Abstract
Galectins regulate cellular functions by binding to glycan ligands on cell surface glycoprotein receptors. Prototype galectins, such as galectin-1, are one carbohydrate recognition domain (CRD) monomers that noncovalently dimerize, whereas tandem-repeat galectins, such as galectin-9, have two non-identical CRDs connected by a linker domain. Dimerization of prototype galectins, or both CRDs in tandem-repeat galectins, is typically required for the crosslinking of glycoprotein receptors and subsequent cellular signaling. Several studies have found that tandem-repeat galectins are more potent than prototype galectins in triggering many cell responses, including cell death. These differences could be due to CRD specificity, the presence or absence of a linker domain between CRDs, or both. To interrogate the basis for the increased potency of tandem-repeat galectins compared with prototype galectins in triggering cell death, we created three tandem-repeat galectin constructs with different linker regions joining identical galectin-1 CRDs, so that any differences we observed would be due to the contribution of the linker region rather than due to CRD specificity. We found that random-coil or rigid α-helical linkers that permit separation of the two galectin-1 CRDs facilitated the formation of higher-order galectin multimers and that these galectins were more potent in binding to glycan ligands and cell surface glycoprotein receptors, as well as triggering T cell death, compared with native galectin-1 or a construct with a short rigid linker. Thus, the increased potency of tandem-repeat galectins compared with prototype galectins is likely due to the ability of the linker domain to permit intermolecular CRD interactions, resulting in the formation of higher-order multimers with increased valency, rather than differences in CRD specificity.
Keywords: apoptosis, galectin, glycan microarray, lattice, tandem repeat
Introduction
Galectins regulate many critical cell processes, including differentiation, maturation, activation, migration and apoptosis (Hernandez and Baum 2002; Rabinovich and Toscano 2009). Although galectins have homologous carbohydrate recognition domains (CRDs) (Barondes et al. 1994), different members of the galectin family are differentially expressed in various cells and tissues, recognize unique complements of glycan ligands, signal through different intracellular pathways, have distinct cellular and molecular targets, and trigger a wide range of cellular responses, including cell proliferation, maturation, migration and death. Moreover, several studies have demonstrated that galectins differ in potency in triggering the same cellular response; for example, tandem-repeat galectins-4, -8 and -9 are more potent than galectin-3, which is more potent than galectin-1, in triggering signaling in T cells and neutrophils (Kashio et al. 2003; Sturm et al. 2004; Stillman et al. 2006; Lu et al. 2007; Stowell et al. 2007; Tribulatti et al. 2007; Bi et al. 2008; Stowell, Arthur, et al. 2008; Stowell, Qian, et al. 2008).
All members of the galectin family are functionally oligomeric, but the mechanism for oligomerization differs among the subfamilies of galectins. In the prototype subfamily, which includes galectin-1, the CRD exists as a monomer that noncovalently dimerizes in solution, and the dimeric form is required for effective binding and signaling through cell surface glycoproteins (Giudicelli et al. 1997; Levroney et al. 2005). In tandem-repeat galectins, there are two distinct CRDs joined by a random-coil linker, which is required for the full function (Tureci et al. 1997; Levy et al. 2006). Thus, functional multivalency, allowing the crosslinking of glycan ligands, is a common feature of galectins and is required for many cellular effects (Brewer 2002).
The ability of tandem-repeat galectins to induce cell signaling at lower concentrations than prototype galectins has been proposed to result from the constitutive bivalency of tandem-repeat galectins (Levy et al. 2006; Carlsson et al. 2007; Bi et al. 2008). However, the longer flexible linker domain of the tandem-repeat galectins may also contribute to the increased potency, as creating a constitutive dimeric galectin-1 with a rigid glycine–glycine linker offered only a 3-fold increase in potency over wild-type galectin-1, compared with the >20-fold increase in potency of galectin-9 vs. galectin-1 in triggering T cell death (Battig et al. 2004; Bi et al. 2008). Here, we investigate how the linker region between galectin-1 CRDs affects galectin multimerization, and thus binding and signaling. Although the linker region did not alter galectin-1 binding specificity or the downstream signaling pathway, the presence of a long linker that allows rotational freedom of the CRDs promoted higher-order multimerization, a mechanism by which increased signaling potency may be achieved.
