Abstract
Implantation of skeletal myoblasts to the heart has been investigated as a means to regenerate and protect the myocardium from damage after myocardial infarction. While several animal studies utilizing skeletal myoblasts have reported positive findings, results from clinical studies have been mixed. In this study we utilize a newly developed bioreducible polymer system to transfect skeletal myoblasts with a plasmid encoding vascular endothelial growth factor (VEGF) prior to implantation into acutely ischemic myocardium. VEGF has been demonstrated to promote revascularization of the myocardium following myocardial infarction. We report that implanting VEGF expressing skeletal myoblasts into acutely ischemic myocardium produces superior results compared to implantation of untransfected skeletal myoblasts. Skeletal myoblasts expressing secreted VEGF were able to restore cardiac function to non-diseased levels as measured by ejection fraction, to limit remodeling of the heart chamber as measured by end systolic and diastolic volumes, and to prevent myocardial wall thinning. Additionally, arteriole and capillary formation, retention of viable cardiomyocytes, and prevention of apoptosis was significantly improved by VEGF expressing skeletal myoblasts compared to untransfected myoblasts. This work demonstrates the feasibility of using bioreducible cationic polymers to create engineered skeletal myoblasts to treat acutely ischemic myocardium.
1. Introduction
Myocardial infarction (MI) is the leading cause of death in developed nations and one of the most common causes of death in the world. Unfortunately, current pharmacological treatment regimens for myocardial infarction do not reliably limit remodeling of the left ventricle (LV) post-infarction and prevent progression to heart failure. Novel potential treatments, including gene and cell therapies, offer a means to directly treat the pathophysiology underlying the long-term complications of myocardial infarction—loss of cardiomyocytes due to necrosis and apoptosis.
Implantation of cells to the myocardium has long been investigated as a means to recover myocardial tissue and improve outcomes post-MI. Skeletal myoblasts are a class of progenitor muscle cells that may recover infarcted myocardium and limit remodeling of the left [1–3] and the right ventricle [4]. Several studies have demonstrated the ability of skeletal myoblasts to regenerate myocardium through mechanisms including in situ proliferation and fusion with resident myotubes and myofibers [5,6]. While initial results using skeletal myoblasts for implantation to the myocardium have been positive, the long-term benefits remain uncertain. Implantation of cells is limited by the rapid loss of cells from the injection site. With the majority of cells being lost by mechanical means soon after injection, the primary benefit of skeletal myoblast implantation is thought to derive from the paracrine effects of the growth factors and cytokines secreted by the injected cells [7,8].
In addition to cell-based approaches, other investigators have focused on angiogenic therapies to treat myocardial infarction. Therapies using angiogenic factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), have demonstrated the beneficial effects of angiogenesis on protection of endogenous cardiomyocytes and on the retention of functionally contractile myocardium [9–11]. The most common technique for expressing angiogenic factors has been the utilization of viral vectors to deliver VEGF into endogenous cardiomyocytes [12]. In addition to direct transduction of myocardial tissue, examples of viral transduction of skeletal myoblasts have been published [13–15]. While viral gene therapy offers high transfection efficiencies, its clinical utility is limited by host immune responses, oncogenic potential, limitations in viral loading, and difficulty in large-scale manufacturing. For these reasons, the development of safer, non-viral methods for gene delivery is increasingly important.
Non-viral polymer gene therapy is a technique that has been rapidly advancing over the past 10 years. Polymer gene carriers are non-immunogenic, stable, have a large DNA loading capacity and are also easily manufactured. They are, however, when compared to viral vectors, less efficient at transfecting cells and producing prolonged gene expression. Among cationic polymers for gene therapy, polyethyeneimine (PEI) has recently been used to transfect human skeletal myoblasts with VEGF for implantation into the myocardium for cardiac repair following myocardial infarction [16]. While PEI has long been considered the gold standard for polymer transfection, it is known to be highly toxic to most cell types and it lacks the ability to rapidly release its DNA cargo upon internalization to the cell. We have recently reported the synthesis and validation of disulfide-containing bioreducible polymers which improve upon PEI by allowing for the rapid release of DNA cargo upon internalization to the cell. These newly described polymers, poly(cystaminebisacrylamide-diaminohexane) [poly(CBA-DAH)] and poly(cystaminebisacrylamide-diaminohexane-arginine) [poly(CBA-DAH-R)], showed 11- and 16-fold higher expression of luciferase respectively with significantly less toxicity than branched PEI. We have also demonstrated that these polymers can efficiently transfect the functional gene VEGF165, the predominant isoform of VEGF which possesses high angiogenic potency, into primary myoblasts [17]. The production of therapeutic levels of secreted VEGF by these transfected skeletal myoblasts suggests that transfected primary skeletal myoblasts can be used as bioreactors for angiogenic VEGF expression in vivo [17].
In this study, we utilized an in vivo animal model to evaluate the therapeutic efficacy of polymer-transfected primary skeletal myoblasts in limiting the size of myocardial infarction. Rats were treated with skeletal myoblasts that had been transfected with poly(CBA-DAH) containing a plasmid encoding human VEGF165. These VEGF165-expressing skeletal myoblast- treated animals were compared to: 1) treatment with untransfected myoblasts; 2) no treatment (ligation only); and 3) thoracotomy only. Magnetic resonance imaging (MRI), histology and immuno-histochemistry were used to determine differences in cardiac function, infarct size, arteriole and/or capillary density, and apoptosis between the study groups.
2. Materials and methods
All experiments were approved by the University of Utah’s Institutional Animal Care and Use Committee and followed the guidelines provided by the National Institutes of Health in Guide for the Care and Use of Laboratory Animals.
2.1 Isolation, culture and characterization of rat primary skeletal myoblasts
The process of isolating, culturing and characterizing rat skeletal primary myoblasts was reported in our previous work [17] with slight modifications from published protocols [18,19].
