Abstract
Neurospora crassa utilizes DNA methylation to inhibit transcription of heterochromatin. DNA methylation is controlled by the histone methyltransferase DIM-5, which trimethylates histone H3 lysine 9, leading to recruitment of the DNA methyltransferase DIM-2. Previous work demonstrated that the histone deacetylase (HDAC) inhibitor trichostatin A caused a reduction in DNA methylation, suggesting involvement of histone deacetylation in DNA methylation. We therefore created mutants of each of the four classical N. crassa HDAC genes and tested their effect on histone acetylation levels and DNA methylation. Global increases in H3 and H4 acetylation levels were observed in both the hda-3 and the hda-4 mutants. Mutation of two of the genes, hda-1 and hda-3, caused partial loss of DNA methylation. The site-specific loss of DNA methylation in hda-1 correlated with loss of H3 lysine 9 trimethylation and increased H3 acetylation. In addition, an increase in H2B acetylation was observed by two-dimensional gel electrophoresis of histones of the hda-1 mutant. We found a similar increase in the Schizosaccharomyces pombe Clr3 mutant, suggesting that this HDAC has a previously unrecognized substrate and raising the possibility that the acetylation state of H2B may play a role in the regulation of DNA methylation and heterochromatin formation.
METHYLATION of selected cytosines in DNA is common in animals, plants, and fungi and serves in both genome defense and gene regulation (Selker 2004; Zilberman 2008). Despite recent progress in understanding the mechanisms of DNA methylation, its regulation remains largely undefined. For example, the extent to which various histone modifications affect DNA methylation has not been established. The filamentous fungus Neurospora crassa provides a particularly favorable model system to investigate such issues. DNA methylation in N. crassa is governed by DIM-5, a histone methyltransferase (HMTase) that trimethylates H3 lysine 9 (H3K9) (Tamaru and Selker 2001; Tamaru et al. 2003). Methylated H3K9 is bound by heterochromatin protein-1 (HP1) (Freitag et al. 2004a), which directly recruits the DNA methyltransferase (DMTase) DIM-2 (Honda and Selker 2008). Phosphorylation of H3 serine 10 (H3S10) interferes with methylation of H3K9 by DIM-5 (Adhvaryu and Selker 2008). This study was motivated by an early indication that the acetylation state of one or more histones bears on DNA methylation. In particular, we noted that the histone deacetylase (HDAC) inhibitor trichostatin A (TSA) inhibited some DNA methylation in N. crassa (Selker 1998). TSA can also influence DNA methylation in plant and animal systems (Chen et al. 1998; Cameron et al. 1999; Ou et al. 2007). Because methylation of H3K9 is required for DNA methylation, a simple hypothesis was that removal of an acetyl group from this residue is required to generate a substrate for the H3K9 MTase, DIM-5. Moreover, it seemed possible that acetylation of other residues, such as H3K14, might indirectly influence DNA methylation, for example, by interference with the binding of HP1 (Mateescu et al. 2004). Other possibilities exist of course.
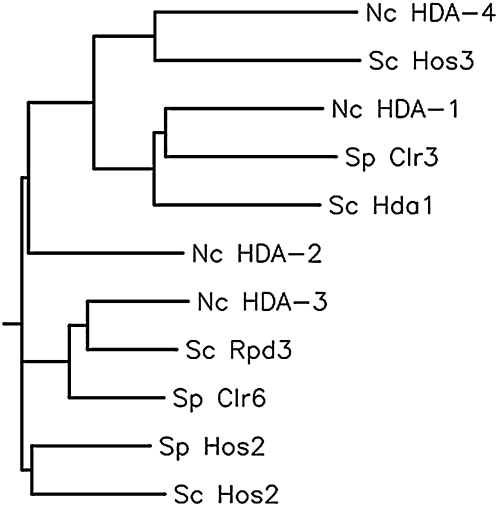
Histone deacetylases have been shown to play prominent roles in the regulation of gene expression (Cunliffe 2008; Hollender and Liu 2008; McDonel et al. 2009). Extensive work in yeasts, which lack DNA methylation, has linked particular histone acetylation to cases of both gene activation and repression (Robyr et al. 2002; Wiren et al. 2005; Sinha et al. 2006). A phylogenetic tree based on a single alignment of protein sequences of Saccharomyces cerevisiae and Schizosaccharomyces pombe HDACs and their N. crassa homologs is shown in Figure 1. Actively transcribed chromatin regions in S. cerevisiae are enriched for H3 acetylation on K9, K18, and K27 and lack acetylation on H4 K16 and H2B K11 and -K16 (Kurdistani et al. 2004). Rpd3 and Hda1 are responsible for deacetylation of the promoters of distinct sets of genes (Robyr et al. 2002). Rpd3 is a global repressor, and mutants lacking this enzyme show increased acetylation of H4 K5 and -K12 and H3 K18 at derepressed genes (Robyr et al. 2002). Hda1 deacetylates subtelomeric regions as well as the promoters of a large set of genes, most of which do not depend on Rpd3.
Figure 1.—
Phylogenetic tree based on a single alignment of predicted protein sequences of N. crassa (Nc) and related S. cerevisiae (Sc) and S. pombe (Sp) HDACs. Even though N. crassa HDA-2 is not monophyletic with Hos2 in the tree, pairwise BLAST comparisons indicate that Hos2 is indeed the closest homolog of Neurospora HDA-2.
How HDACs would be involved in DNA methylation is not obvious, but similarities between known components of the heterochomatin/DNA methylation machinery of Neurospora and the heterochomatin machinery of S. pombe (which lacks DNA methylation) suggest that information from S. pombe may provide clues. As in Neurospora, heterochromatin formation in S. pombe requires an H3 K9 HMTase (Clr4) and an HP1 homolog (Swi6), as well as additional factors. Notably, the HDAC Clr3 is a component of the SHREC complex found at all heterochromatic loci (Sugiyama et al. 2007) and is important for heterochromatin formation. Clr3 deacetylase activity has been shown to recruit Clr4 to establish and maintain heterochromatin (Yamada et al. 2005).
We found four Neurospora genes with hallmarks of classical histone deacetylases (de Ruijter et al. 2003) and named them hda-1–hda-4 (Borkovich et al. 2004). As a step in assessing their possible role in DNA methylation, we attempted to disrupt each of the hda genes but found indications that one, hda-3, is essential for viability. Interestingly, a partial loss-of-function allele, hda-3RIP1, resulted in partial loss of DNA methylation. Mutation of two other genes, hda-2 and hda-4, had no discernible effect on DNA methylation. Finally, a null allele of hda-1 resulted in a regional DNA methylation defect, with some chromosomal regions showing no loss of methylation, some a partial loss, and others an apparent complete loss of methylation. We explored the mechanism of this loss of DNA methylation by investigating histone modification changes in the hda mutants.
