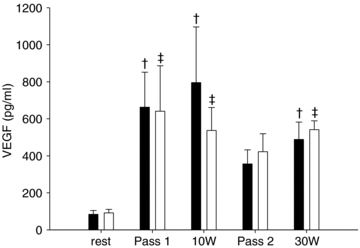
The concentration of VEGF protein was measured in the dialysate and the concentration in the interstitium was estimated by determination of relative loss of tritium labelled adenosine for each probe. Microdialysate samples were collected at rest, during two bouts of passive movement (Pass 1 and Pass 2, respectively), and exercise at 10 and 30 W in the untrained (filled bars) and passively trained (open bars) leg. Values are means ±s.e.m. (n= 7). †P < 0.05 vs. rest untrained leg; ‡P < 0.05 vs. rest passively trained leg.
Effect of muscle interstitial fluid on proliferation
Analysis of the effect of interstitial fluid on cultured endothelial cells by BrdU incorporation into cells showed that dialysate obtained from the passively trained or the untrained leg during passive movement induced an ∼16-fold higher (P < 0.05) proliferative effect than the perfusate (Fig. 3). Exercise at 10 or 30 W resulted in a similar increase in the proliferative effect of the interstitial fluid of both legs as passive exercise. There was no difference in the interstitial VEGF level between the untrained and trained leg.
Figure 3. Effect of skeletal muscle microdialysate on proliferation of cultured endothelial cells.
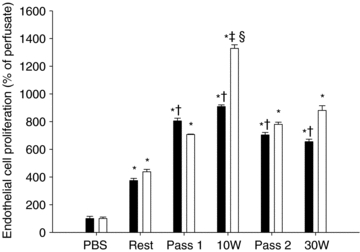
Proliferation of human umbilical vein endothelial cells (HUVECs) determined by incorporation of bromodeoxyurdine (BrdU), after addition of skeletal muscle microdialysate from the untrained (filled bars) and passively trained (open bars) leg. Microdialysate samples were collected at rest, during two bouts of passive movement (Pass 1 and Pass 2, respectively) and during exercise at 10 or 30 W. Values are means ±s.e.m. *P < 0.05 vs. PBS; †P < 0.05 vs. rest untrained leg; ‡P < 0.05 vs. rest passively trained leg; §P < 0.05 vs. Pass 1, Pass 2, and 30 W passively trained leg (n= 7). PBS: proliferation of endothelial cells with addition of the microdialysis perfusate consisting of PBS.
Probe recovery
The relative recovery of the probes determined from radioactively labelled adenosine was higher (P < 0.05) during passive movement, and exercise at 10 and 30 W than at rest (37%, 34%, 34%vs. 21%, respectively). There were no differences in recovery between the two legs.
Muscle mRNA content of angiogenic factors
The levels of eNOS, MMP-9 and Tie-2 mRNA were higher (P < 0.05) in the passively trained leg after 4 weeks of training compared to before (Fig. 4B, D and G, respectively). There was a general effect of the training period (P < 0.05) on the levels of MMP-2 and TIMP-1 mRNA for both legs (P < 0.05) (Fig. 4C and E, respectively). There was no difference in mRNA levels for VEGF, TIMP2, Ang-1, Ang-2 or in the Ang-1/Ang-2 ratio after compared to before training in either of the legs.
Figure 4. Content of VEGF, eNOS, MMP-2, MMP-9, TIMP1-, TIMP-2, Tie-2, Ang-1 and Ang-2 mRNA in human skeletal muscle tissue with passive movement training of the leg.