Briefly, rat skeletal primary myoblasts were obtained from young rat hindlimbs. Isolated rat skeletal primary myoblasts were maintained in growth medium consisting of Ham’s F-10 supplemented with 20% FBS, 2.5 ng/mL human bFGF, penicillin (200 μg/mL), and streptomycin (200 μg/mL) and incubated at 37°C in 5% CO2. Primary cultures derived from skeletal muscle consisting of myoblasts and fibroblasts were incubated in Ham’s F-10 medium, giving a growth advantage to myoblasts over fibroblasts. Primary myoblasts were separated from contaminating cells, such as fibroblasts, by taking advantage of their differential adherence to the flask over several passages. After two weeks of selective growth, cells were trypsinized, suspended in 90% serum and 10% dimethyl sulfoxide (DMSO) and frozen in liquid nitrogen.
To select the primary myoblasts, cells were immunostained using the primary antibody (mouse-anti-human desmin IgG1/κ) and the secondary antibody (Streptavidin-horseradish peroxidase, SAv-HRP conjugated biotinylated anti-mouse IgG), fixed by 2% paraformaldehyde in PBS for 30 min, washed with PBS and then with blocking buffer (3% BSA with 0.5% Triton X-100 in PBS) for 1 h at room temperature. The samples were incubated with 1:100 primary anti-desmin IgG at 4°C overnight. The next day, cells were washed with PBS, diluted in secondary antibody (1:100 biotinylated anti-mouse IgG) for 1 h at room temperature, and washed three times in PBS. SAv-HRP was applied and the samples were incubated for 30 min at room temperature. DAB substrate solution was applied to the slides until the desired color intensity was reached (around 5 min). The cells were dehydrated using 95% alcohol and xylene and covered with Fluoromount ™ Mount Gel (Sigma-Aldrich, St. Louis, MO). After several weeks in culture, the vast majority of the cells stained positive for the myoblast specific protein desmin.
2.2 Preparation of polymer, poly(CBA-DAH), and plasmid, pCMV-VEGF165
The poly(disulfide amine) polymer, poly(CBA-DAH), was synthesized as detailed previously [20]. Briefly, poly(CBA-DAH) was synthesized by Michael reaction of N-Boc-DAH and CBA in MeOH/H2O solution (9:1, v/v) and the reaction was protected from light under nitrogen at 60 °C for 3 days with stirring. After the third day, 10% molar of N-Boc-DAH was added to the reaction mixture to consume any unreacted acrylamide functional groups and the reaction was allowed to proceed for an additional 2 h. The reaction products were precipitated with anhydrous diethyl ether followed by deprotection of the N-Boc groups with the reagent solution (TFA:triisobutylsilane:H2O =95:2.5:2.5, v/v) in an ice bath for 30 min. The crude product was again precipitated with anhydrous diethyl ether and dried under vacuum. The final product was dialyzed against deionized water overnight (MWCO=1000, Spectrum Laboratories, Inc., Rancho Dominguez, CA), and lyophilized to obtain poly(CBA-DAH). The poly(CBA-DAH) was analyzed by 1H NMR (400 MHz, D2O) to confirm successful synthesis of the desired product.
The plasmid, pCMV-VEGF165, containing the vascular endothelial growth factor gene 165 was amplified in E. coli DH5a and purified by standard Maxiprep kit (Invitrogen, Carlsbad, CA).
2.3 Rat myocardial infarction model
Male Sprague-Dawley rats were anesthetized under 4% isoflurane for induction, intubated and kept under 2% isoflurane for maintenance of anesthesia. The left chest was shaved and a thoracotomy was performed in the 4th or 5th intercostal space, exposing the heart. The left anterior descending coronary artery (LAD) was permanently ligated using a single stitch of 6-0 prolene suture (Ethicon). After confirming successful ligation of the LAD by observation of visible blanching of the left ventricle, the animals were assigned to one of three treatment groups: 1) Ligation only (n=7), 2) injection of untreated skeletal myoblasts (n=7), and 3) injection of VEGF165-transfected skeletal myoblasts (n=7). Animals assigned to cell injection groups received 2×106 cells in 200 μL PBS in three separate injections to the border zone of the infarct. After the injection of cells, the chest was closed in layers and the animal was allowed to recover under a warming lamp. Additionally, some animals received a full thoracotomy with exposure of the heart, but no ligation of the LAD to act as sham operation controls (n=5).
Primary myoblasts were transfected with poly(CBA-DAH)/pCMV-VEGF165 at the ratio of 20:1(w/w) 24h prior to the surgery. Cells were injected at the border zone of the infarct with three injections for a total number of cells ~2×106/200 μl in PBS.
2.4 VEGF expression study
To verify expression of VEGF in the heart following injection of VEGF-transfected myoblasts into the myocardium, VEGF levels were measured three days after surgery using a commercially available human VEGF165 ELISA kit (R&D Systems, Minneapolis, MN). A total of eight animals were treated with myoblasts transfected with VEGF, eight animals were treated with untransfected myoblasts, while eight control animals received no myoblasts and underwent ligation only. Hearts were weighed, and homogenized in 1× TE buffer containing protease inhibitors. 100 μL of the supernatant was used for determination of VEGF levels by ELISA assay.
The quantification of VEGF protein was determined using a UV absorbance plate reader (Model 680, Bio-Rad Lab, Hercules, CA). All experiments were performed in triplicate and quantitated using the supplied VEGF standard.
2.5 Magnetic resonance imaging (MRI)
Magnetic resonance imaging (MRI) was performed to determine changes in indices of heart function in all animals. Four weeks following surgery, the animals were analyzed by cardiac MRI to measure parameters of heart function. Anesthesia induction was achieved in each animal by exposure to 4% isoflurane and maintained with 2% isoflurane. The rats were placed prone on a pressure sensor to measure respiration, and a pulse oximeter was attached to the rat’s foot. The heart was imaged using a Bruker Biospec 7T/30 cm system operated with Bruker AVANCE II electronics (Bruker BioSpin MRI GmbH, Ettlingen, Germany). A Bruker birdcage quadrature resonator (72 mm inner diameter) was used for signal transmission and reception (Bruker BioSpin MRI GmbH, Ettlingen, Germany). The images were acquired using black blood FLASH CINE parameters with double-gating off respiration and ECG. The instrument settings were as follows: repetition time (TR) = 31 ms, echo time (TE) = 2.8 ms, number of averages (NEX) = 4, field of view (FOV) = 8 cm × 8 cm, in-plane resolution = 408 × 312 microns, slice thickness = 2 mm, number of movie frames = 5. Prospective gating was achieved using SAII monitoring and gating systems (SA Instruments, Stony Brook, NY, USA).