Results and discussion
Long linkers in galectin-1 constructs confer increased potency
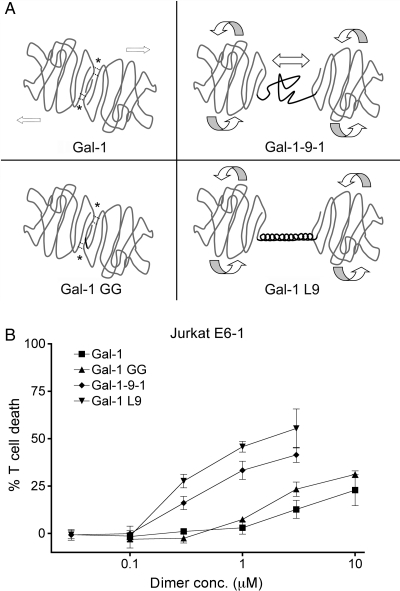
To investigate the influence of linker length and structure on galectin binding, signaling and multimerization, we used the galectin-1 constructs shown in Figure 1A. Wild-type galectin-1 dimerizes noncovalently via hydrophobic interactions between the monomers; this structure is relatively rigid and fixes the CRD orientation (Liao et al. 1994). To examine the effect of constitutive dimerization, we used the gal-1GG construct (Battig et al. 2004; Bi et al. 2008), which has two galectin-1 CRDs joined by a glycine–glycine linker that maintains the CRD orientation of wild-type galectin-1. To examine the effect of a flexible tandem-repeat linker region, we used the gal-1-9-1 construct, which has the native 14 amino acid galectin-9 short isoform random-coil linker between two galectin-1 CRDs; we reasoned that this structure would allow the crosslinking of glycan ligands, and also allow the lateral movement of the CRDs and more flexible CRD orientation (Bi et al. 2008). A fourth construct, gal-1L9, links two galectin-1 CRDs with a 34 amino acid rigid α-helix derived from the bacterial ribosomal L9 protein (Kuhlman et al. 1998). This linker has a similar maximal length (about 50 Å) as the galectin-9 flexible linker domain. We reasoned that this structure would, like gal-1-9-1, allow free CRD rotation, but that the rigid α-helix would allow less lateral movement of CRDs compared with gal-1-9-1. While native tandem-repeat galectins have two different CRDs, these constructs with two identical galectin-1 CRDs do maintain the orientation of tandem-repeat galectins, so that we could distinguish the contributions of the linker domains from those of the CRDs. Galectin-1, gal-1GG, gal-1-9-1 and gal-1L9 were purified by lactose affinity chromatography, and all four proteins agglutinated T cell lines in a lactose-inhibitable manner (data not shown), indicating that the constructs retained carbohydrate-dependent binding to T cells.
Fig. 1.
(A) Schematic of galectin-1 constructs. Upper left: galectin-1 forms homodimers via hydrophobic interactions (*). Lower left: gal-1GG has a glycine–glycine linker (black line) between the C-terminus of one CRD and the N-terminus of the second CRD. Hydrophobic interactions at the dimer interface are maintained. Upper right: gal-1-9-1 has two galectin-1 CRDs joined by the galectin-9 short random-coil linker region (black line), which would allow the rotation and lateral movement of the CRDs. Lower right: gal-1L9 has two galectin-1 CRDs joined by the bacterial ribosomal L9 rigid α-helix peptide (black line). Gal-1L9 would allow the rotation of CRDs, but not lateral movement. (B) Potency of galectin-1 constructs. Galectins with long linker regions are more potent in T cell death assays. Jurkat E6-1 T cells were incubated with the indicated dimer concentration of galectin-1 (filled square), gal-1GG (filled triangle), gal-1-9-1 (filled diamond), gal-1L9 (filled inverted triangle) or buffer control for 6 h. Data represent mean ± SD of triplicate samples.
We compared the ability of the galectin-1 constructs to induce death of a human T cell line (Figure 1B). Wild-type galectin-1 began to induce the cell death of Jurkat E6-1 cells at 3 µM dimer (6 µM monomer), which is close to the reported Kd for galectin-1 and consistent with our previous observations (Cho and Cummings, 1995; Bi et al. 2008). Gal-1GG began to induce the cell death of Jurkat E6-1 cells at 1 µM, a 3-fold enhancement that is similar to previous reports comparing wild-type galectin-1 with gal-1GG (Battig et al. 2004; Bi et al. 2008). Gal-1-9-1 began to trigger death of Jurkat E6-1 cells at 0.3 µM (Bi et al. 2008); moreover, we found that gal-1L9, with a linker region of similar length, was similar in potency to gal-1-9-1. Indeed, cell death induced by gal-1-9-1 and gal-1L9 at 0.3 µM was comparable to death induced by galectin-1 at 10 µM homodimer, i.e. approximately a 30-fold enhancement over wild-type galectin-1. Thus, despite having identical CRDs, the constructs demonstrated significant differences in potency that were directly related to the length of the linker domain.
Linkers do not change glycan-binding specificity
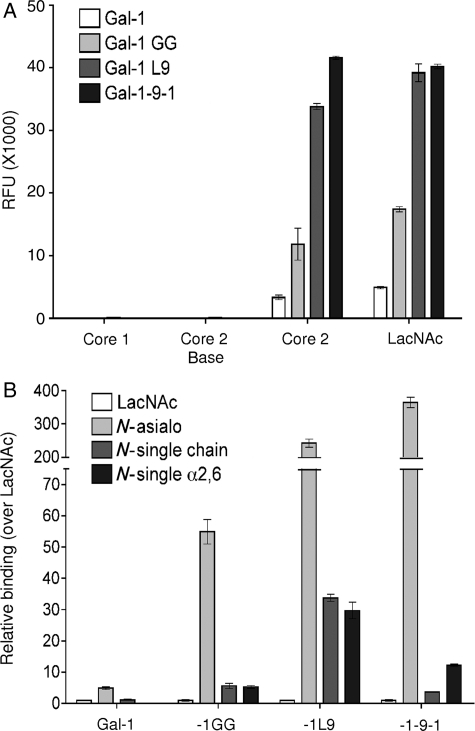
To confirm that the differences in potency we observed were not due to changes in carbohydrate specificity of the constructs, we asked if the four constructs bound N- and O-glycans in a similar manner, using a glycan microarray. As shown in Figure 2A, wild-type galectin-1 bound preferentially to core 2 O-glycans bearing N-acetyllactosamine (LacNAc) (Core 2), compared with binding to LacNAc alone, with no measurable binding to core 1 or non-extended core 2 structures (Core 2 base). Although gal-1GG, gal-1-9-1 and gal-1L9 showed increased binding to both core 2 and LacNAc structures compared with wild-type galectin-1, none of these constructs significantly bound core 1 or non-extended core 2 structures. Thus, all constructs bound O-glycans with the same relative specificity.