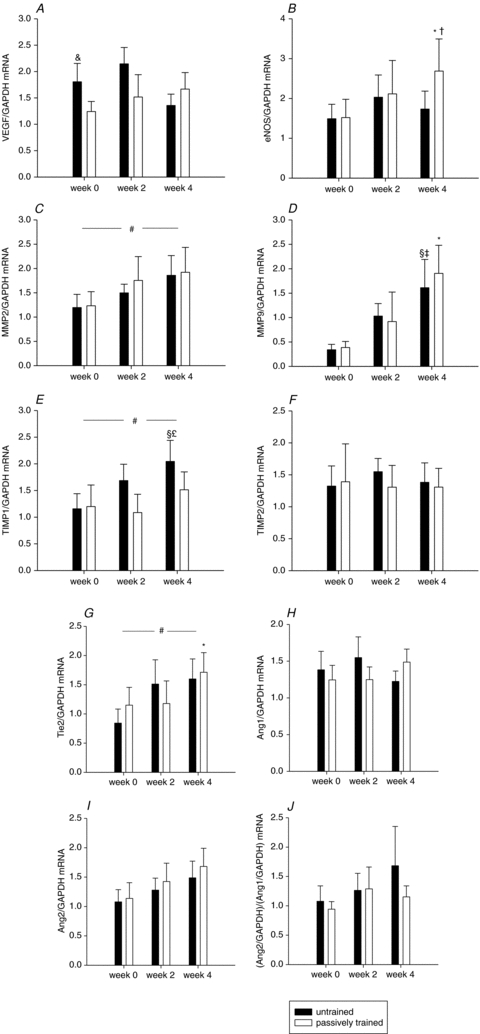
The mRNA content of VEGF (A), eNOS (B), MMP-2 (C), MMP-9 (D), TIMP-1 (E), TIMP-2 (F),Tie-2 (G), Ang-1 (H), Ang-2 (I), and the ratio Ang-2/Ang-1 (J) were determined in skeletal muscle tissue before, and after 2 and 4 weeks of passive training in the untrained (filled bars) and passively trained (open bars) leg. Muscles biopsies were obtained from m.v. lateralis. mRNA levels were determined with real-time PR-PCR, and data are presented relative to GAPDH mRNA. Values are means ±s.e.m. (n= 7). A, &P < 0.05 vs. rest passively trained leg; B, *P < 0.005 vs. rest passively trained leg, †P < 0.05 vs. 2 weeks passively trained leg; C, #P= 0.05 vs. rest; D, §P= 0.001 vs. rest untrained leg, ‡P= 0.001 vs. 2 weeks untrained leg, *P < 0.05 vs. rest passively trained leg; E, §P < 0.005 vs. rest untrained leg, £P < 0.05 vs. 4 weeks passively trained leg, #P < 0.05 vs. rest; G, *P < 0.05 vs. rest passively trained leg, #P < 0.05 vs. rest.
Leg blood flow and oxygen uptake during passive movement
Thigh blood flow at rest was 0.45 ± 0.06 l min−1 and increased 2-fold during the first 30 s of passive movement to 0.90 ± 0.14 l min−1 (P < 0.05) and the blood flow remained elevated during the subsequent 9.5 min of passive movement (Fig. 5A). Thigh oxygen extraction (a-v O2 difference) at rest of 70.8 ± 13.3 ml l−1 was lowered to 59.6 ± 10.6 ml l−1 after 30 of passive movement and remained unaltered during the subsequent 9.5 min of passive movement (Fig. 5B). Oxygen uptake was 29.1 ± 4.5 ml min−1 at rest and increased (P < 0.05) to 48.0 ± 6.2 ml min−1 at 30 s and remained at this level during the bout (Fig. 5C).
Figure 5. Thigh blood flow, arterio-venous oxygen difference, and oxygen uptake during passive movement of the leg.