MATERIALS AND METHODS
Protein sequence alignments:
N. crassa HDAC protein sequences were aligned against S. cerevisiae and S. pombe sequences using CLUSTALW, and a PHYLIP rooted phylogenetic tree based on this alignment (Figure 1) was generated at the Biology WorkBench (http://seqtool.sdsc.edu/CGI/BW.cgi). Protein accession numbers are listed in Table 1.
TABLE 1.
Histone deacetylases in Neurospora and their closest yeast homologs
| N. crassa | S. cereviseae | S. pombe |
|---|---|---|
| HDA-1 (XP_956974) | Hda1 (NP_014377) | Clr3 (NP_595104) |
| HDA-2 (XP_964451) | Hos2 (NP_011321) | Hos2 (NP_594079) |
| HDA-3 (XP_964367) | Rpd3 (NP_014069) | Clr6 (NP_595333) |
| HDA-4 (XP_961839)a | Hos3 (NP_015209) |
Protein accession numbers are in parentheses.
The Neurospora database NCU07018 predicts a different start codon, which adds 139 N-terminal amino acids compared to this National Center for Biotechnology Information reference sequence.
Strains and growth conditions:
All N. crassa and S. pombe strains used in this study are listed in Table 2. Standard conditions were used for N. crassa growth and maintenance (Davis 2000). S. pombe strains were grown in YES medium (5 g/liter yeast extract, 30 g/liter glucose supplemented with 225 mg/liter adenine, histidine, leucine, uracil, and lysine hydrochloride) to mid-log phase using standard conditions.
TABLE 2.
Strains used in this study
| Strain | Genotype | Source/reference |
|---|---|---|
| N150 | matA | FGSC 2489 |
| N411 | matahis-3 | FGSC 6524 |
| N617 | mata; am RIP8 | Singer et al. (1995) |
| N623 | matA his-3 | FGSC 6103 |
| N1167 | mata; mtrRIP2; trp-2 | Rountree and Selker (1997) |
| N1673 | matA his-3; am132 inl | Hays et al. (2002) |
| N1674 | matA his-3; lys-1 am132 inl; amRIP:hph:amRIP | Hays et al. (2002) |
| N1877 | matahis-3; Δdim-2∷hph | Kouzminova and Selker (2001) |
| N2531 | matahis-3; hda-4RIP1 | This study |
| N2540 | matA ridRIP1 his-3+∷ccg-1-hpo+sgfp+ | This study |
| N2627 | matA hda-2RIP1 | This study |
| N2670 | matA; amRIP8 hda-1RIP1 | This study |
| N2666 | matA his-3+∷ccg-1-hpo+sgfp+;hda-3RIP1 | This study |
| N2849 | mata; hda-1RIP1; mtrRIP2; amRIP8; trp-2 | This study |
| N3152 | mata; Δhda-1∷hph | FGSC 12003 |
| N3349 | matA Δhda-2∷hph | FGSC 11158 |
| N3351 | mata; Δhda-4∷hph | FGSC 11175 |
| N3610 | mata; Δhda-1∷hph | This study |
| N3406 | matA; qde-2RIP; sms-2RIP | Y. Liu |
| N3721 | mata; am RIP8 mtrRIP2 trp-2 | This study |
| N4063 | Δhda-1∷hph; qde-2RIP; sms-2RIP | This study |
| N4065 | Δhda-1∷hph; qde-2RIP; sms-2RIP | This study |
| SPG 17 | h90 leu1-32 ade6-216 his2 ura4 | S. Grewal |
| SPT 381 | h+ leu1-32 ade6-216 ura4DS/E, otr1R∷ura4+clr3Δ∷kan | S. Grewal |
Creation of mutants using repeat-induced point mutation:
The hda-1, hda-2, and hda-3 genes were isolated by PCR amplification of gene fragments using degenerate primers for conserved regions (Table 3) that were used as probes of the Orbach-Sachs cosmid library and Neurospora λ genomic library. The hda-4 gene was identified by BLAST-searching the Neurospora genome database (http://www.broad.mit.edu/annotation/genome/neurospora/Home.html) using known histone deacetylase domains from S. cerevisiae, Homo sapiens, and Drosophila melanogaster as query sequences.
TABLE 3.
Primer sequences for histone deacetylase detection and amplification
| No. | Name | Sequence |
|---|---|---|
| 1297 | hda-1 deg 5′ | TCCGGCCTCCCGGNCAYCAYGC |
| 1298 | hda-1 deg 3′ | CCGTTGCCGTTGTTGAYRTCCCARTC |
| 503 | hda-2 deg 5′ | CAGTTCAACGTCGGCGANGAYTGYCC |
| 504 | hda-2 deg 3′ | CCNAARACRATRCAGCAGTTGCTGTAGCAGGAGCC |
| 503 | hda-3 deg 5′ | CAGTTCAACGTCGGCGANGAYTGYCC |
| 605 | hda-3 deg 3′ | NCCNGGRAARAAYTCNCCRTA |
Genes were subcloned into his-3 targeting vectors (Margolin et al. 1997) and transformed into his-3 strains N623 (hda-1 and -3), N1673 (hda-2), or N1674 (hda-4) by electroporation (Margolin et al. 1997). His-3+ transformants were selected and crossed to N617 (hda-1, -2, and -3) or N411 (hda-4). Genomic DNA from the progeny was analyzed for RFLPs indicative of mutations from repeat-induced point mutation (RIP) (Cambareri et al. 1989) by Southern hybridization analyses. Mutant strains were backcrossed to N617 (hda-1), N2540 (hda-3), or N623 (hda-2 and hda-4). Sequencing of the mutant alleles was performed on strains showing RFLPs.
Southern hybridization analyses:
Genomic DNA was isolated following 2 days of growth in Vogel's 2% sucrose media as described (Miao et al. 2000). Strains used were dim-2 (N1877), wild type (N617), hda-1RIP1 (N2670), Δhda-1 (N3152), hda-2RIP1 (N2627), Δhda-2 (N3349), hda-3RIP1 (N2666), hda-4RIP1 (N2531), Δhda-4 (N3351), Δhda-1 (N3610), qde-2RIP; sms-2RIP (N3406), and Δhda-1; qde-2RIP; sms-2RIP (N4063 and N4065). All deletion strains were generated by the Neurospora functional genomics program (Colot et al. 2006) and provided by the Fungal Genetics Stock Center (FGSC). Approximately 0.5 μg of DNA was digested overnight with the designated restriction endonuclease and fractionated by electrophoresis on 0.8% agarose gels. Transfer to membranes and blotting were performed as previously described (Miao et al. 2000). When TSA was used, it was added to a final concentration of 1 μm, and the strains were grown with or without the drug for only 27 hr (Selker 1998). All blots were reprobed to confirm complete DNA digestion (data not shown).