Fig. 2.
Galectin-1 constructs share binding specificities. Biotinylated galectin-1, gal-1GG, gal-1-9-1 and gal-1L9 were assayed on a glycan microarray. (A) Galectin-1 constructs bind specifically to LacNAc, either alone or on a core 2 O-glycan. Binding, assayed by relative fluorescence units (RFUs), are shown for glycans 131 (Core 1, Galb1-3GalNAca-Sp8), 182 [Core 2 base, GlcNAcb1-6(Galb1-3)GalNAca-Sp8], 157 [Core 2, Galb1-4GlcNAc1-6(Galb1-3)GalNAca-Sp8] and 161 (LacNAc, Galb1-4GlcNAc-Sp0). Data are mean RFU ± SE of six replicate assays. (B) Galectin-1 constructs bind to biantennary N-glycans; binding is reduced on single-chain or α2,6-sialylated N-glycans. Binding in RFU is shown for glycans 161 (LacNAc, Galb1-4GlcNAc-Sp0), 50 (N-asialo, Galb1-4GlcNAcb1-2Mana1-3(Galb1-4GlcNAcb1-2Mana1-6)Manb1-4GlcNAcb1-4GlcNAcb-Sp12), 360 [N-single chain, Mana1-3(Galb1-4GlcNAcb1-2Mana1-6)Manb1-4GlcNAcb1-4GlcNAcb-Sp12], and 314 [N-single α2,6, Neu5Aca2-6Galb1-4GlcNAcb1-2Mana1-3(Galb1-4GlcNAcb1-2Mana1-6)Manb1-4GlcNAcb1-4GlcNAcb-Sp12], relative to LacNAc (glycan 161). Data are mean RFU over LacNAc RFU ± S.E. of six replicate assays.
We also analyzed the binding of the constructs to N-glycans. Because of the high binding of the constructs to biantennary N-glycans, we used a lower concentration of gal-1GG, gal-1-9-1 and gal-1L9, compared with that used for wild-type galectin-1. As shown in Figure 2B, galectin-1 preferentially bound to biantennary N-glycans bearing two LacNAc sequences (N-asialo); the loss of one LacNAc chain (N-single chain) or the addition of a single-terminal α2,6-linked sialic acid (N-single α2,6) significantly reduced galectin-1 binding. Similar binding patterns were seen with gal-1GG, gal-1-9-1 and gal-1L9. The increase in binding of gal-1-9-1 and gal-1L9 to the asialo biantennary N-glycan over LacNAc (>200-fold increase) was striking and was significantly greater than that of wild-type galectin-1 (5-fold increase). Nevertheless, the relative preference for asialo biantennary N-glycans over α2,6-linked sialic acid capped N-glycans was maintained. Thus, as we observed for O-glycans, the introduction of a long linker between the CRDs significantly increased the relative binding of a tandem-repeat-type galectin to N-glycan ligands, but did not change the overall specificity of the CRDs for specific glycans.
In addition to examining CRD specificity, we also examined the ability of the four constructs to kill T cells via the galectin-1 death pathway (Bi et al. 2008). Signaling through CD7 and CD45, and the expression of core 2 O-glycans, was required for optimal T cell killing by all four constructs, and susceptibility to cell death was reduced by the addition of α2,6-linked sialic acids to N-glycans (data not shown), confirming that all four constructs bind via galectin-1 CRDs to utilize the same signaling pathway to trigger cell death. Thus, the differences in potency among the four constructs (Figure 1B) cannot be attributed to differences in binding specificity among CRDs for particular glycans or glycoprotein receptors, or to differences in cell death pathways, and must be due to the effects of the linkers. Therefore, we asked if differential multimerization of the galectin constructs, altering functional valency, would be affected by different linkers and thus account for the differences in potency that we observed.
Multimerization of galectins with long linker domains
As mentioned in the Introduction, the gal-1GG construct, in which the short Gly–Gly linker prohibits the dissociation of the galectin-1 CRDs, is approximately 3-fold more potent than wild-type galectin-1 in various assays (Figure 1B) (Battig et al. 2004; Bi et al. 2008). However, the longer linkers in the gal-1-9-1 and gal-1L9 constructs could allow more movement and rotation of the CRDs in these tandem-repeat-type galectins. This CRD mobility could allow the hydrophobic faces of the galectin-1 CRDs to be exposed to solvent, and thus facilitate dimerization between CRDs on different gal-1-9-1 or gal-1L9 molecules. This would be similar to the interactions that have been proposed for native tandem-repeat galectins (Nagae et al. 2006; Carlsson et al. 2007; Miyanishi et al. 2007).