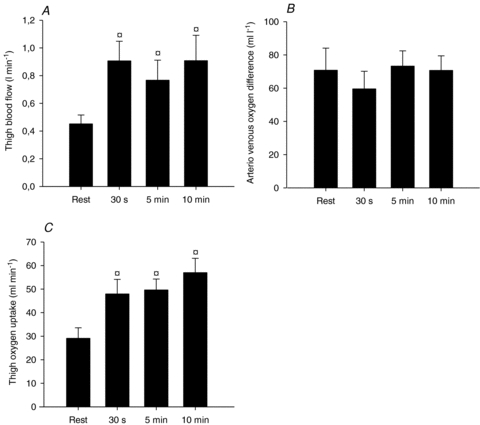
Thigh blood flow (l min−1, A), arterio-venous oxygen difference (ml l−1, B), and oxygen uptake (ml min−1, C) were determined at rest and during 30 s, 5 min, and 10 min of passive movement of the leg. Blood flow values were time-matched with blood sample measurements using a linear connection of consecutive data points. Values are means ±s.e.m. (n= 6). †P < 0.05 vs. rest.
Muscle stretch and EMG activity during passive movement
The average fascicle lengthening was 0.3 mm deg−1 and the total magnitude of muscle lengthening during movement from 95 deg to 35 deg amounted to ∼20 mm (see Fig. 6 in the online Supplemental Material).
EMG measurements showed negligible activity in m. v. lateralis at 5, 15, 60, and 90 min of passive movement of the leg (see Fig. 7 in the online Supplemental Material).
Discussion
The main findings in the current study were that after 2 weeks of passive movement training there was a higher number of proliferating cells co-localized with endothelial cells and a higher number of capillaries around a fibre compared to before training. The training period also induced an increase in the mRNA level of eNOS, Tie-2 and MMP-9 in the passively trained leg and there was a main effect of the training period on MMP-2 and TIMP-1 mRNA levels. Moreover, acute passive movement induced an increase in interstitial VEGF as well as in the cell-proliferative effect of the interstitial fluid that was similar for the trained and the untrained leg. Combined, the results demonstrate that passive movement provides sufficient stimuli to initiate angiogenic processes allowing for capillary endothelial cell proliferation and a small but significant increase in capillarization.
The hypothesis of the present study was that the increase in blood flow and stretch of the muscle tissue with passive movement training would lead to repeated stimulation of angiogenic processes and consequent growth of capillaries. Long-term enhanced blood flow, and thus an increased level of shear stress, as well as passive muscle stretch combined with overload, have previously been shown to stimulate capillary growth in laboratory animals, although the experimental conditions in these animal models were chronic and the stretch model more severe than in the present study (Dawson & Hudlicka, 1989; Egginton et al. 1998). In our study, passive movement of the leg elevated blood flow about 3-fold, which is similar to the increase observed with prazosin treatment of rats (Egginton et al. 1998). An enhanced level of Ki67 positive cells, co-localized with endothelial cells, was observed after 2 weeks of training with passive movement sessions, indicating an enhanced proliferative activity of capillary endothelial cells in the muscle. The increase in Ki67 at 2 weeks was paralleled by a significant increase in the number of capillaries around a fibre (CAF) at 2 weeks and, although not significantly different, six out of the seven subjects showed an increase in C:F ratio with training with the level being numerically the highest at 2 weeks (mean 2.82 vs. 2.41 pre-training). The increase in Ki67 and CAF mean was no longer present after 4 weeks of training whereas CD was enhanced at this time. The transient increase in Ki67 is similar to that observed with active training, where an enhanced number of Ki67 positive cells was evident during but not at the end of a 6 week training period (Jensen et al. 2004). As Ki67 indicates proliferation of capillary endothelial cells it is likely that the increase in Ki67 precedes capillary growth and in the study by Jensen et al. (2004) the decline in Ki67 was associated with a lack of increase in capillarization with further training. Our interpretation of the data is, therefore, that passive movement training initiated angiogenic processes and capillary growth, although the magnitude of growth was less than that generally observed with active exercise. Growth of capillaries has been proposed to be dependent on a balance between positive and negative angiogenic factors, and only when the positive factors overcome the negative factors, growth occurs (Egginton, 2009). Thus, the positive angiogenic factors induced by the passive movement in the current study may have been sufficiently strong to initiate growth of capillaries, but not strong enough to proceed to the magnitude of capillary growth seen with active exercise training.