Resultant CINE MRI sequences were analyzed for left ventricular ejection fraction, wall thickness, and volume using the freely available software Segment v1.8 (Segment; http://segment.heiberg.se) [21]. The left ventricle was analyzed in a semi-automated manner by an investigator blinded to the treatment groups. Wall thickness was measured at the mid-ventricular level in a short-axis view at end diastole and end systole. Measurements of left ventricle end diastolic and end systolic volume were calculated from one 2 mm slice at the height of the base of the papillary muscles in order to normalize across groups.
2.6 Histology and immunohistochemistry
The day after MRI, rats were sacrificed by overdose of isoflurane gas inhalation. The hearts were excised, perfused with 20 mL of Heparin-PBS, 20 mL of 2.56 M KCl, 20 mL of 10% formalin, and fixed in 10% formalin at 4°C till sectioning. Hearts were sectioned sagittally via the LV into 4 micron sections. Immunohistochemical staining was performed on the sections of formalin-fixed, paraffin-embedded tissue. Sections were air-dried at room temperature and then placed in a 60°C oven for 30 minutes to melt the paraffin. All of the staining steps were performed at 37°C using an automated immunostainer (BenchMark® XT, Ventana Medical Systems, Tucson, AZ).
Collagen in the heart sections was stained using Masson’s trichrome. The infarct size of myocardium was calculated by the total infarction area divided by the total LV area using a Zeiss Stemi 2000-C Stereo Microscope (Edmund Optics Inc. Barrington, NJ, USA). To evaluate the arteriolar and capillary density and the loss of cardiomyocytes with the myocardial infarction, heart sections were immunohistochemically stained using α-smooth muscle actin, endocardial cell specific CD31 (Purified Mouse Anti-Rat CD31, Abcam), and cardiomyocyte specific cTnT (Monoclonal Anti-Troponin T antibody, Sigma). The sections were detected using the IView DAB detection kit-research (Ventana Medical Systems), which is an open secondary, Streptavidin-HRP system, utilizing DAB (3-3′ diaminobenzidine) as the chromogen. The sections were counterstained with hematoxylin (Ventana Medical Systems) for 4 minutes.
Capillaries positive for CD31 and α-smooth muscle actin–positive arterioles over the infarcted region were counted in five random high-power fields (Nikon Eclipse TE300 Microscope) per specimen as the average number of capillaries per field. Arterioles were defined as vessels with an internal diameter of <50 μm; vessels with a diameter of <10 μm were considered capillaries. The loss or recovery of cardiomyocytes by cTnT staining was determined throughout the border zone of five hpf per specimen. The determination of apoptosis was performed using a commercially available kit (ApopTag Apoptosis Detection Kit, Intergen). Apoptosis in the infarcted regions was examined in five random hpf per section. For all the histology every hpf was randomly chosen within the infarct border zone by an investigator blinded to the treatment groups. Analysis of all images was carried out with NIH ImageJ software (NIH, Bethesda, MD).
2.7 Statistical analysis
Results are expressed as the mean value ± standard error of the mean (SEM). Differences between groups were identified by one-way analysis of variance (ANOVA) followed by Tukey post hoc analysis to identify significance between groups using GraphPad Prizm 4.03 software (GraphPad Software, La Jolla, CA).
3. Results
An acute myocardial infarction (MI) model was used to evaluate the therapeutic efficacy of VEGF transfected skeletal primary myoblasts. Myocardial infarction in Sprague-Dawley rats was induced by ligation of the left anterior descending coronary (LAD) artery as previously described[22]. Animals were randomly placed into one of four groups: thoracotomy only (n=5 short-term study, n=5 long-term study), thoracotomy followed by ligation only (n=5 short-term study, n=7 long-term study), thoracotomy with ligation followed by injection of primary untransfected myoblasts (n=5 short-term study, n=7 long-term study), and thoracotomy with ligation followed by injection with primary myoblasts transfected with poly(CBA-DAH)/pCMV-VEGF165 (n=5 short-term study, n=7 long-term study). The primary myoblasts were transfected with poly(CBA-DAH)/pCMV-VEGF165 at a 20:1 w/w ratio 24 hours before surgery and the transfected cells were injected at the border zone of the infarction. Our previously published results have identified the efficacy of this approach in successfully transfecting primary myoblasts and achieving sustained VEGF expression and secretion [17].
3.1 Short-Term VEGF Expression Study
To determine whether transfected primary myoblast transplantation affected VEGF expression, the levels of vascular endothelial growth factor in hearts with acute myocardial infarction were measured using an ELISA assay three days following surgery.
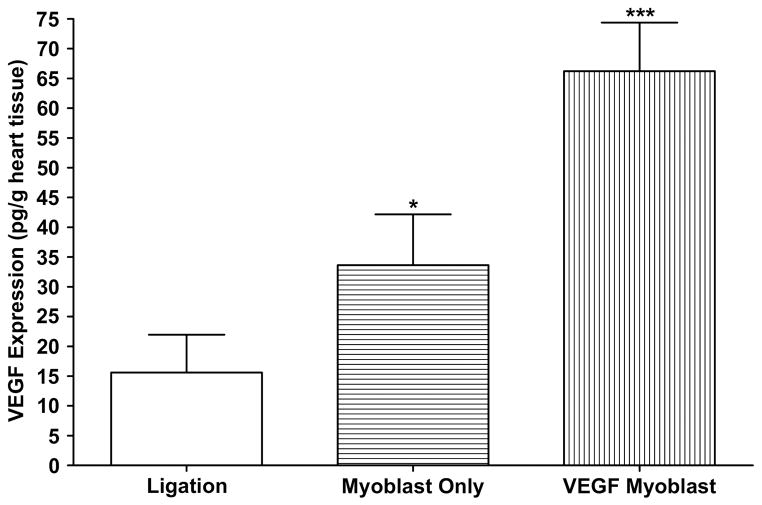
VEGF levels in the ligation only group averaged 15.6±6.3 pg/g heart tissue, while levels in the untransfected myoblast group averaged 33.7±8.5 pg/g heart tissue. The VEGF transfected myoblast transplantation group showed a much higher level of VEGF expression than both the ligation only and untransfected myoblast groups (66.3±8.1 pg/g, P<0.001 vs ligation only, P<0.05 vs myoblast only) (Figure 1). Detection of human VEGF165 in the ligation only and untransfected myoblast groups was most likely due to cross reactivity between human VEGF and endogenous rat VEGF.