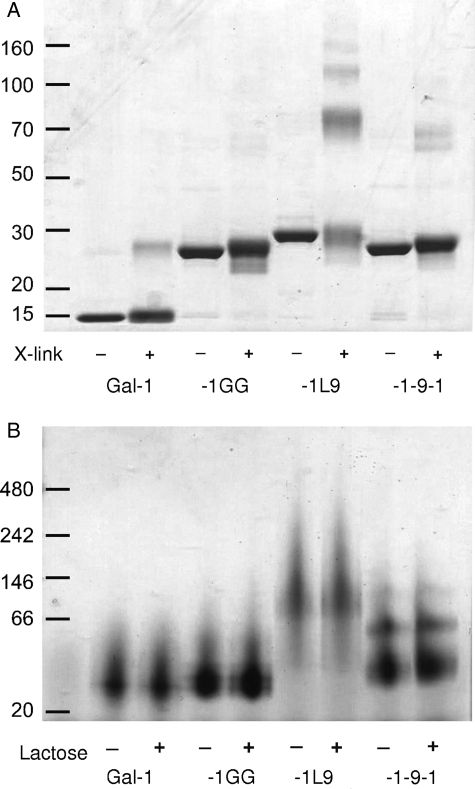
To determine whether gal-1-9-1 and gal-1L9 were capable of forming dimers and higher-order multimers, we treated the galectins with a nonreducible crosslinker and analyzed the proteins by sodium dodecyl sulfate – polyacrylamide gel electrophoresis (SDS–PAGE) (Figure 3A). As expected, monomeric (14.5 kDa) and dimeric (29 kDa) galectin-1 were detected, and gal-1GG migrated at approximately 29 kDa, similar to the galectin-1 dimer. However, both gal-1-9-1 and gal-1L9 formed higher-order multimers. While a significant fraction of gal-1-9-1 migrated as a monomer at 32 kDa, we also detected dimers and some trimers of gal-1-9-1 at 64 and 96 kDa, respectively. The majority of gal-1L9 formed higher-order multimers, with complexes of up to five units (165 kDa) observed. This suggests that the random-coil linker in gal-1-9-1 can allow self-association of two linked galectin-1 CRDs, but can also allow intermolecule association of CRDs. In contrast, gal-1L9, because of its rigid linker, may not promote the association of linked CRDs, and intermolecule dimerization may be favored.
Fig. 3.
Gal-1-9-1 and gal-1L9 form higher order multimers. (A) BS3 crosslinking of constructs reveals that gal-1L9 forms multimers up to pentamers, whereas gal-1-9-1 forms dimers and trimers. Proteins were incubated with crosslink reagent or buffer control and analyzed by SDS–PAGE. (B) Multimerization of galectins occurs under native conditions and does not occur through the CRD-active site. Proteins in 50 mM lactose or buffer control were run on a 4–12% Bis–Tris native gel.
To confirm that multimers were formed in the absence of a crosslinker and did not result from intermolecular disulfide bonding through CRDs (Tracey et al. 1992; Bourne et al. 1994), the constructs were analyzed by native gel electrophoresis in the presence or absence of lactose, to block the CRD. Both gal-1-9-1 and gal-1L9 formed multimers in native solution, which were unaffected by the presence of lactose (Figure 3B). Thus, intermolecular interactions between CRDs of gal-1-9-1 and gal-1L9 that preserve the glycan-binding capacity of the CRDs account for the formation of higher-order multimers, creating galectins with tetrameric or higher valency.