It should be mentioned that whereas CD was significantly increased at 4 weeks with passive training, the C:F ratio was not. This statistical discrepancy could probably be explained by one out of the seven subjects not showing a higher C:F ratio after training but a higher CD due to a small alteration in fibre area.
In accordance with a previous study from our laboratory (Hellsten et al. 2008), acute passive movement led to an enhanced VEGF concentration in the muscle interstitium. A novel finding in this study was, however, that the concentration of interstitial VEGF in response to passive movement was similar for the passively trained and the untrained leg. A parallel observation was that the marked 16-fold increase in endothelial cell proliferative effect of the muscle dialysate with acute passive and active exercise was similar in the trained and untrained leg. These findings are somewhat in contrast to findings on the effect of exercise training where a period of aerobic training was shown to lead to a decrease in the exercise induced muscle interstitial VEGF levels in young individuals (Gavin et al. 2007b) and a period of intense intermittent training reduced the proliferative effect of muscle interstitial fluid obtained during exercise (Jensen et al. 2004). A lower exercise induced response in angiogenic factors after training is believed to be due to a negative feed-back occurring when capillarization has increased. Therefore, it may be concluded that an increase in metabolism in combination with shear stress or passive stretch, as occurs during active exercise training, may be needed to alter the VEGF response. Nevertheless, the increase in interstitial VEGF with passive movement was similar to that observed during active exercise at 10 and 30 W in the current study and to that observed with active exercise in previous studies (Hoffner et al. 2003a; Jensen et al. 2004). These findings would suggest that the acute release of VEGF from skeletal muscle cells is not directly dependent on either active contraction or exercise intensity.
The passive movement training resulted in an increase in mRNA expression of several angiogenic genes: eNOS, Tie-2, MMP-2 and 9 and TIMP-1. These genes have previously been reported to be up-regulated by electrostimulation (Rivilis et al. 2002) and by endurance training (Frandsen et al. 2000; McAllister et al. 2005; Rullman et al. 2007). eNOS has been shown to be an important mediator of angiogenesis and the expression is induced by both shear stress and muscle contraction (Baum et al. 2004; Williams et al. 2006). In human skeletal muscle, eNOS is located in the vascular endothelial cells (Frandsen et al. 1996) and therefore it appears likely that, during exercise, physiological or biochemical signals originating from the luminal side, i.e. shear stress induced signalling, would induce the expression of this enzyme, rather than muscle derived metabolic signals. Our current and previous (Hellsten et al. 2008) findings on the effect of passive exercise support this notion by showing that eNOS mRNA is up-regulated by enhanced shear stress and passive tension, with little contribution by metabolism. Our findings also suggest that passive movement provides sufficient stimuli to sustain elevated levels of eNOS mRNA.
Matrix metalloproteinases (MMPs) have been shown to be important for contraction induced angiogenesis (Haas et al. 2000), but in contrast to eNOS, MMPs have been shown to be induced by stretch/overload induced by surgical extirpation but not by shear stress (Rivilis et al. 2002). Muscle stretch induces growth by sprouting where one of the first steps is degradation of the basement membrane, a process in which MMPs are involved (Egginton, 2009). Shear stress, on the other hand, is believed to induce capillary growth by longitudinal splitting where the lumen of the capillary is divided, a process that does not require significant alteration of the basement membrane. It therefore seems likely that the observed increase in MMP-2 and -9 mRNA levels was induced by the stretch component of the passive movement. The observed responses in the various angiogenic factors in this study suggest that passive movement activates capillary growth both by longitudinal splitting and sprouting processes.
Angiopoietin 1 and 2 remained unaffected by the passive movement training, but the expression of Tie-2 mRNA was higher after the period, suggesting that the angiopoietin system had been stimulated. This finding is in accordance with a study on the effect of resistance training in humans where Tie-2 mRNA was increased without an effect on the Ang-1/Ang-2 ratio (Gavin et al. 2007a). In some studies, training has been found to enhance the level of Ang-2 and reduce the level of Ang-1 (Lloyd et al. 2003); however, chronic shear stress induced by prazosin has been found to enhance Tie-2, down-regulate Ang-2 and not affect Ang-1 (Chlench et al. 2007). As Ang-1 stabilizes capillaries and Ang-2 prevents the effect of Ang-1 and promotes capillary growth it is probable that their levels will change transiently over the course of a training period, similar to VEGF. This could explain some of the discrepancies.