Figure 1.
Human VEGF expression in rat hearts three days after surgery as measured by ELISA. VEGF levels were significantly higher in the VEGF165-transfected myoblast group than the ligation and myoblast only groups (P<0.001 vs ligation; P<0.05 vs myoblast only). All data are presented as mean values ± SEM (n=8 ligation, n=8 myoblast only, n=8 VEGF myoblast group).
3.2 Cardiac MRI Analysis
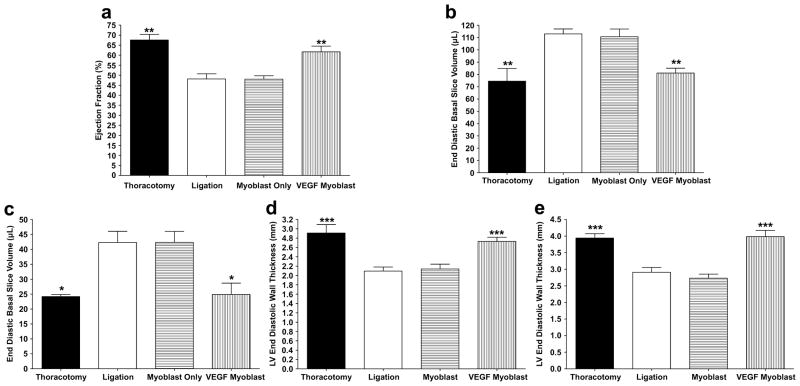
Four weeks following treatment, cardiac MRI was performed on all animals. The thoracotomy only group had an LV ejection fraction of 67.56 ± 2.86%, which falls within the range of expected ejection fractions for normal rat hearts [23]. The hearts that underwent ligation of the LAD to induce a myocardial infarction followed by injection of VEGF-transfected myoblasts showed no significant decrease in LV ejection fraction (61.68 ± 2.83%) compared to the control, i.e. thoracotomy only, animals. Compared to both the thoracotomy only and VEGF165-transfected myoblast injection groups, the LV ejection fraction was significantly decreased in both the ligation only (48.18 ± 2.53%, P < 0.01) and untransfected myoblast injection (48.02 ± 1.68%, P < 0.01) groups (Figure 2a).
Figure 2.
Cardiac parameters measured by MRI four weeks after treatment. (a) Cardiac ejection fraction. Both the VEGF myoblast and thoracotomy control groups displayed significantly higher ejection fractions than the ligation and myoblast only groups (P<0.01). There was no significant difference in ejection fraction between the thoracotomy control group and the VEGF165-transfected myoblast group. (b) End diastolic volume (EDV). EDV significantly increased in the ligation and myoblast only groups compared to both thoracotomy controls and VEGF165-transfected myoblasts (P<0.01). There was no significant increase in EDV between thoracotomy controls and the VEGF myoblast group. (c) End systolic volume (ESV). ESV significantly increased in the ligation and myoblast only groups compared to both thoracotomy controls and VEGF165-transfected myoblasts (P<0.05). There was no significant increase in ESV between thoracotomy controls and the VEGF myoblast group. (d) End diastolic wall thickness (EDWT) four weeks post treatment. The EDWT significantly decreased in the ligation and myoblast only groups compared to both thoracotomy controls and VEGF165-transfected myoblasts (P<0.001). There was no significant decrease in EDWT between thoracotomy controls and the VEGF myoblast group. (e) End systolic wall thickness (ESWT). ESWT significantly decreased in the ligation and myoblast only groups compared to both thoracotomy controls and VEGF165- transfected myoblasts (P<0.001). There was no significant decrease in ESWT between thoracotomy controls and the VEGF myoblast group. All data are presented as mean ± SEM (n=5 thoracotomy, n=7 ligation, n=7 myoblast only, and n=7 VEGF myoblast group).
Measurement of end diastolic volume (EDV) and end systolic volume (ESV) revealed a similar trend, with the VEGF-transfected myoblast treatment group showing no significant increase in EDV or ESV compared to the thoracotomy only control group (EDV, 81.2±3.9 μL vs. 75.55±10.3 μL, respectively and ESV 24.9 ± 3.8 μL vs. 24.2±0.7 μL, respectively). The ligation only control group (EDV, 113.0±4.0 μL; ESV 42.3±3.7 μL) and untransfected myoblast treatment group (EDV, 110.7±6.2 μL; ESV 42.4±3.7 μL) displayed significant increases in EDV and ESV over the 4-week time period compared to both the thoracotomy only and VEGF-transfected myoblast treatment groups (P < 0.01, both groups) (Figures 2b, c).
The final cardiac parameters measured from MRI were end diastolic wall thickness (EDWT) and end systolic wall thickness (ESWT). The thoracotomy only control group and VEGF-transfected myoblast treatment group showed no difference in wall thickness in both diastole and systole (EDWT, 2.91±0.18 mm vs. 2.73±0.09 mm, respectively; ESWT, 3.94±0.13 mm vs. 3.98±0.19 mm, respectively). The ligation only and the untransfected myoblast treatment groups displayed significant thinning of the left ventricular wall at both end diastole (EDWT, 2.09±0.09 mm and 2.14±0.10 mm, respectively), and end systole (ESWT, 2.91±0.14 mm and 2.73±0.12 mm, respectively) compared to the thoracotomy only (P < 0.001) and VEGF-transfected myoblast treatment groups (P < 0.001), (Figure 2d).