Galectins with long linker domains form cell surface lattices at lower concentrations
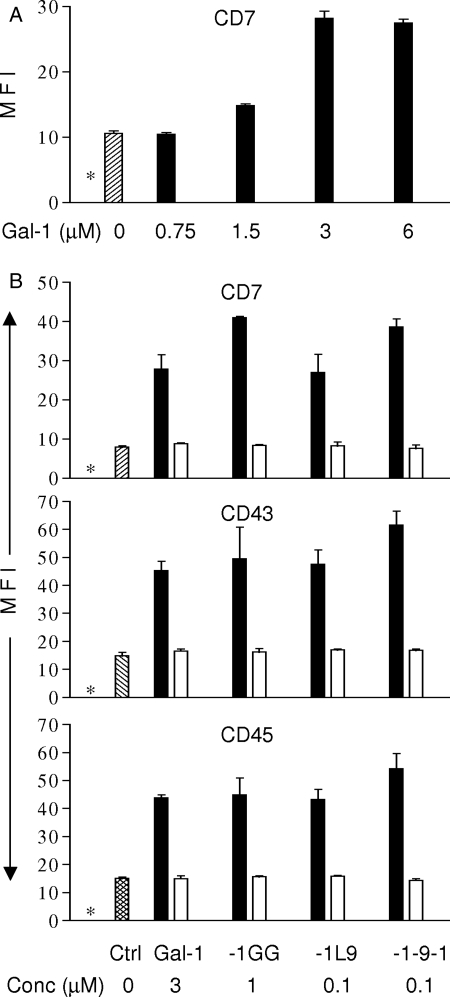
An important mechanism by which galectins regulate cellular events is by complexing with cell surface glycoprotein receptors to create galectin–glycoprotein lattices (Brewer 2002; Garner and Baum 2008; Rabinovich and Toscano 2009). These lattices can affect cell signaling in several ways. Galectin lattices can retain glycoproteins on the cell surface by retarding endocytosis and thus increase the effective concentration of the receptor, or galectins can alter signaling by the receptors (Chen et al. 2007; Lau et al. 2007; Abbott et al. 2008). To determine whether the galectins with longer linkers are also more potent in creating cell surface glycoprotein lattices, we examined the four galectins for the ability to retain the glycoproteins CD7, CD43 and CD45, receptors for galectin-1, on the surface of T cells. As shown in Figure 4A, wild-type galectin-1 increased the abundance of CD7 at the cell surface in a dose-dependent manner. To demonstrate the retention of cell surface receptors, three micromolar dimeric galectin-1 was the optimal concentration for demonstrating retention of cell surface receptors, with no significant increase in receptor abundance above that concentration, and a sub-optimal effect below that concentration. Similar results were observed for retention of CD43 and CD45 on the cell surface by wild-type galectin-1 (data not shown). Thus, we compared the ability of wild-type galectin-1, gal-1GG, gal-1-9-1 and gal-1L9 to increase the abundance of CD7, CD43 and CD45 at the cell surface (Fig. 4B). One micromolar gal-1GG was sufficient to retain all three glycoprotein receptors at the level observed for 3 µM dimeric galectin-1, consistent with the 3-fold increase in potency we observed for gal-1GG in triggering T cell death (Figure 1). In contrast, only 0.1 µM gal-1-9-1 and gal-1L9 were required to achieve the same effect on CD7, CD43 and CD45 cell surface expression, indicating that the galectins with longer linkers promote the formation of multivalent intermolecular multimers that can enhance the “lattice effect” on the cell surface. This was consistent with both the increased potency of these constructs in triggering T cell death (Figure 1B) as well as the increase in binding to biantennary N-glycans with two LacNAc branches (Figure 2B), compared with galectin-1. Although Figures 3A and B indicate that gal-1L9 formed a greater fraction of higher-order multimers compared with gal-1-9-1, we did not see a significant difference in the ability of the two constructs to retain glycoprotein receptors on the cell surface, even at lower concentrations (0.03 μM, data not shown), suggesting that additional factors, such as rigidity or flexibility of the linker, are also important in forming glycoprotein lattices. All effects on cell surface glycoprotein receptors were abrogated by the addition of lactose, demonstrating that the effect is carbohydrate-dependent. This suggests that the formation of multimers by tandem-repeat galectins may promote enhanced binding to and retention on multiantennary glycans presented on multiple glycan branches on highly glycosylated cell surface receptors. Moreover, the presentation and clustering of multivalent glycans on cell surface receptors may further potentiate binding and crosslinking of tandem-repeat galectins and enhance receptor retention.
Fig. 4.
Linker length influences the ability of galectins to retain glycoprotein receptors on the T cell surface. (A) Galectin-1 retains CD7 on the T cell surface. Maximal effect was observed at 3 µM galectin-1 dimer. CD7 expression in the absence of galectin-1 is indicated by the hatched bar. The asterisk (*) indicates the level of binding by an isotype matched control. (B) Insertion of a linker domain between the CRDs decreases the effective concentration at which gal-1GG, gal-1-9-1 and gal-1L9 retain CD7, CD43 and CD45 on the T cell surface (closed bars). The concentration of each galectin construct is indicated (Conc µM). Increased retention of glycoprotein receptors was abolished by co-incubation with 100 mM lactose (open bars). Antigen expression in the absence of galectins is indicated by the hatched bar. The asterisk (*) indicates the level of binding by an isotype-matched control. Data represent mean ± SD of triplicate samples from one of three replicate experiments.