Western blots:
Nuclei were isolated as described (Baum and Giles 1986) with the inclusion of HDAC inhibitors TSA (1 μm; Wako) (Selker 1998) and butyrate (50 mm; Sigma) in all buffers. Nuclear proteins were separated by 10% SDS-PAGE. Following transfer to PVDF membrane in 10 mm N-cyclohexyl-3-aminopropanesulfonic acid, pH 11, 20% methanol, blots were probed as previously described (Tamaru et al. 2003) in PBS including 3% nonfat dry milk powder using the following antibodies: α-H3 (Abcam ab1791), α-H3K4me2 (Upstate 07-030), α-H3 acetyl K9 (Abcam ab4441), α-H3 acetyl K14 (Upstate 06-911), α-H3 acetyl K9/K14 (Upstate 06-599), α-H3 acetyl K18 (Upstate 07-354), α-H3 acetyl K23 (Upstate 07-355), α-H3 acetyl K27 (Upstate), and α-H4 tetra-acetyl (Upstate 06-866). Strains used were wild type (N617), hda-1 (N2670), hda-2 (N2627), hda-3 (N2666), and hda-4 (N2531).
Chromatin immunoprecipitation:
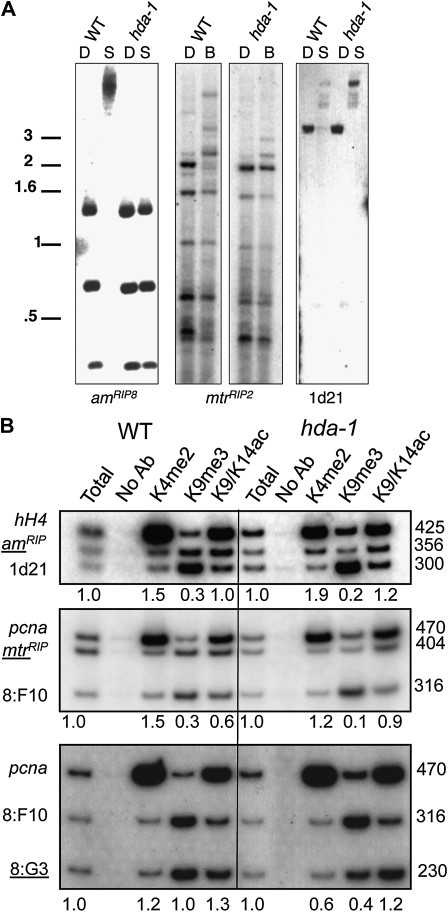
Chromatin immunoprecipitation (ChIP) experiments were performed as previously described (Tamaru et al. 2003) with the antibodies listed above and α-H3 trimethyl K9 (Cowell et al. 2002) on wild type (N150), hda-1 (N2849), Δhda-1 (N3610), qde-2RIP; sms-2RIP (N3406), and αhda-1; qde-2RIP; sms-2RIP (N4063 and N4065) strains. HDAC inhibitors described above were included in the ChIP lysis buffer for the ChIP experiments shown in Figure 4. Primer sequences not published elsewhere (Adhvaryu and Selker 2008; Smith et al. 2008) are for amRIP8 (468: GATCCGATGTCACGGACAAG and 469: GCCTGCTCGAACTCGGGCTC).
Figure 4.—
Chromatin immunoprecipitation shows increased H3 acetylation at regions that lose methylation in hda-1RIP1. (A) Southern analysis showing loss of DNA methylation in hda-1RIP1 at amRIP8 and mtrRIP2, but not 1d21. Strains are N617 (WT) and N2670 (hda-1) for amRIP8 and 1d21 blots and digests are DpnII (D) and Sau3AI (S). For the mtrRIP2 blot, strains are N3721 (WT) and N2849 (hda-1), and the 5mC-sensitive isoschizomer BfuCI (B) was used instead of Sau3AI. (B) Triplex PCR reactions were performed with ChIP'd DNA as template in wild-type (WT) and hda-1RIP1 strains, with antibodies specific for H3 K4me2, H3 K9me3, and H3 K9/K14acetyl. hH4 and pcna are euchromatic controls, and the other two regions shown in each panel are methylated in wild type. Regions that lose DNA methylation in hda-1RIP1 are underlined. Sizes of PCR products are shown on the left, and relative enrichment is shown under the top, middle, and bottom. Relative enrichment is calculated as the ratio of amRIP8:1d21, mtrRIP2:8:F10, and 8:G3: 8:F10, normalized to this ratio in the total input DNA.
Two-dimensional gel electrophoresis:
Nuclei were isolated as for Western blots above and resuspended in an equal volume of 0.15 m NaCl, 10 mm Tris–Cl (pH 8.0), and 1 mg/ml protamine sulfate. Histones were extracted from the nuclear suspensions as previously described (Green and Do 2009). An equal volume of ice-cold 0.4 m H2SO4 was added to the nuclear suspension, followed by incubation on ice for 24 hr with occasional mixing. Acid-insoluble material was pelleted by centrifugation at 14,000 × g for 10 min. The supernatant was mixed with 100% trichloroacetic acid (TCA) to a final concentration of 20% and incubated on ice for 1 hr. TCA-insoluble proteins were collected by centrifugation at 14,000 × g at 4° for 10 min. The protein pellet was resuspended in ice-cold acetone, sonicated in a bath sonicator, and recovered by centrifugation at 14,000× g at 4° for 10 min. The acetone precipitate was air-dried at room temperature and then dissolved in acetic acid–urea loading buffer (8.0 m urea, 5% acetic acid, 5% β-mercaptoethanol, 0.2 mg/ml crystal violet) to a concentration of 5 mg/ml. Histones were resolved by two-dimensional gel electrophoresis (2DGE) as described (Green and Do 2009). The first-dimension gel contained acetic acid–urea–Triton X-100 (12% polyacrylamide, 5% acetic acid, 6 mm Triton X-100, and 5.0 m urea). The second-dimension gel contained acetic acid–urea (12% acrylamide, 5% acetic acid, and 8 m urea). Gels were stained in 0.05% Coomassie blue R-250, 40% ethanol, and 5% acetic acid; destained in 20% ethanol and 0.5% acetic acid; dried between two cellophane sheets, and scanned with a flatbed scanner.
RESULTS
Characterization of Neurospora hda genes:
We identified and characterized Neurospora genes encoding HDACs to study the potential role that histone acetylation may play in controlling DNA methylation. At the time that this study began, the N. crassa genome sequence was unavailable. We therefore identified Neurospora HDAC genes by PCR amplification using degenerate primers. We isolated three genes, which we named hda-1–hda-3. When the genome sequence was released, we discovered a fourth HDAC gene, hda-4. In addition, seven NAD-dependent deacetylases, named nst-1–nst-7, are found in the N. crassa genome (Borkovich et al. 2004). The nst genes do not appear to play a role in DNA methylation, although at least three of the seven are required for transgene silencing by heterochromatin at Neurospora telomeres (Smith et al. 2008). Since the HDAC inhibitor TSA inhibits some DNA methylation (Selker 1998), we predicted that increased histone acetylation caused by a deficiency in one or more HDACs would result in reduced DNA methylation. We therefore disrupted each of the four HDAC genes and analyzed the mutants for defects in DNA methylation and histone acetylation.