3.3 Effect of transfected myoblast transplantation on infarct size
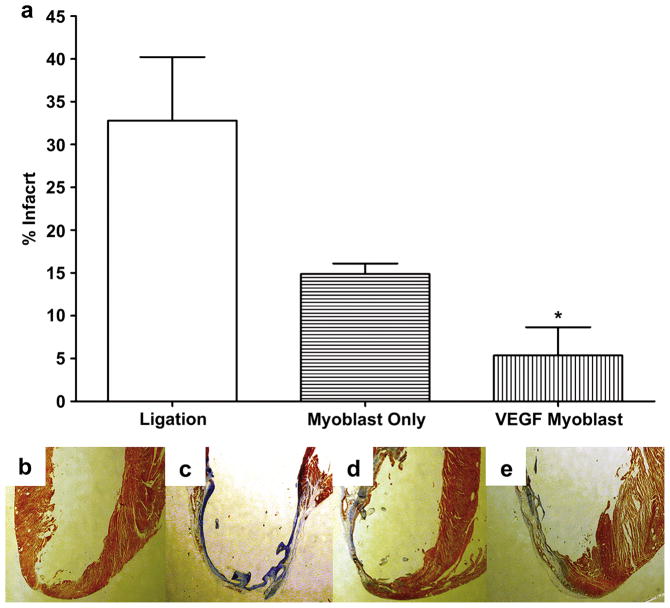
Four weeks following surgery, hearts were cut sagittally and stained with Masson’s trichrome. Figures 3b–e show representative Masson’s trichrome staining in rat hearts from the following groups: thoracotomy only (Figure 3b); ligation only (no injection, Figure 3c); injection of untransfected primary myoblasts only (Figure 3d); and injection of primary myoblasts transfected with poly(CBA-DAH)/pCMV-VEGF165 (Figure 3e). On Masson’s trichrome staining, the infarct scar, characterized by high collagen content, is indicated by bluish staining. Ligation only, without treatment, resulted in infarction of 32.8±7.4% of the left ventricle (Figure 3a). With injection of untransfected primary myoblasts, the infarct size decreased to 14.9±1.2% of the left ventricle (P<0.05 versus ligation only group). With injection of VEGF transfected myoblasts, the infarct size was further reduced to only 5.4 ± 3.3% of the left ventricle, (P<0.001 versus the ligation only group).
Figure 3.
Masson’s trichrome staining for infarct size, four weeks after surgery. (a) Infarcted area as a percentage of the left ventricle. P<0.05 for ligation only versus VEGF myoblast group (b–e) Representative myocardial tissue sections stained with Masson’s trichrome. Bluish staining indicates high collagen content, indicating infarction; (b) Thoracotomy only, control group, (c) ligation only, (d) untransfected myoblast injection group, (e) VEGF165- transfected myoblast injection group. All data are presented as mean ± SEM (n=5 thoracotomy, n=7 ligation, n=7 myoblast only, and n=7 VEGF myoblast group).
3.4 Enhancement of vascular density in ischemic myocardium
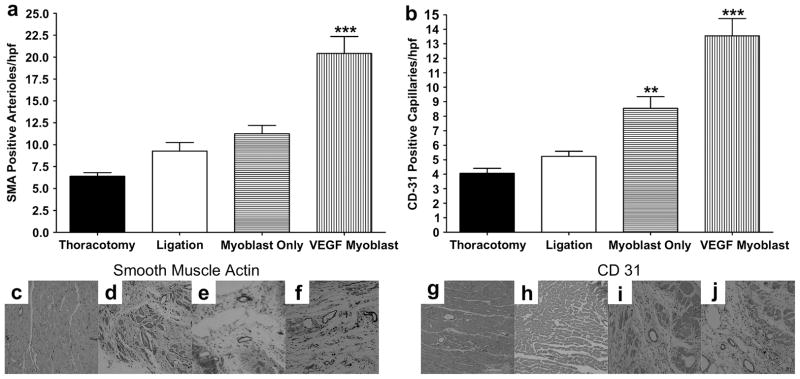
Capillary endothelial cells were detected by immunostaining of the infarcted myocardium with antibodies to CD31. Capillaries positive for CD31 were counted in 5 random microscope high power fields (hpf) in the ischemic border zone of each section, and capillary density was expressed as the average number of capillaries per field. As shown in representative micrographs of sections from each treatment group (Figures 4c–j), an increased number of capillaries and arterioles were present in the VEGF165-transfected myoblast injection group than in the thoracotomy only, ligation only or untransfected myoblast injection groups. As shown in Figure 4a, immunohistochemical staining for smooth muscle α-actin (SMA) revealed more arterioles present in the VEGF transfected myoblast treatment group than any of the other groups. Mean arterioles positive for SMA per hpf increased from 9.2±1.0 in the ligation only group to 20.4±1.9 in the VEGF transfected myoblast group (P<0.05). Mean capillaries positive for CD31 per hpf in the VEGF transfected myoblast group (13.6±1.2) were significantly higher (P<0.05) than in the thoracotomy only (4.1±0.3), ligation only (5.2±0.4) and untransfected myoblast (8.5±0.8) groups (Figure 4b).
Figure 4.
Representative immunohistochemistry stains for SM α-actin and CD31 in the infarct border zone, 4 weeks after myocardial infarction. (c,g) Thoracotomy only, (d,h) ligation only, (e,i) untransfected myoblast injection and (f,j) VEGF165-transfected myoblast injection group; (a). Average number of smooth muscle actin (SMA)-positive arterioles from five random high-power fields (hpf) per animal from the ischemic border zone of the infarct. The number of arterioles in the VEGF165-transfected myoblast group was significantly greater than all other groups P<0.001. (b) Average number of CD31-positive capillaries from five random high-power fields (hpf) per animal from the ischemic border zone of the infarct. The number of capillaries in the VEGF165-transfected myoblast group was significantly greater than all other groups P<0.001. The average number of capillaries in the myoblast only group was significantly higher than both thoracotomy and ligation groups; P<0.01 and P<0.05 respectively. All data are presented as mean ± SEM (n=5 thoracotomy, n=7 ligation, n=7 myoblast only, and n=7 VEGF myoblast group).