Several roles for linker regions in tandem-repeat galectins have been proposed, including protein–protein interactions (Levy et al. 2006; Delacour et al. 2009), membrane insertion (Lipkowitz et al. 2001) and regulation of CRD presentation (Sato et al. 2002; Bi et al. 2008). Here we demonstrate that the insertion of a linker region between two CRDs in a tandem-repeat galectin confers increased signaling potency by allowing intermolecular interaction of CRDs, thus promoting higher-order multimerization, which may occur in tandem-repeat galectins-9 and -8 (Nagae et al. 2006; Stowell, Arthur, et al. 2008), and increased lattice formation on the cell surface. This linker function is not sequence-specific, as there were similar effects between a galectin-9-derived linker protein and a bacterial-derived linker protein, although there may be some variation in effect due to differences in linker secondary structure. Both gal-1-9-1 and gal-1L9 constructs would be expected to allow relatively free rotation of the CRDs, although the flexible linker in gal-1-9-1 would also allow intramolecular lateral movement of CRDs, which the rigid linker in gal-1L9 would not. However, gal-1-9-1 showed no advantage in cell death assays, compared with gal-1L9 (Figure 1). This suggests that CRD rotational freedom and the intermolecular multimerization possible for gal-1-9-1 and gal-1L9 are more important factors in regulating signaling potency than intramolecular CRD lateral movement. The ability of the α-helical linker of gal-1L9 to promote intermolecular multimerization (Figure 3) may relate directly to native tandem-repeat galectins. Tandem-repeat galectin CRDs have little intramolecular dimerization; rather, it has been suggested that, although both CRDs in native tandem-repeat galectins can bind glycans, one CRD may have the majority of glycan-binding specificity, and the other may act as a dimerization motif (Sato et al. 2002; Carlsson et al. 2007; Miyanishi et al. 2007), allowing the formation of higher-order multimers that could be more effective in creating a cell surface lattice with glycoprotein receptors. Thus, in vivo, tandem-repeat galectins that can form higher-order multivalent multimers may have significant biological effects at much lower tissue concentrations than have been observed for prototype galectins. As multiple isoforms of tandem-repeat galectins, with different linker regions, have been conserved among species (Bidon et al. 2001; Spitzenberger et al. 2001; Sato et al. 2002), the specific linker regions may contribute to differences in function and potency among the isoforms, to allow fine-tuning of signaling thresholds.
Materials and methods
Galectin construction, expression and biotinylation
Galectin-1, gal-1GG and gal-1-9-1 were constructed, expressed and purified as described (Pace et al. 2003; Bi et al. 2008). Gal-1L9 was constructed by serial PCR reactions to ligate cDNA encoding the galectin-1 CRD with the first half of the L9 linker (sequence from Kuhlman et al. 1998) and the second half of the L9 linker with a second galectin-1 CRD, which were each cloned into pCR-XL-TOPO vector (Invitrogen). TOPO vector with the N-terminal CRD and L9 linker cDNA was cut with restriction enzymes XbaI and BsmI (New England Biolabs). TOPO vector with the C-terminal CRD and L9 linker cDNA was cut with restriction enzymes BamHI and BsmI. pGEMEX vector was cut with restriction enzymes XbaI and BamHI. Vector and reaction products were ligated with T4 DNA ligase and transformed into JM109 cells. cDNA was verified by DNA sequencing (UC Davis Sequencing) and Gal-1 L9 in modified pGEMEX vector was expressed and purified as for galectin-1. All constructs were biotinylated as described (Perillo et al. 1995). Biotinylated proteins at 200 µg/mL or 2 µg/mL were assayed at Core H at the Consortium for Functional Glycomics on the mammalian version 4 slide glycan microarray.
Death assays
For 6 h, 2 × 105 Jurkat cells were incubated in 200 µL of media with the indicated concentrations of galectins in 1.2 mM dithiothreitol (Fisher Scientific) or buffer control. Cells were dissociated in 0.1 M lactose, washed twice in annexin V-binding buffer, then stained with 1:100 diluted annexin V-fluoroscein isothiocyanate (FITC) (Molecular Probes) and counterstained with 2 µg/mL of 7-aminoactinomycin D (7-AAD; Invitrogen) or propidium iodide (PI; Invitrogen) in annexin V-binding buffer (100 mM HEPES, 1.4 M NaCl, 25 mM CaCl2). FITC, PI and 7-AAD fluorescence were read on a BD FACScan or BD LSR I flow cytometer. Cell death was calculated by normalizing % annexin V-positive cells in galectin treated cells to % annexin V-positive control-treated cells.
Detection of multimers
Purified galectins at 40 µg/mL were incubated with 5 mM Bis(sulfosuccinimidyl) suberate (BS3) crosslink reagent (Pierce) for 15 min in phosphate buffered saline (PBS) at room temperature. The reaction was stopped by the addition of Tris pH 7.5 to a concentration of 160 mM. Samples were denatured and run on 4–12% Bis–Tris NuPAGE Novex gels (Invitrogen) in 3-[N-morpholino]propanesulfonic acid (MOPS) buffer under reducing conditions. Gels were stained with Novex Colloidal Blue (Invitrogen). For native gels, 2 µg of purified protein in 1× PBS with or without 50 mM lactose was run on 4–16% Bis–Tris NativePAGE gels (Invitrogen) and stained with Novex Colloidal Blue.
Cell surface glycoprotein receptor expression
Jurkat E6-1T cells (6 × 105) were incubated with PBS or the indicated concentrations of galectins in 24-well tissue culture plates for 30 min with or without 100 mM lactose, followed by 30 min fixation with 4% paraformaldehyde on ice. Fixed cells were stained with 1 µg of anti-CD7-FITC (Becton Dickinson), 0.01 µg of anti-CD43-PE (Caltag) or 0.01 µg of anti-CD45-PE (Caltag) mouse anti-human mAb for 1 h at 4°C. Cells were analyzed on a BD FACScan and mean fluorescence intensity was determined. Isotype-matched antibody served as controls.
Funding
This work was supported by NIH R01GM63281 (to L.G.B.), NIH T32AI52031 (to L.A.E.) and the UC Cancer Research Coordinating Committee (to L.G.B.).
Conflict of interest statement
None declared.