An unexpected observation in the present study was that alterations in mRNA levels of MMP-2, MMP-9 and TIMP-1 occurred also in the collateral leg that had not been passively moved. Moreover, there was an increase in the capillarization in the untrained leg. This observation would suggest that the training was associated with an increase in a plasma-borne compound that affected also the collateral leg. Such compounds could be cytokines, e.g. interleukin-6 (IL-6) or interleukin-8 (IL-8) as they have been found to affect MMP-2, MMP-9 and TIMP-1 expression (Kralisch et al. 2005; Lin et al. 2007; Thirumangalakudi et al. 2007). IL-6 and IL-8 has been shown to increase in muscle tissue, and IL-6 also in interstitial fluid and plasma in response to contraction (Steensberg et al. 2000; Akerstrom et al. 2005; Rosendal et al. 2005), and it is possible that passive movement would induce a similar response.
The model of passive movement was selected in order to examine the role of enhanced blood flow/shear stress and passive muscle stretch without significant increases in metabolism. The ability of the model to fulfil these criteria was examined by measurement of change in passive fibre length, determination of muscle EMG activity, muscle blood flow and oxygen uptake. These determinations revealed that blood flow to the passively moved limb increased about 3-fold above resting values. Oxygen uptake was negligibly enhanced, in particular considering that the blood flow and oxygen uptake corresponded to the whole leg, of which only about 20% represents the active muscle. A lack of muscle activity was also verified by the EMG response not being elevated above rest. Therefore, signals stemming from metabolism are unlikely to be main contributors to the angiogenic response during passive exercise.
The muscle fibres were stretched approximately 20% as determined by ultrasound transducer methodology. This magnitude of stretch is of similar magnitude as that seen in rat EDL in the chronic stretch model by surgical extirpation of the tibialis anterior (Egginton et al. 1998). The relative contribution of the increase in shear stress versus the enhanced passive stretch of the tissue is not possible to deduce but, based on the previously observed effect of chronic stretch and chronic shear stress on angiogenesis (Rivilis et al. 2002), it is likely that both shear stress and passive stretch of the tissue contributed to the angiogenic response. As shear stress is believed to induce growth by longitudinal splitting whereas stretch induces growth by sprouting it is likely that both of these growth processes were stimulated during passive leg movement, as also supported by our specific findings at the MRNA level.
The present study of the effect of a period of passive movement training provides basic knowledge about the physiological signals responsible for angiogenesis in skeletal muscle which is important for our understanding of what makes capillaries in skeletal muscle grow or degenerate, respectively. In addition, the passive movement model may prove to be a highly useful model for individuals who are unable to exercise due to poor blood supply to the leg, such as in peripheral arterial disease, or for maintaining capillarization in muscle during a period of inactivity due to bed-rest or injury.
In conclusion, the present study demonstrates that a period of regular passive movement training, which primarily enhances shear stress and passive stretch, provides sufficient stimuli to induce an angiogenic response as evidenced by enhanced proliferation of capillary endothelial cells, an increase in the number of capillaries around a fibre and capillary density as well as an up-regulation of several angiogenic factors in the muscle.
Acknowledgments
The Financial support of The Danish Ministry of Culture is gratefully acknowledged. The skillful technical assistance of Gemma Martinez Rubio Kroos, and Karina Olsen is gratefully acknowledged.