Creation of Neurospora hda mutant strains by RIP:
We used RIP to generate mutations in the four hda genes by targeting a second copy of each gene to the his-3 locus and then crossing the duplication strains to induce RIP (Selker et al. 1989). We recovered mutant progeny for each of the four hda loci. The hda-1RIP1 sequence revealed a Q179 to stop mutation (Figure S1). Therefore, the gene product should not include a complete deacetylase domain, implying that this should be a null allele. We found indications that hda-3 is essential for viability of Neurospora, and successfully isolated an apparent partial loss-of-function allele, hda-3RIP1. Most of the ascospores from the cross to induce RIP of hda-3 were white and nonviable, evidence of lethal mutations. Sequencing of the hda-3RIP1 allele revealed several nonconservative substitutions in conserved residues within the deacetylase domain and a stop codon downstream of the deacetylase domain at Q385 (Figure S2). The stop codon at Q385 is upstream of a C-terminal domain that is conserved in filamentous fungi and required for in vitro deacetylase activity of the Aspergillus nidulans homolog, RpdA (Tribus et al. 2010). We expect that the mutant protein generated from the hda-3RIP1 allele retains some activity because we recovered few progeny and failed to isolate a null allele by RIP, and later, the Neurospora Genome Project (Colot et al. 2006) could not isolate a homokaryotic strain containing a knockout allele of this gene (http://www.fgsc.net/). Sequencing of the hda-2RIP1 and hda-4RIP1 alleles revealed stop codons upstream of the deacetylase domains in each case (data not shown). We subsequently obtained knockout strains for hda-1, hda-2, and hda-4 generated by the Neurospora Genome Project (Table 2).
DNA methylation defects in Neurospora hda mutants:
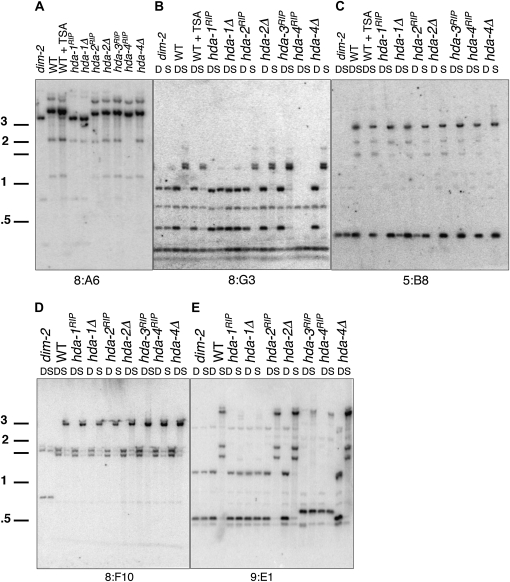
To assess the effect of HDAC mutations on DNA methylation, we performed Southern hybridization analyses using restriction enzymes that are sensitive to 5-methyl-cytosine (5mC) in their recognition sites. In Neurospora, DNA methylation is found almost exclusively in RIP-generated, AT-rich regions scattered throughout the genome and concentrated at centromeres and telomeres (Smith et al. 2008; Lewis et al. 2009). We analyzed the methylation status of several well-studied heterochromatic loci in hda mutants by Southern analyses using genomic DNA digested with various restriction enzymes, including the isoschizomers Sau3AI (5mC-sensitive) and DpnII (5mC-insensitive) (Figure 2, B–E). We included the dim-2 mutant as a control for complete loss of methylation (Kouzminova and Selker 2001) and compared the results for the various mutants with the effect of TSA treatment (Figure 2, A–C). Analysis of BglII sites at the 8:A6 region (Figure 2A) revealed that treatment of the control strain with TSA caused a partial loss of methylation, resulting in fragments representing both methylated and unmethylated sites (compare with results for dim-2 and wild-type strains). Interestingly, DNA from both the hda-1RIP1 and Δhda-1 strains produced a band indicative of loss of methylation. None of the other hda mutants showed evidence of altered DNA methylation in the 8:A6 region. TSA treatment caused no detectable loss of methylation in the 8:G3 or 5:B8 regions (Figure 2, B and C), consistent with the previous finding that this HDAC inhibitor causes significant loss of DNA methylation in relatively few loci (see above and Selker 1998). Similarly, we found that loss of methylation in hda-1 is locus-specific. Striking loss of cytosine methylation was detected at 8:A6 (Figure 2A), 9:E1 (Figure 2E), and RIP'd alleles of am and mtr (see below), but other regions showed a partial loss of methylation (8:G3 and 5:B8 in Figure 2, B and C). No loss of methylation was found in another set of loci, including 8:F10 (Figure 2D) and 1d21 (see below). In every case, the hda-1RIP1 mutant and the knockout mutant showed identical methylation phenotypes, consistent with our conclusion that both are null alleles.
Figure 2.—
DNA methylation is lost at some loci in hda-1. Southern blots showing genomic DNAs digested with 5mC-sensitive BglII (A) or with 5mC-insensitive DpnII (lane D) and 5mC-sensitive Sau3AI (lane S). Probes are indicated at the bottom of each autoradiogram for panels A–E.
A partial loss of methylation in the hda-3RIP1 mutant was detected at the amRIP:hph:amRIP and 8:F8 loci but not at other regions tested (Figure S3). Methylation at 8:A6, 8:G3, 5:B8, and 8:F10 (Figure 2, A–D) appeared similar to that of wild-type strains. We cannot conclude, however, that hda-3 is not important for DNA methylation elsewhere because it was not possible to generate a null allele. The hda-3RIP1 and hda-4RIP1 mutants have a different allele of 9:E1 than wild type, accounting for the RFLPs in these two strains (Figure 2E); the hda-4RIP1 mutant lacks the 8:G3 sequence (Figure 2B). The hda-2 and hda-4 mutations did not affect DNA methylation at any region tested, and again, the deletion and RIP-mutated alleles gave identical results.