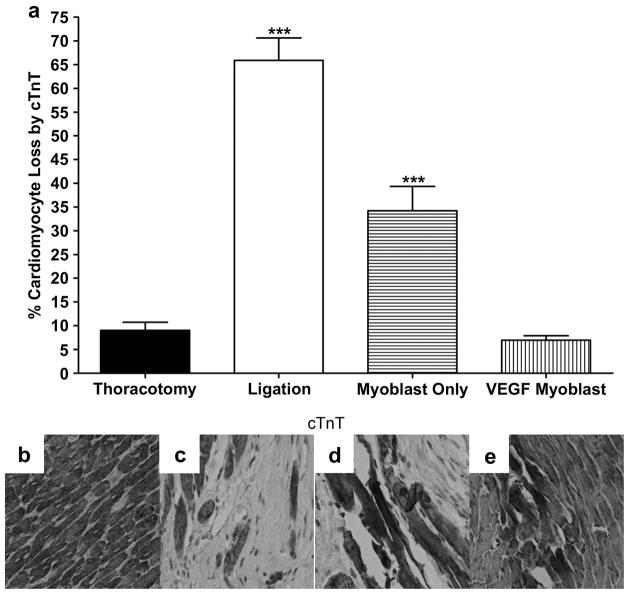
3.5 Cardiomyocyte loss in myocardial infarction
Immunohistochemical staining 4 weeks after surgery was used to identify troponin T (cTnT) in rat heart myocardium. With troponin-T (cTnT) immunostaining, necrotic cardiomyocytes lose their normal brownish staining. As shown in Figure 5, cTnT in the left ventricular infarct zone was barely visible in the primary VEGF165-transfected myoblast group (6.9±0.9%), and not significantly different than the thoracotomy only control group (9.0±1.7%). There was a significant increase in the number of cardiomyocytes lost in the ligation only (65.9±4.8%) and untransfected myoblast (34.2±5.1%) groups.
Figure 5.
The loss of cardiomyocytes, 4 weeks after surgery as indicated by loss of immunohistochemical staining for cTnT; (a) Loss of cardiomyocytes from the infarct border zone 4 weeks after surgery identified from cTnT staining and represented as percent cardiomyocytes lost per hpf. Five random hpf were counted per animal. The loss of cardiomyocytes was significantly prevented in both the myoblast only group (P<0.001 vs ligation) and the VEGF myoblast group (P<0.001 vs ligation and myoblast only). Additionally, the ligation and myoblast only groups were statistically greater than the thoracotomy control (P<0.001), whereas the VEGF myoblast treatment group was not statistically different from thoracotomy control. Representative micrographs of cTnT staining for (b) thoracotomy control group, (c) ligation, (d) myoblast only injection group and (e) VEGF165-transfected myoblast injection group. With cell death, myofibers lose their usual normal brownish staining. All data are presented as mean ± SEM (n=5 thoracotomy, n=7 ligation, n=7 myoblast only, and n=7 VEGF myoblast group).
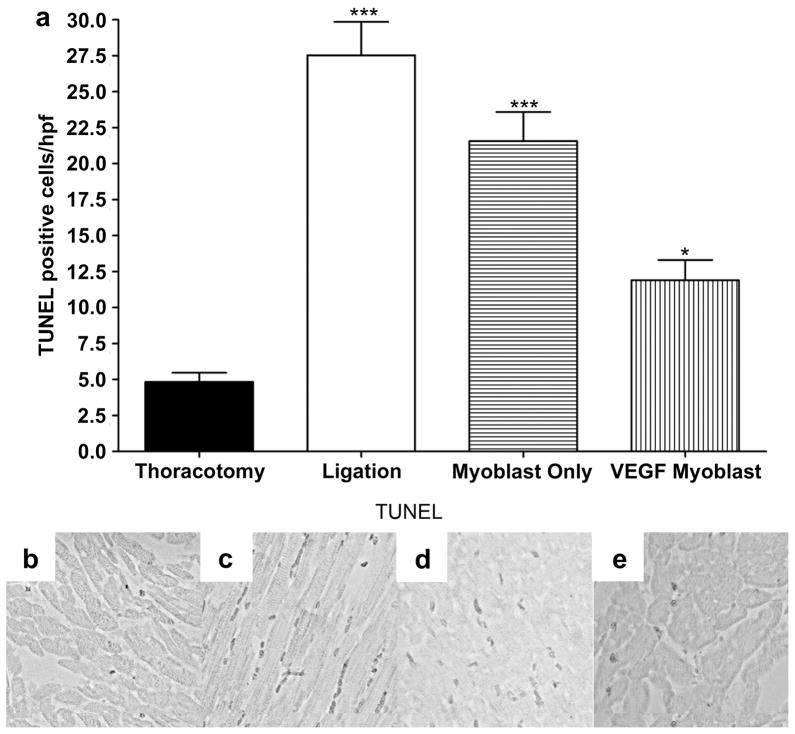
3.6 Reduction of apoptotic activity in ischemic myocardium
Apoptotic activity in the infarcted myocardium after treatment was measured by TUNEL staining. Figure 6 compares representative images of the TUNEL staining from the ischemic region in the left ventricle following thoracotomy only, ligation only, untransfected myoblast injection and VEGF165-transfected myoblast injection. Five random hpf in the ischemic border zone were selected and TUNEL-positive cells were counted. TUNEL-positive cells per hpf were 27.5±2.3 in the ligation only group and 21.6±2.0 in the untransfected myoblast group. The number of TUNEL-positive cells in the infarcted zone was significantly reduced in the VEGF-transfected myoblast group (11.9±1.4, P<0.001 versus ligation only).
Figure 6.