Glossary
Abbreviations
- Ang
angiopoietin
- CAF
capillaries around a fibre
- CD
capillary density
- C:F
capillaries per fibre
- eNOS
endothelial nitric oxide synthase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IL
interleukin
- MMP
matrix metalloproteinase
- NO
nitric oxide
- TIMP
tissue inhibitor of MMPs
- VEGF
vascular endothelial growth factor
Author contributions
The work was carried out at the Department of Exercise and Sports Sciences, University of Copenhagen, and Department of Sports Medicine, Bispebjerg Hospital, Copenhagen, Denmark. Conception and design of the experiments: B.H., N.R., J.B., Y.H. Collection, analysis and interpretation of data: B.H., N.R., J.B.-M., J.B.-M., J.B., Y.H. Drafting the article or revising it critically for important intellectual content: B.H., N.R., J.B.-M., J.B., Y.H. The final version was approved by all authors.
Supplement Information
References
- Akerstrom T, Steensberg A, Keller P, Keller C, Penkowa M, Pedersen BK. Exercise induces interleukin-8 expression in human skeletal muscle. J Physiol. 2005;563:507–516. doi: 10.1113/jphysiol.2004.077610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270:677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum O, Da Silva-Azevedo L, Willerding G, Wockel A, Planitzer G, Gossrau R, Pries AR, Zakrzewicz A. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol. 2004;287:H2300–H2308. doi: 10.1152/ajpheart.00065.2004. [DOI] [PubMed] [Google Scholar]
- Bojsen-Moller J, Hansen P, Aagaard P, Kjaer M, Magnusson SP. Measuring mechanical properties of the vastus lateralis tendon-aponeurosis complex in vivo by ultrasound imaging. Scand J Med Sci Sports. 2003;13:259–265. doi: 10.1034/j.1600-0838.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- Chlench S, Mecha DN, Hohberg M, Hoffmann C, Pohlkamp T, Beyer G, Bongrazio M, Da Silva-Azevedo L, Baum O, Pries AR, Zakrzewicz A. Regulation of Foxo-1 and the angiopoietin-2/Tie2 system by shear stress. FEBS Lett. 2007;581:673–680. doi: 10.1016/j.febslet.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Dawson JM, Hudlicka O. The effects of long term administration of prazosin on the microcirculation in skeletal muscles. Cardiovasc Res. 1989;23:913–920. doi: 10.1093/cvr/23.11.913. [DOI] [PubMed] [Google Scholar]
- Egginton S. Invited review: activity-induced angiogenesis. Pflugers Arch. 2009;457:963–977. doi: 10.1007/s00424-008-0563-9. [DOI] [PubMed] [Google Scholar]
- Egginton S, Hudlicka O, Brown MD, Walter H, Weiss JB, Bate A. Capillary growth in relation to blood flow and performance in overloaded rat skeletal muscle. J Appl Physiol. 1998;85:2025–2032. doi: 10.1152/jappl.1998.85.6.2025. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Hoffner L, Betak A, Saltin B, Bangsbo J, Hellsten Y. Endurance training does not alter the level of neuronal nitric oxide synthase in human skeletal muscle. J Appl Physiol. 2000;89:1033–1038. doi: 10.1152/jappl.2000.89.3.1033. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Lopez-Figueroa M, Hellsten Y. Localization of nitric oxide synthase in human skeletal muscle. Biochem Biophys Res Commun. 1996;227:88–93. doi: 10.1006/bbrc.1996.1472. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Drew JL, Kubik CJ, Pofahl WE, Hickner RC. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol (Oxf) 2007a;191:139–146. doi: 10.1111/j.1748-1716.2007.01723.x. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Ruster RS, Carrithers JA, Zwetsloot KA, Kraus RM, Evans CA, Knapp DJ, Drew JL, McCartney JS, Garry JP, Hickner RC. No difference in the skeletal muscle angiogenic response to aerobic exercise training between young and aged men. J Physiol. 2007b;585:231–239. doi: 10.1113/jphysiol.2007.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TL, Milkiewicz M, Davis SJ, Zhou AL, Egginton S, Brown MD, Madri JA, Hudlicka O. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H1540–H1547. doi: 10.1152/ajpheart.2000.279.4.H1540. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R975–R982. doi: 10.1152/ajpregu.00677.2007. [DOI] [PubMed] [Google Scholar]