The stabilization of heterochromatin in S. pombe involves both the RNAi-mediated RITS complex and the Clr3 HDAC-mediated SHREC complex (Grewal 2010). Previous work from our lab demonstrated that RNAi components in Neurospora are dispensable for the establishment and maintenance of heterochromatin and DNA methylation (Freitag et al. 2004b). We considered the possibility that redundant systems, similar to S. pombe, were at work maintaining heterochromatin and DNA methylation in Neurospora. To investigate this possibility, we created a strain that was mutant for both of Neurospora's argonaute proteins (Catalanotto et al. 2002; D. W. Lee et al. 2003; H. C. Lee et al. 2010), qde-2RIP and sms-2RIP, in combination with the hda-1Δ allele. We then assayed DNA methylation and histone H3 K9me3 at regions that showed little to no change in the hda-1Δ strain alone. DNA methylation at the 5:B8 and 8:F10 regions (Figure S4, A and B) and histone H3 K9me3 at the 8:F10 region (Figure S4C) remained unaltered in the hda-1Δ; qde-2RIP;sms-2RIP triple mutant. These results are consistent with our previous observations that the classical RNAi machinery does not play a role in the maintenance of heterochromatin and DNA methylation in Neurospora.
Global changes in site-specific acetylation of histones H3 and H4 in Neurospora hda mutants revealed by Western blotting:
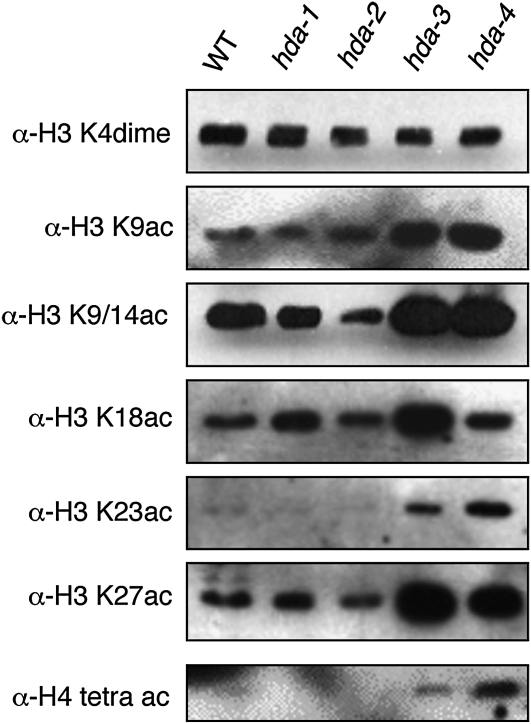
To investigate how hda mutations interfere with DNA methylation, we first tested the overall acetylation status at histone residues that can be assessed by Western blotting of nuclear proteins with available antibodies for specific histone H3 and H4 acetylation sites (Figure 3). The hda-3RIP1 and hda-4RIP1 mutants showed increased acetylation at every site assayed except at H3 K18 in hda-4RIP1, suggesting that Neurospora HDA-3 and HDA-4 have broad specificities on histones H3 and H4. The hda-2RIP1 mutation, which showed no effect on DNA methylation, caused no significant global increase in acetylation of either H3 or H4 by the Western analysis although there may be a small decrease in acetylation in this mutant at H3 K9/14 and K18. Interestingly, the mutant that showed markedly reduced DNA methylation, hda-1RIP1, showed a possible small increase in H3 K18 acetylation but did not show a prominent global change in acetylation with any of the available antibodies against acetylated H3 and H4. Mutation of H3 K18 has no effect on DNA methylation in vivo and does not affect DIM-5 activity in vitro (K. Adhvaryu, E. Berge, H. Tamaru, M. Freitag and E. Selker, unpublished results).
Figure 3.—
Histone hyper-acetylation in hda mutants. Western blots of nuclear proteins from wild type (WT) or from the indicated hdaRIP mutant strains were probed with the antibody designated at the left of the blots. Antibodies to H3K4me2 (top) and unmodified H3 (not shown) were used to verify equal loading of histones.
Local changes in H3 K9/K14 acetylation in Neurospora HDAC mutants revealed by chromatin immunoprecipitation:
The Western blots did not provide clear evidence for a change in global acetylation of H3 and H4 that might account for the effect of hda-1RIP on DNA methylation at selective chromosomal regions. We therefore turned to ChIP experiments to test for altered acetylation at representative affected loci. ChIP/PCR experiments failed to show increased H3 K18 acetylation at regions that lose DNA methylation (data not shown), but subtle effects were observed elsewhere (Figure 4). We directly compared two regions that are normally methylated, including one that loses methylation (underlined in Figure 4B) and one that is unaffected. PCR amplification of regions with DNA methylation is challenging because these regions are typically repetitive and AT-rich. We therefore generated strains carrying additional methylated low-copy-number regions, RIP'd alleles of am (amRIP8) and mtr (mtrRIP2) that we found lost their DNA methylation in an hda-1RIP1 background (Figure 4A). An additional methylated region unaffected by hda-1RIP1, 1d21, was also studied. We performed triplex PCR, including primers to a euchromatic gene as a control (hH4 or pcna) and primers for two methylated regions. Direct comparison of a methylated region to the euchromatic control can give misleading results because global increases in acetylation may also change the level of acetylation at euchromatic regions. Therefore we used triplex PCRs, including a euchromatic region as an additional internal control, but for quantification of enrichment we calculated the ratio of the methylated region that loses methylation (underlined in Figure 4B) to the methylated region that retains methylation (1d21 or 8:F10).
As expected, in wild-type strains, heterochromatic regions showed high H3K9me3 and low H3K4me2, while the euchromatic regions showed the opposite pattern (Figure 4B). Heterochromatic regions that showed loss of DNA methylation in hda-1RIP (amRIP8, mtrRIP2, and 8:G3) showed specific loss of H3K9me3, and regions that retained DNA methylation (1d21 and 8:F10) also retained methylation of H3K9. The hda-1 mutation had no obvious effect on K4me2. Little change in relative enrichment of H3 K9/K14 acetylation was seen at regions that had lost DNA methylation; the enrichment at amRIP8 increased from 1.0 to 1.2 relative to 1d21 (Figure 4B, top), and the ratio of H3 K9/K14 acetylation increased from 0.6 to 0.9 at mtrRIP2 relative to 8:F10 (Figure 4B, middle); the relative enrichment of H3 acetylation at 8:G3 did not increase, but it is noteworthy that while both amRIP8 and mtrRIP2 completely lose DNA methylation in hda-1RIP1 (Figure 4A), 8:G3 shows only a partial loss of DNA methylation (Figure 2B). The slightly increased acetylation at amRIP8 and mtrRIP2 was observed only with the antibody that detects H3 acetylated at either K9 or K14. No change was observed with an antibody specific for acetylation of only H3K9 acetylation. This suggests that this modest effect was specific to K14, consistent with results for the closely related S. pombe HDAC Clr3 (Sugiyama et al. 2007), but no specific antibodies were available to confirm this.