Abbreviations
CRD, carbohydrate recognition domain; LacNAc, N-acetyllactosamine; RFU, relative fluorescence units; DTT, dithiothreitol; 7-AAD, 7-aminoactinomycin D; PI, propidium iodide; MFI, mean fluorescence intensity; SDS-PAGE, sodium dodecyl sulfate — polyacrylamide gel electrophoresis; FITC, fluorescein isothiocyanate; BS3, Bis(sulfosuccinimidyl) suberate; PBS, phosphate buffered saline; MOPS - 3-[N-morpholino]propanesulfonic acid.
Acknowledgements
We thank Omai Garner, Paula Cabrera, Mary Clark and Mabel Pang for helpful suggestions, James Bowie for valuable advice in choosing linker structures and David Smith and Jamie Heinberg-Molinaro of the Glycan-Protein Interaction Core of The Consortium for Functional Glycomics (NIH GM62116) at Emory University School of Medicine, Atlanta, GA, for glycan array analysis. We thank the UCLA Jonsson Comprehensive Cancer Center Flow Cytometry Resource (NIH CA16042, AI28697).
References
- Abbott KL, Matthews RT, Pierce M. Receptor tyrosine phosphatase beta (RPTPbeta) activity and signaling are attenuated by glycosylation and subsequent cell surface galectin-1 binding. J Biol Chem. 2008;283:33026–33035. doi: 10.1074/jbc.M803646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Battig P, Saudan P, Gunde T, Bachmann MF. Enhanced apoptotic activity of a structurally optimized form of galectin-1. Mol Immunol. 2004;41:9–18. doi: 10.1016/j.molimm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bi S, Earl LA, Jacobs L, Baum LG. Structural features of galectin-9 and galectin-1 that determine distinct T cell death pathways. J Biol Chem. 2008;283:12248–12258. doi: 10.1074/jbc.M800523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidon N, Brichory F, Bourguet P, Le Pennec JP, Dazord L. Galectin-8: a complex sub-family of galectins (Review) Int J Mol Med. 2001;8:245–250. doi: 10.3892/ijmm.8.3.245. [DOI] [PubMed] [Google Scholar]
- Bourne Y, Bolgiano B, Liao DI, Strecker G, Cantau P, Herzberg O, Feizi T, Cambillau C. Crosslinking of mammalian lectin (galectin-1) by complex biantennary saccharides. Nat Struct Biol. 1994;1:863–870. doi: 10.1038/nsb1294-863. [DOI] [PubMed] [Google Scholar]
- Brewer CF. Binding and cross-linking properties of galectins. Biochim Biophys Acta. 2002;1572:255–262. doi: 10.1016/s0304-4165(02)00312-4. [DOI] [PubMed] [Google Scholar]
- Carlsson S, Oberg CT, Carlsson MC, Sundin A, Nilsson UJ, Smith D, Cummings RD, Almkvist J, Karlsson A, Leffler H. Affinity of galectin-8 and its carbohydrate recognition domains for ligands in solution and at the cell surface. Glycobiology. 2007;17:663–676. doi: 10.1093/glycob/cwm026. [DOI] [PubMed] [Google Scholar]
- Chen IJ, Chen HL, Demetriou M. Lateral compartmentalization of T cell receptor versus CD45 by galectin-N-glycan binding and microfilaments coordinate basal and activation signaling. J Biol Chem. 2007;282:35361–35372. doi: 10.1074/jbc.M706923200. [DOI] [PubMed] [Google Scholar]
- Cho M, Cummings RD. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. I. Physical and chemical characterization. J Biol Chem. 1995;270:5198–5206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- Delacour D, Koch A, Jacob R. The role of galectins in protein trafficking. Traffic. 2009;10:1405–1413. doi: 10.1111/j.1600-0854.2009.00960.x. [DOI] [PubMed] [Google Scholar]
- Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem Soc Trans. 2008;36:1472–1477. doi: 10.1042/BST0361472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicelli V, Lutomski D, Levi-Strauss M, Bladier D, Joubert-Caron R, Caron M. Is human galectin-1 activity modulated by monomer/dimer equilibrium? Glycobiology. 1997;7:viii–x. doi: 10.1093/glycob/7.3.323-a. [DOI] [PubMed] [Google Scholar]
- Hernandez JD, Baum LG. Ah, sweet mystery of death! Galectins and control of cell fate. Glycobiology. 2002;12:127R–136R. doi: 10.1093/glycob/cwf081. [DOI] [PubMed] [Google Scholar]
- Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, Yoshida N, Nakamura T, Hirashima M. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol. 2003;170:3631–3636. doi: 10.4049/jimmunol.170.7.3631. [DOI] [PubMed] [Google Scholar]
- Kuhlman B, Boice JA, Fairman R, Raleigh DP. Structure and stability of the N-terminal domain of the ribosomal protein L9: evidence for rapid two-state folding. Biochemistry. 1998;37:1025–1032. doi: 10.1021/bi972352x. [DOI] [PubMed] [Google Scholar]
- Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell. 2007;129:123–134. doi: 10.1016/j.cell.2007.01.049. [DOI] [PubMed] [Google Scholar]