TUNEL staining for apoptotic activity, 4weeks after surgery. (a) TUNEL staining for apoptotic cells 4weeks after surgery. TUNEL-positive apoptotic cells were counted in the five random high-power fields (hpf) per animal in the ischemic border zone. The average number of apoptotic cells was statistically greater between thoracotomy control and all groups with less significance between thoracotomy and the VEGF myoblast group. (P<0.001 thoracotomy vs ligation and myoblast only; P<0.05 between thoracotomy and VEGF myoblast group.) The VEGF myoblast group had significantly fewer apoptotic cells than the ligation group (P<0.001) and the myoblast only group (P<0.01). There was no significant difference between the myoblast only treatment group and ligation controls. Representative micrographs of apoptotic nuclei by TUNEL staining in (b) thoracotomy control group, (c) ligation, (d) myoblast only injection group and (e) VEGF165-transfected myoblast injection group. All data are presented as mean ± SEM (n=5 thoracotomy, n=7 ligation, n=7 myoblast only, and n=7 VEGF myoblast group).
4. Discussion
Exogenous primary myoblast transplantation into ischemic myocardium offers many potential benefits for the treatment of myocardial infarction, including improvement and restoration of cardiac function. The improvement in cardiac function is thought to be due to the enhancement of the contractile properties of endogenous cardiomyocytes and the differentiation and proliferation of implanted myoblasts into functional myocardium within ischemic hearts. Recent studies have demonstrated the feasibility of primary myoblast transplantation for the treatment of ischemic heart disease [18,24]. In addition, we have previously reported that VEGF gene therapy, particularly ischemia-inducible VEGF gene therapy, significantly improves myocardial function and reduces left ventricular infarct size following acute myocardial infarction [25,26]. Because ischemic heart disease is characterized by reduced blood supply to the myocardium, therapeutic angiogenesis using angiogenic growth factors like VEGF may restore blood supply by stimulating neovascularization. Excessive VEGF expression, however, has been associated with heart failure and death, due to vascular tumor formation instead of the formation of functioning vessels [27]. We have reported that the new poly(disulfide amine)s, poly(CBA-DAH) and poly(CBA-DAH-R), are efficient and non-toxic polymeric gene carriers for the transfection of primary myoblasts and that they mediate controlled and optimal VEGF expression. Our previous studies laid the foundation for this current study demonstrating the functional benefit of this approach for treating acute myocardial infarction in an in vivo rat model [17].
In the present study, VEGF transfected myoblasts induced angiogenesis in a paracrine fashion. Immunostaining for capillaries in the ischemic zone revealed that neovascularization was more pronounced around the injection sites in the infarct border zone. This enhancement of capillary and arteriolar density in the left ventricle by VEGF transfected myoblasts correlated with VEGF expression levels (Fig. 1), and resulted in a decrease in infarct size and preservation of ventricular function.
Mechanisms by which implanted myoblasts may improve ischemic heart function include: 1) reinforcement of the ventricular wall and 2) limitation of post-infarct scar expansion through strengthening and enhancement of the extracellular matrix [1,24]. In addition, one of the major routes of cardiomyocyte loss in myocardial infarction is via apoptosis and necrosis, resulting in heart failure. There are several reports which show that cTnT staining levels in the infarcted myocardium are highly correlated with infarct size and can be used as a useful biomarker to detect myocardial injury [28,29]. As a result of its high cardiac specificity, cTnT immuno-staining can identify myocardial damage, especially necrosis [30]. As shown by our cTnT immunostaining results, the transplantation of skeletal primary myoblasts transfected with VEGF more efficiently protects against the loss of cardiomyocytes than does injection with untransfected myoblasts. VEGF transfected myoblast transplantation into ischemic myocardium increases VEGF expression, increases the vascular density of arterioles and capillaries, protects endogenous cardiomyocytes from death by apoptosis and necrosis, and ultimately preserves cardiac function to a significantly greater degree than untransfected myoblast transplantation.
Consistent with our previous in vitro study using poly disulfide polymers and bPEI for the transfection of primary myoblasts, transplantation of poly(CBA-DAH)-VEGF myoblasts demonstrated the utility of a dual therapeutic approach to maintain cardiac function and inhibit left ventricular remodeling after myocardial infarction. The sustained VEGF release from skeletal myoblasts resulted in:1) increased vessel density and 2) decreased apoptosis and cardiomyocyte loss.
5. Conclusions
Our study demonstrates the enhanced angiogenic efficacy of VEGF-transfected myoblasts using the bioreducible cationic polymer poly(CBA-DAH) as a transfection agent. Treatment with poly(CBA-DAH)/VEGF-transfected myoblasts preserved cardiac wall thickness, restored left ventricular function, induced neovascularization and reduced cardiac apoptosis more effectively than untransfected myoblasts. This work demonstrates that combining cellular implantation therapies with gene therapy can potentially increase the efficacy of surgical implantation of cells and produce better patient outcomes. Additionally, this work illustrates the in vivo safety and efficacy of poly(CBA:DAH) when used in combination with implanted cells.
Acknowledgments
This work was supported by NIH grants HL071541 (D.A.B.) and HL065477 (S.W.K.). The authors would also like to thank Sheryl Tripp of ARUP laboratories and Aida Garzarelli for their assistance in the preparation of the hearts for immunohistochemistry and histological analysis.