Global changes in histone modification state in Neurospora hda mutants revealed by 2DGE:
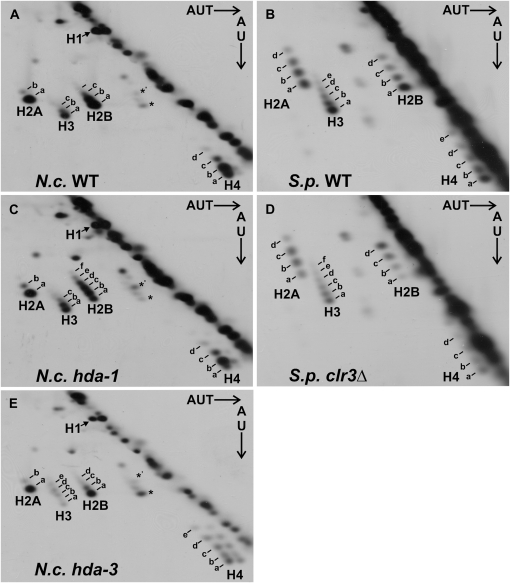
Considering that the effects of the hda-1 and hda-3 mutations on sites of acetylation that could be assayed with available antibodies were subtle, we turned to an approach to examine all four core histones of the hda mutants for aberrant acetylation. In particular, we employed high-resolution 2DGE to resolve histones according to their relative charge/mass ratios (Green and Do 2009). In this system, charge-altering modifications such as acetylation and phosphorylation reduce migration of the protein spot in both dimensions, yielding derivative species (spots labeled “b,” “c,” “d,” etc. in Figure 5) that migrate diagonally to the top left of the parent spot “a.” Recent studies in our laboratories indicate that the origin of most, if not all, derivative spots observed in Neurospora H3 and H2B histones are generated by histone acetylation, as opposed to histone phosphorylation (Anderson et al. 2010). Our results revealed a number of effects of the hda mutations. First, the results confirmed the small increase in acetylation of H3 detected by Western analysis of the hda-1RIP1 mutant (Figure 3), as demonstrated by increased intensity of the derivative H3 spot labeled “b” and decreased intensity of the parent H3 spot (“a”) in the hda-1RIP1 mutant (Figure 5C) relative to the wild-type strain (WT; Figure 5A). The shifted spot is indicative of charge neutralization, presumably due to acetylation, resulting in slower mobility in the first and second dimensions.
Figure 5.—
Global changes in histone modification state revealed by two-dimensional gel electrophoresis (2DGE). (A) Wild-type (WT) (N617) Neurospora core histones; all have additional diagonal spots above the bottom parent spot, which represent additional acetylated states of each histone. (B) WT S. pombe (SPG 17) histones. (C) Neurospora hda-1RIP1 mutant (N2670) shows increased acetylation of H2B and a slight increase in H3 acetylation compared to WT (A). (D) S. pombe clr3 mutant (SPT 381) shows increased H2B acetylation. (E) Neurospora hda-3RIP1 mutant (N2666) shows more extensive acetylation of H3 and H4 than WT (A). Protein spots are labeled “a,” “b,” “c,” “d,” etc. A minor H2B subtype observed in N. crassa (labeled *) also appeared to be hyperacetylated in the hda1 mutant (labeled *′).
While little change in the distribution of modified forms was observed for histones H3 and H4 in the hda-1RIP1 mutant, a dramatic increase in acetylation of histone H2B was indicated by the 2DGE analysis (Figure 5, A and C). Thus, in Neurospora, the primary target of the HDA-1 enzyme appears to be histone H2B rather than histone H3. This observation is consistent with our Western analysis, which failed to detect substantial changes in site-specific modification of histone H3 in the hda-1 mutant (Figure 3). These results suggest the possibility that the observed decreases in DNA methylation in the hda-1 mutant may be due to failure of HDA-1 to deacetylate histone H2B. This possibility is consistent with the observation that the S. cerevisiae HDA-1 homolog, Hda1p, is active on both H3 and H2B (Wu et al. 2001). Although H3K14 is thought to be the pertinent target of the S. pombe HDA-1 homolog Clr3 (Bjerling et al. 2002; Sugiyama et al. 2007), the possibility of effects on histone H2B had not been examined in this organism.
As a step in examining the possible generality of our findings, we analyzed histones from an S. pombe Clr3 mutant by 2DGE. As observed in the Neurospora hda-1 mutant, we found evidence of increased H2B acetylation (Figure 5, B and D). In wild-type S. pombe, H2B was predominantly unmodified (Figure 5B) whereas H2B appeared multiply acetylated in the clr3 mutant (Figure 5D). The major H2B spot, labeled “d,” apparently has three charged residues masked, likely by acetylation (Anderson et al. 2010). In addition, the 2DGE revealed that S. pombe Clr3 also targets H2A, as indicated by the redistribution of H2A spots in the clr3 mutant toward the less mobile derivative spots b, c, and d (Figure 5, B and D).
We also performed 2DGE analysis on the Neurospora hda-3RIP1 mutant, which also affected DNA methylation, although not as dramatically as hda-1RIP1 (Figure 2). In contrast to the 2DGE data obtained for Neurospora hda-1 and S. pombe Clr3 (Figure 5C and D, respectively), no global change in acetylation was detected by 2DGE for the H2A or H2B histones in the hda-3RIP1 mutant (Figure 5E). However, the 2DGE analysis did show a shift in the distribution of acetylation states of histones H3 and H4 in the hda-3RIP1 mutant, as indicated by the accumulations of di-, tri-, and tetra-acetylated H3 and H4 forms (spots b, c, d, and e, respectively, in Figure 5E). These observations are consistent with our Western analysis of the hda-3RIP1 strain, which revealed increased acetylation at multiple sites in histone H3 and a modest increase in tetra-acetylation of histone H4. Thus DNA methylation defects in hda-3RIP1 may be attributed to defective activity of the HDA-3 enzyme.
DISCUSSION
This study followed up an early indication that HDACs play a role in DNA methylation in Neurospora, namely that the HDAC inhibitor TSA caused a selective loss of DNA methylation (Selker 1998). We identified four genes encoding HDACs in N. crassa and disrupted them to assess their possible role in the control of DNA methylation. One of the four genes, hda-3, appears to be essential for viability of N. crassa and other filamentous fungi (Tribus et al. 2010). We managed to generate a partial loss-of-function allele, hda-3RIP1, by exploiting the genome defense mechanism RIP. Analysis of this mutant suggested that HDA-3 has broad specificity for acetylated lysines on H3 and H4 (Figures 3 and 5) and produced evidence that this HDAC plays a modest role in DNA methylation. The S. pombe homolog of HDA-3, Clr6, is also essential for viability (Grewal et al. 1998), has broad specificity for acetylated lysines in H3 and H4 (Bjerling et al. 2002), and is required to prevent spurious transcription of both genes and heterochromatic repeats (Nicolas et al. 2007). Interestingly, Arabidopsis thaliana HDA6, which is the closest homolog of Neurospora HDA-3, is required for full RNA-directed DNA methylation (Aufsatz et al. 2002) and transcriptional gene silencing (Probst et al. 2004). It may be possible to generate temperature-sensitive hda-3 alleles in Neurospora or to conditionally express hda-3 to further decrease HDA-3 activity to better elucidate its function in DNA methylation.