- Hoffner L, Nielsen JJ, Langberg H, Hellsten Y. Exercise but not prostanoids enhance levels of vascular endothelial growth factor and other proliferative agents in human skeletal muscle interstitium. J Physiol. 2003;550:217–225. doi: 10.1113/jphysiol.2002.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson PA, Veneman T, Nurjhan N, Gerich J. An improved method to calculate adipose tissue interstitial substrate recovery for microdialysis studies. Life Sci. 1994;54:1621–1624. doi: 10.1016/0024-3205(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Jensen L, Bangsbo J, Hellsten Y. Effect of high intensity training on capillarization and presence of angiogenic factors in human skeletal muscle. J Physiol. 2004;557:571–582. doi: 10.1113/jphysiol.2003.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M. Proinflammatory adipocytokines induce TIMP-1 expression in 3T3-L1 adipocytes. FEBS Lett. 2005;579:6417–6422. doi: 10.1016/j.febslet.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Hellsten Y, Bangsbo J. Intense interval training enhances human skeletal muscle oxygen uptake in the initial phase of dynamic exercise at high but not at low intensities. J Physiol. 2004;559:335–345. doi: 10.1113/jphysiol.2004.062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Zhang W, Fan Y, Mulholland M. Interleukin-1β and interleukin-6 stimulate matrix metalloproteinase-9 secretion in cultured myenteric glia. J Surg Res. 2007;137:38–45. doi: 10.1016/j.jss.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Lloyd PG, Prior BM, Yang HT, Terjung RL. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. Am J Physiol Heart Circ Physiol. 2003;284:H1668–H1678. doi: 10.1152/ajpheart.00743.2002. [DOI] [PubMed] [Google Scholar]
- McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol. 2005;98:753–761. doi: 10.1152/japplphysiol.01263.2003. [DOI] [PubMed] [Google Scholar]
- Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J. Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol. 2003;285:R143–R148. doi: 10.1152/ajpregu.00029.2003. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior SJ, McKenzie MJ, Joseph LJ, Ivey FM, Macko RF, Hafer-Macko CE, Ryan AS. Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation. 2009;16:203–212. doi: 10.1080/10739680802502423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H1430–H1438. doi: 10.1152/ajpheart.00082.2002. [DOI] [PubMed] [Google Scholar]
- Rosendal L, Sogaard K, Kjaer M, Sjogaard G, Langberg H, Kristiansen J. Increase in interstitial interleukin-6 of human skeletal muscle with repetitive low-force exercise. J Appl Physiol. 2005;98:477–481. doi: 10.1152/japplphysiol.00130.2004. [DOI] [PubMed] [Google Scholar]
- Rullman E, Rundqvist H, Wagsater D, Fischer H, Eriksson P, Sundberg CJ, Jansson E, Gustafsson T. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol. 2007;102:2346–2351. doi: 10.1152/japplphysiol.00822.2006. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Steensberg A, van HG, Osada T, Sacchetti M, Saltin B, Klarlund PB. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumangalakudi L, Yin L, Rao HV, Grammas P. IL-8 induces expression of matrix metalloproteinases, cell cycle and pro-apoptotic proteins, and cell death in cultured neurons. J Alzheimers Dis. 2007;11:305–311. doi: 10.3233/jad-2007-11307. [DOI] [PubMed] [Google Scholar]
- Tsurumi Y, Murohara T, Krasinski K, Chen D, Witzenbichler B, Kearney M, Couffinhal T, Isner JM. Reciprocal relation between VEGF and NO in the regulation of endothelial integrity. Nat Med. 1997;3:879–886. doi: 10.1038/nm0897-879. [DOI] [PubMed] [Google Scholar]
- Williams JL, Cartland D, Hussain A, Egginton S. A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. J Physiol. 2006;570:445–454. doi: 10.1113/jphysiol.2005.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