- Levroney EL, Aguilar HC, Fulcher JA, Kohatsu L, Pace KE, Pang M, Gurney KB, Baum LG, Lee B. Novel innate immune functions for galectin-1: galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J Immunol. 2005;175:413–420. doi: 10.4049/jimmunol.175.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y, Auslender S, Eisenstein M, Vidavski RR, Ronen D, Bershadsky AD, Zick Y. It depends on the hinge: a structure-functional analysis of galectin-8, a tandem-repeat type lectin. Glycobiology. 2006;16:463–476. doi: 10.1093/glycob/cwj097. [DOI] [PubMed] [Google Scholar]
- Liao DI, Kapadia G, Ahmed H, Vasta GR, Herzberg O. Structure of S-lectin, a developmentally regulated vertebrate beta-galactoside-binding protein. Proc Natl Acad Sci U S A. 1994;91:1428–1432. doi: 10.1073/pnas.91.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz MS, Leal-Pinto E, Rappoport JZ, Najfeld V, Abramson RG. Functional reconstitution, membrane targeting, genomic structure, and chromosomal localization of a human urate transporter. J Clin Invest. 2001;107:1103–1115. doi: 10.1172/JCI12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Nakagawa R, Kashio Y, Ito A, Shoji H, Nishi N, Hirashima M, Yamauchi A, Nakamura T. Characterization of galectin-9-induced death of Jurkat T cells. J Biochem. 2007;141:157–172. doi: 10.1093/jb/mvm019. [DOI] [PubMed] [Google Scholar]
- Miyanishi N, Nishi N, Abe H, Kashio Y, Shinonaga R, Nakakita S, Sumiyoshi W, Yamauchi A, Nakamura T, Hirashima M, et al. Carbohydrate-recognition domains of galectin-9 are involved in intermolecular interaction with galectin-9 itself and other members of the galectin family. Glycobiology. 2007;17:423–432. doi: 10.1093/glycob/cwm001. [DOI] [PubMed] [Google Scholar]
- Nagae M, Nishi N, Murata T, Usui T, Nakamura T, Wakatsuki S, Kato R. Crystal structure of the galectin-9 N-terminal carbohydrate recognition domain from Mus musculus reveals the basic mechanism of carbohydrate recognition. J Biol Chem. 2006;281:35884–35893. doi: 10.1074/jbc.M606648200. [DOI] [PubMed] [Google Scholar]
- Pace KE, Hahn HP, Baum LG. Preparation of recombinant human galectin-1 and use in T-cell death assays. Methods Enzymol. 2003;363:499–518. doi: 10.1016/S0076-6879(03)01075-9. [DOI] [PubMed] [Google Scholar]
- Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature. 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nature Reviews Immunology. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- Sato M, Nishi N, Shoji H, Seki M, Hashidate T, Hirabayashi J, Kasai Ki K, Hata Y, Suzuki S, Hirashima M, et al. Functional analysis of the carbohydrate recognition domains and a linker peptide of galectin-9 as to eosinophil chemoattractant activity. Glycobiology. 2002;12:191–197. doi: 10.1093/glycob/12.3.191. [DOI] [PubMed] [Google Scholar]
- Spitzenberger F, Graessler J, Schroeder HE. Molecular and functional characterization of galectin 9 mRNA isoforms in porcine and human cells and tissues. Biochimie. 2001;83:851–862. doi: 10.1016/s0300-9084(01)01335-9. [DOI] [PubMed] [Google Scholar]
- Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Slanina KA, Horton JR, Smith DF, Cummings RD. Dimeric Galectin-8 induces phosphatidylserine exposure in leukocytes through polylactosamine recognition by the C-terminal domain. J Biol Chem. 2008;283:20547–20559. doi: 10.1074/jbc.M802495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109:219–227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180:3091–3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- Sturm A, Lensch M, Andre S, Kaltner H, Wiedenmann B, Rosewicz S, Dignass AU, Gabius HJ. Human galectin-2: novel inducer of T cell apoptosis with distinct profile of caspase activation. J Immunol. 2004;173:3825–3837. doi: 10.4049/jimmunol.173.6.3825. [DOI] [PubMed] [Google Scholar]
- Tracey BM, Feizi T, Abbott WM, Carruthers RA, Green BN, Lawson AM. Subunit molecular mass assignment of 14,654 Da to the soluble beta-galactoside-binding lectin from bovine heart muscle and demonstration of intramolecular disulfide bonding associated with oxidative inactivation. J Biol Chem. 1992;267:10342–10347. [PubMed] [Google Scholar]
- Tribulatti MV, Mucci J, Cattaneo V, Aguero F, Gilmartin T, Head SR, Campetella O. Galectin-8 induces apoptosis in the CD4(high)CD8(high) thymocyte subpopulation. Glycobiology. 2007;17:1404–1412. doi: 10.1093/glycob/cwm104. [DOI] [PubMed] [Google Scholar]
- Tureci O, Schmitt H, Fadle N, Pfreundschuh M, Sahin U. Molecular definition of a novel human galectin which is immunogenic in patients with Hodgkin's disease. J Biol Chem. 1997;272:6416–6422. doi: 10.1074/jbc.272.10.6416. [DOI] [PubMed] [Google Scholar]