We found pronounced DNA methylation defects in mutants of hda-1. Indeed, the defects were considerably more extensive than those resulting from TSA treatment. The S. cerevisiae and S. pombe homologs of HDA-1 have been reported to be specific for H3 K14 (Bjerling et al. 2002; Sugiyama et al. 2007) and H3 and H2B (Wu et al. 2001), respectively. The S. pombe homolog of HDA-1, Clr3, is required for recruitment of the H3 K9 HMTase Clr4 to heterochromatin and directly interacts with the HP1 homolog, Swi6 (Yamada et al. 2005). Heterochromatin nucleation and spreading requires Clr3 deacetylase activity, Clr4, and Swi6. Moreover, clr3 mutants have been shown to cause increased acetylation of H3 K14 and increased RNA PolII occupancy at heterochromatic regions (Sugiyama et al. 2007). Mutation of N. crassa hda-1 resulted in a similar, albeit modest, increase in H3 acetylation at the regions of heterochromatin that lost DNA methylation in this mutant (Figure 4B). N. crassa HDA-1 may therefore play a similar role in restricting histone acetylation and transcription of heterochromatin, which may then impact DNA methylation.
Our finding of a sizable increase in H2B acetylation in the hda-1 mutant of Neurospora as well as in the clr3 mutant of S. pombe (Figure 5) raises the interesting possibility that H2B may indirectly impact methylation of H3K9, which is a hallmark of heterochromatin in both organisms and is required for DNA methylation in Neurospora (Tamaru et al. 2003). Together with observations on S. cerevisiae Hda1 (Wu et al. 2001), our findings suggest that H2B is an important and general target of this particular HDAC type. It is noteworthy that there is firm precedent for interplay between modifications on H2B and H3. For example, H2B ubiquitination is required for H3 methylation in yeast (Sun and Allis 2002), and in A. thaliana, H2B ubiquitination interferes with H3 K9 methylation and DNA methylation (Sridhar et al. 2007). There is no previous evidence for H2B acetylation regulating H3 methylation, but we suggest that this possibility deserves investigation.
Acknowledgments
We thank Tamir Khalafallah, Jon Murphy, and Jeewong Choi for technical assistance. The S. pombe strains were generously provided by Shiv Grewal, and Neurospora strain N3406 was a generous gift from Yi Liu. This work was supported by grants from the National Institutes of Health (GM025690) and the National Science Foundation (MCB-0121383) to E.U.S. and by an American Cancer Society Postdoctoral Fellowship to K.M.S. (PF-04-043-01-GMC).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.123315/DC1.
References
- Adhvaryu, K. K., and E. U. Selker, 2008. Protein phosphatase PP1 is required for normal DNA methylation in Neurospora. Genes Dev. 22 3391–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, D. C., G. R. Green, K. Smith and E. U. Selker, 2010. Extensive and varied modifications in histone H2B of wild-type and histone deacetylase 1 mutant Neurospora crassa. Biochemistry 49 5244–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufsatz, W., M. F. Mette, J. Van Der Winden, M. Matzke and A. J. Matzke, 2002. HDA6, a putative histone deacetylase needed to enhance DNA methylation induced by double-stranded RNA. EMBO J. 21 6832–6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum, J. A., and N. H. Giles, 1986. DNase I hypersensitive sites within the inducible qa gene cluster of Neurospora crassa. Proc. Natl. Acad. Sci. USA 83 6533–6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerling, P., R. A. Silverstein, G. Thon, A. Caudy, S. Grewal et al., 2002. Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and in vivo specificity. Mol. Cell. Biol. 22 2170–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich, K. A., L. A. Alex, O. Yarden, M. Freitag, G. E. Turner et al., 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 68 1–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambareri, E. B., B. C. Jensen, E. Schabtach and E. U. Selker, 1989. Repeat-induced G-C to A-T mutations in Neurospora. Science 244 1571–1575. [DOI] [PubMed] [Google Scholar]
- Cameron, E. E., K. E. Bachman, S. Myohanen, J. G. Herman and S. B. Baylin, 1999. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 21 103–107. [DOI] [PubMed] [Google Scholar]
- Catalanotto, C., G. Azzalin, G. Macino and C. Cogoni, 2002. Involvement of small RNAs and role of the qde genes in the gene silencing pathway in Neurospora. Genes Dev. 16 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., J. W. Crabb and J. A. Kinsey, 1998. The Neurospora aab-1 gene encodes a CCAAT binding protein homologous to yeast HAP5. Genetics 148 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot, H. V., G. Park, G. E. Turner, C. Ringelberg, C. M. Crew et al., 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 103 10352–10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell, I. G., R. Aucott, S. K. Mahadevaiah, P. S. Burgoyne, N. Huskisson et al., 2002. Heterochromatin, HP1 and methylation at lysine 9 of histone H3 in animals. Chromosoma 111 22–36. [DOI] [PubMed] [Google Scholar]
- Cunliffe, V. T., 2008. Eloquent silence: developmental functions of class I histone deacetylases. Curr. Opin. Genet. Dev. 18 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, R. H., 2000. Neurospora: Contributions of a Model Organism. Oxford University Press, Oxford.