Footnotes
All work was performed at the University of Utah, Salt Lake City, Utah, USA
All authors have no financial conflicts of interest influencing the work performed in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Menasche P. Skeletal myoblasts and cardiac repair. J Mol Cell Cardiol. 2008;45:545–53. doi: 10.1016/j.yjmcc.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Menasche P, Hagège AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–83. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 3.Taylor DA. Cellular cardiomyoplasty with autologous skeletal myoblasts for ischemic heart disease and heart failure. Curr Control Trials Cardiovasc Med. 2001;2:208–10. doi: 10.1186/cvm-2-5-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoashi T, Matsumiya G, Miyagawa S, Ichikawa H, Ueno T, Ono M, et al. Skeletal myoblast sheet transplantation improves the diastolic function of a pressure-overloaded right heart. J Thorac Cardiovasc Surg. 2009;138:460–7. doi: 10.1016/j.jtcvs.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, et al. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–88. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 6.Reinecke H, Minami E, Poppa V, Murry CE. Evidence for fusion between cardiac and skeletal muscle cells. Circ Res. 2004;94:e56–60. doi: 10.1161/01.RES.0000125294.04612.81. [DOI] [PubMed] [Google Scholar]
- 7.Engelmann MG, Franz WM. Stem cell therapy after myocardial infarction: ready for clinical application? Curr Opin Mol Ther. 2006;8:396–414. [PubMed] [Google Scholar]
- 8.Menasche P. Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis. 2007;50:7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Lei Y, Haider H, Shujia J, Sim ES. Therapeutic angiogenesis. Devising new strategies based on past experiences. Basic Res Cardiol. 2004;99:121–32. doi: 10.1007/s00395-004-0447-x. [DOI] [PubMed] [Google Scholar]
- 10.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–65. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 11.Haider H, Ye L, Jiang S, Ge R, Law PK, Chua T, et al. Angiomyogenesis for cardiac repair using human myoblasts as carriers of human vascular endothelial growth factor. J Mol Med. 2004;82:539–49. doi: 10.1007/s00109-004-0546-z. [DOI] [PubMed] [Google Scholar]
- 12.Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100:468–74. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- 13.Niagara MI, Haider H, Ye L, Koh VS, Lim YT, Poh KK, et al. Autologous skeletal myoblasts transduced with a new adenoviral bicistronic vector for treatment of hind limb ischemia. J Vasc Surg. 2004;40:774–85. doi: 10.1016/j.jvs.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, et al. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001;104:I207–12. doi: 10.1161/hc37t1.094524. [DOI] [PubMed] [Google Scholar]
- 15.Germani A, Di Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P, et al. Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol. 2003;163:1417–28. doi: 10.1016/S0002-9440(10)63499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye L, Haider HKh, Tan R, Toh W, Law PK, Tan W, et al. Transplantation of nanoparticle transfected skeletal myoblasts overexpressing vascular endothelial growth factor-165 for cardiac repair. Circulation. 2007;116:I-113–20. doi: 10.1161/CIRCULATIONAHA.106.680124. [DOI] [PubMed] [Google Scholar]
- 17.Ou M, Kim TI, Yockman JW, Borden BA, Bull DA, Kim SW. Polymer transfected primary myoblasts mediated efficient gene expression and angiogenic proliferation. J Control Release. 2010;142:61–9. doi: 10.1016/j.jconrel.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Degenfeld G, Banfi A, Springer ML, Blau HM. Myoblast-mediated gene transfer for therapeutic angiogenesis and arteriogenesis. Br J Pharmacol. 2003;140:620–6. doi: 10.1038/sj.bjp.0705492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banfi A, Springer ML, Blau HM. Myoblast-mediated gene transfer for therapeutic angiogenesis. Methods Enzymol. 2002;346:145–57. doi: 10.1016/s0076-6879(02)46054-5. [DOI] [PubMed] [Google Scholar]
- 20.Ou M, Wang XL, Xu R, Chang CW, Bull DA, Kim SW. Novel biodegradable poly(disulfide amine)s for gene delivery with high efficiency and low cytotoxicity. Bioconjug Chem. 2008;19:626–33. doi: 10.1021/bc700397x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heiberg E, Wigstrom L, Carlsson M, Bolger A, Karlsson M. Time resolved three-dimensional automated segmentation of the left ventricle. Computers in Cardiology. 2005;2005:599–602. [Google Scholar]
- 22.Huang NF, Sievers RE, Park JS, Fang Q, Li S, Lee RJ. A rodent model of myocardial infarction for testing the efficacy of cells and polymers for myocardial reconstruction. Nat Protocols. 2006;1:1596–609. doi: 10.1038/nprot.2006.188. [DOI] [PubMed] [Google Scholar]
- 23.Montet-Abou K, Daire JL, Ivancevic MK, Hyacinthe JN, Nguyen D, Jorge-Costa M, et al. Optimization of cardiac cine in the rat on a clinical 1.5-T MR system. MAGMA. 2006;19:144–51. doi: 10.1007/s10334-006-0037-z. [DOI] [PubMed] [Google Scholar]
- 24.Menasche P. Skeletal muscle satellite cell transplantation. Cardiovasc Res. 2003;58:351–7. doi: 10.1016/s0008-6363(02)00769-1. [DOI] [PubMed] [Google Scholar]
- 25.Bull DA, Bailey SH, Rentz JJ, Zebrack JS, Lee M, Litwin SE, et al. Effect of terplex/VEGF-165 gene therapy on left ventricular function and structure following myocardial infarction: VEGF gene therapy for myocardial infarction. J Control Release. 2003;93:175–81. doi: 10.1016/j.jconrel.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Yockman JW, Choi D, Whitten MG, Chang CW, Kastenmeier A, Erickson H, et al. Polymeric gene delivery of ischemia-inducible VEGF significantly attenuates infarct size and apoptosis following myocardial infarct. Gene Ther. 2008;16:127–35. doi: 10.1038/gt.2008.146. [DOI] [PubMed] [Google Scholar]
- 27.Ernst RS, Mark TS, Mike P, Sharon SH, Jeffrey MI, Laurence HK, et al. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat—angiogenesis and angioma formation. J Am Coll Cardiol. 2000;35:1323–30. doi: 10.1016/s0735-1097(00)00522-2. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien PJ, Smith DEC, Knechtel TJ, Marchak MA, Pruimboom-Brees I, Brees DJ, et al. Cardiac troponin I is a sensitive, specific biomarker of cardiac injury in laboratory animals. Lab Anim. 2006;40:153–71. doi: 10.1258/002367706776319042. [DOI] [PubMed] [Google Scholar]
- 29.Ricchiuti V, Zhang J, Apple FS. Cardiac troponin I and T alterations in hearts with severe left ventricular remodeling. Clin Chem. 1997;43:990–5. [PubMed] [Google Scholar]
- 30.Hansen SH, Rossen K. Evaluation of cardiac troponin I immunoreaction in autopsy hearts: a possible marker of early myocardial infarction. Forensic Sci Int. 1999;99:189–96. doi: 10.1016/s0379-0738(98)00193-5. [DOI] [PubMed] [Google Scholar]