- de Ruijter, A. J., A. H. van Gennip, H. N. Caron, S. Kemp and A. B. van Kuilenburg, 2003. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 370 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag, M., P. C. Hickey, T. K. Khlafallah, N. D. Read and E. U. Selker, 2004. a HP1 is essential for DNA methylation in Neurospora. Mol. Cell 13 427–434. [DOI] [PubMed] [Google Scholar]
- Freitag, M., D. W. Lee, G. O. Kothe, R. J. Pratt, R. Aramayo et al., 2004. b DNA methylation is independent of RNA interference in Neurospora. Science 304 1939. [DOI] [PubMed] [Google Scholar]
- Green, G. R., and D. P. Do, 2009. Purification and analysis of variant and modified histones using 2D PAGE. Methods Mol. Biol. 464 285–302. [DOI] [PubMed] [Google Scholar]
- Grewal, S. I., 2010. RNAi-dependent formation of heterochromatin and its diverse functions. Curr. Opin. Genet. Dev. 20 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal, S. I., M. J. Bonaduce and A. J. Klar, 1998. Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics 150 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays, S. M., J. Swanson and E. U. Selker, 2002. Identification and characterization of the genes encoding the core histones and histone variants of Neurospora crassa. Genetics 160 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollender, C., and Z. Liu, 2008. Histone deacetylase genes in Arabidopsis development. J. Integr. Plant Biol. 50 875–885. [DOI] [PubMed] [Google Scholar]
- Honda, S., and E. U. Selker, 2008. Direct interaction between DNA methyltransferase DIM-2 and HP1 is required for DNA methylation in Neurospora crassa. Mol. Cell. Biol. 28 6044–6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzminova, E., and E. U. Selker, 2001. dim-2 encodes a DNA methyltransferase responsible for all known cytosine methylation in Neurospora. EMBO J. 20 4309–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdistani, S. K., S. Tavazoie and M. Grunstein, 2004. Mapping global histone acetylation patterns to gene expression. Cell 117 721–733. [DOI] [PubMed] [Google Scholar]
- Lee, D. W., R. J. Pratt, M. McLaughlin and R. Aramayo, 2003. An argonaute-like protein is required for meiotic silencing. Genetics 164 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. C., L. Li, W. Gu, Z. Xue, S. K. Crosthwaite et al., 2010. Diverse pathways generate microRNA-like RNAs and dicer-independent small interfering RNAs in fungi. Mol. Cell 38 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, Z. A., S. Honda, T. K. Khlafallah, J. K. Jeffress, M. Freitag et al., 2009. Relics of repeat-induced point mutation direct heterochromatin formation in Neurospora crassa. Genome Res. 19 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin, B. S., M. Freitag and E. U. Selker, 1997. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Newsl. 44 34–36. [Google Scholar]
- Mateescu, B., P. England, F. Halgand, M. Yaniv and C. Muchardt, 2004. Tethering of HP1 proteins to chromatin is relieved by phosphoacetylation of histone H3. EMBO Rep. 5 490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonel, P., I. Costello and B. Hendrich, 2009. Keeping things quiet: roles of NuRD and Sin3 co-repressor complexes during mammalian development. Int. J. Biochem. Cell Biol. 41 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, V. P., M. Freitag and E. U. Selker, 2000. Short TpA-rich segments of the zeta-eta region induce DNA methylation in Neurospora crassa. J. Mol. Biol. 300 249–273. [DOI] [PubMed] [Google Scholar]
- Nicolas, E., T. Yamada, H. P. Cam, P. C. Fitzgerald, R. Kobayashi et al., 2007. Distinct roles of HDAC complexes in promoter silencing, antisense suppression and DNA damage protection. Nat. Struct. Mol. Biol. 14 372–380. [DOI] [PubMed] [Google Scholar]
- Ou, J. N., J. Torrisani, A. Unterberger, N. Provencal, K. Shikimi et al., 2007. Histone deacetylase inhibitor Trichostatin A induces global and gene-specific DNA demethylation in human cancer cell lines. Biochem. Pharmacol. 73 1297–1307. [DOI] [PubMed] [Google Scholar]
- Probst, A. V., M. Fagard, F. Proux, P. Mourrain, S. Boutet et al., 2004. Arabidopsis histone deacetylase HDA6 is required for maintenance of transcriptional gene silencing and determines nuclear organization of rDNA repeats. Plant Cell 16 1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robyr, D., Y. Suka, I. Xenarios, S. K. Kurdistani, A. Wang et al., 2002. Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell 109 437–446. [DOI] [PubMed] [Google Scholar]
- Rountree, M. R., and E. U. Selker, 1997. DNA methylation inhibits elongation but not initiation of transcription in Neurospora crassa. Genes Dev. 11 2383–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker, E. U., 1998. Trichostatin A causes selective loss of DNA methylation in Neurospora. Proc. Natl. Acad. Sci. USA 95 9430–9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker, E. U., 2004. Genome defense and DNA methylation in Neurospora. Cold Spring Harb. Symp. Quant. Biol. 69 119–124. [DOI] [PubMed] [Google Scholar]
- Selker, E. U., E. B. Cambareri, P. W. Garrett, B. C. Jensen, K. R. Haack et al., 1989. Use of RIP to inactivate genes in Neurospora crassa. Fungal Genet. Newsl. 36 76–77. [Google Scholar]
- Singer, M. J., B. A. Marcotte and E. U. Selker, 1995. DNA methylation associated with repeat-induced point mutation in Neurospora crassa. Mol. Cell. Biol. 15 5586–5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha, I., M. Wiren and K. Ekwall, 2006. Genome-wide patterns of histone modifications in fission yeast. Chromosome Res. 14 95–105. [DOI] [PubMed] [Google Scholar]
- Smith, K. M., G. O. Kothe, C. B. Matsen, T. K. Khlafallah, K. K. Adhvaryu et al., 2008. The fungus Neurospora crassa displays telomeric silencing mediated by multiple sirtuins and by methylation of histone H3 lysine 9. Epigenetics Chromatin 1 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar, V. V., A. Kapoor, K. Zhang, J. Zhu, T. Zhou et al., 2007. Control of DNA methylation and heterochromatic silencing by histone H2B deubiquitination. Nature 447 735–738. [DOI] [PubMed] [Google Scholar]
- Sugiyama, T., H. P. Cam, R. Sugiyama, K. Noma, M. Zofall et al., 2007. SHREC, an effector complex for heterochromatic transcriptional silencing. Cell 128 491–504. [DOI] [PubMed] [Google Scholar]
- Sun, Z. W., and C. D. Allis, 2002. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418 104–108. [DOI] [PubMed] [Google Scholar]
- Tamaru, H., and E. U. Selker, 2001. A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414 277–283. [DOI] [PubMed] [Google Scholar]
- Tamaru, H., X. Zhang, D. McMillen, P. B. Singh, J. Nakayama et al., 2003. Trimethylated lysine 9 of histone H3 is a mark for DNA methylation in Neurospora crassa. Nat. Genet. 34 75–79. [DOI] [PubMed] [Google Scholar]
- Tribus, M., I. Bauer, J. Galehr, G. Rieser, P. Trojer et al., 2010. A novel motif in fungal class 1 histone deacetylases is essential for growth and development of Aspergillus. Mol. Biol. Cell 21 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiren, M., R. A. Silverstein, I. Sinha, J. Walfridsson, H. M. Lee et al., 2005. Genomewide analysis of nucleosome density histone acetylation and HDAC function in fission yeast. EMBO J. 24 2906–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., N. Suka, M. Carlson and M. Grunstein, 2001. TUP1 utilizes histone H3/H2B-specific HDA1 deacetylase to repress gene activity in yeast. Mol. Cell 7 117–126. [DOI] [PubMed] [Google Scholar]
- Yamada, T., W. Fischle, T. Sugiyama, C. D. Allis and S. I. Grewal, 2005. The nucleation and maintenance of heterochromatin by a histone deacetylase in fission yeast. Mol. Cell 20 173–185. [DOI] [PubMed] [Google Scholar]
- Zilberman, D., 2008. The evolving functions of DNA methylation. Curr. Opin. Plant Biol. 11 554–559. [DOI] [PubMed] [Google Scholar]