Abstract
Passive transfer of high-titered antiviral neutralizing IgG, known to confer sterilizing immunity in pig-tailed monkeys, has been used to determine how soon after virus exposure neutralizing antibodies (NAbs) must be present to block a simian immunodeficiency virus (SIV)/HIV chimeric virus infection. Sterilizing protection was achieved in three of four macaques receiving neutralizing IgG 6 h after intravenous SIV/HIV chimeric virus inoculation as monitored by PCR analyses of and attempted virus isolations from plasma, peripheral blood mononuclear cell, and lymph node specimens. In the fourth animal, the production of progeny virus was suppressed for >4 weeks. A delay in transferring NAbs until 24 h after virus challenge resulted in infection in two of two monkeys. These results suggest that even if a vaccine capable of eliciting broadly reactive NAbs against primary HIV-1 were at hand, the Abs generated must remain at, or rapidly achieve, high levels within a relatively short period after exposure to virus to prevent the establishment of a primate lentivirus infection.
Previous studies have shown that passively transferred neutralizing antibodies (NAbs), when present at sufficient titers before virus challenge, can confer sterilizing immunity to macaque monkeys against simian immunodeficiency virus (SIV)/HIV chimeric virus (SHIV) infections (1–5). The principal targets of such Abs are the heavily glycosylated and genetically heterogeneous trimeric envelope spikes on the surface of virus particles. A major focus of current HIV-1 vaccine research has been a search for immunogens capable of generating broadly reacting NAbs against primary viral isolates of diverse geographic origin. An equally important but often overlooked issue pertaining to the development of an effective HIV-1 vaccine is the maintenance of sufficiently high levels of virus neutralizing activity to suppress the establishment of infection if and when virus is subsequently encountered. In this study, a critical parameter relating to this second issue, the time interval during which neutralizing Abs must appear in a hypothetical vaccine to prevent infection, has been examined by transferring potent anti-HIV-1 NAbs to macaque monkeys at various times after SHIV inoculation.
Materials and Methods
Virus and Animals. The origin and preparation of the tissue culture-derived SHIVDH12 stock has been described (6). Pig-tailed macaques (Macaca nemestrina) were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (7) and were housed in a biosafety level 2 facility; biosafety level 3 practices were followed. Phlebotomies and virus inoculations [75 tissue culture 50% infective dose (TCID50) of SHIVDH12 i.v.] were performed as described (5). EDTA-treated blood specimens and acid citrate-dextrose-A-treated samples of blood were used for the preparations of plasma and peripheral blood mononuclear cell (PBMC), respectively.
Antibodies. The purification of Ig fractions from HIV-1-infected or naïve chimpanzees has been reported (8). The purified neutralizing IgGs were administered i.v. (150 mg/kg) at 6 or 24 h after SHIVDH12 challenge.
Virus Neutralization Assay. Neutralizing activities in the plasma of passively immunized monkeys were determined in an assay that measures 100% neutralization against known amounts of virus as described (5, 9). Individual plasma samples were serially diluted (2- or 3-fold, starting at a dilution of 1:4 or 1:6) by using pre-passive immunization plasma from each of the pig-tailed macaques as diluent. A 30-μl aliquot of each plasma dilution was incubated with 30 μl of the SHIVDH12 challenge stock (1.5 × 104 TCID50/ml) at room temperature for 1 h, and the mixture was then used to infect MT-4 cells (10) in quadruplicate. MT-4 cells (5 × 104 in 0.25 ml) were then incubated with 10 μl of the virus/plasma mixture, which contained 75 TCID50 of SHIVDH12. Infected cultures were maintained for 2 weeks, and virus replication was monitored by 32P reverse transcriptase (RT) assays (11). Any infectious SHIVDH12 generated during the 2 weeks of incubation in MT-4 cells would be amplified to levels detectable by the assay. Neutralization antibody titers were calculated by the method of Reed and Muench (12).
Quantitation of Proviral DNA Copies and Plasma Viral RNA Levels. The number of proviral DNA copies in PBMCs and lymph node cells was measured by quantitative DNA PCR as described (6). Plasma viral RNA levels were determined by real-time PCR (ABI Prism 7700 sequence detection system, Perkin–Elmer), employing gag primers and probes as reported (13).
Virus Isolation from Lymph Nodes and PBMCs of Passively Immunized Macaques. Inguinal lymph node samples were collected at weeks 10 or 32 postchallenge. Suspensions of >5 × 105 lymph node cells were cocultivated with MT-4 cells in RPMI medium 1640 supplemented with 10% heat-inactivated FBS (HyClone). Virus production was monitored by RT assay during 4 weeks of culture. PBMC samples (2 × 106 cells) collected at weeks 3 and 6 were cocultivated with naïve pig-tailed monkey PBMCs and virus production was monitored by RT assay during 4 weeks of culture. The resulting virus stocks were titered in MT-4 cells before their use in neutralization assays.
Results
Passive Transfer of Neutralizing IgG to Pig-Tailed Macaques at Various Times After SHIVDH12 Challenge. We previously reported that passively transferred high-titered neutralizing IgG, purified from chimpanzee 4750, chronically infected with the primary HIV-1 isolate, HIVDH12, can confer sterilizing protection against SHIVDH12, if present at sufficient levels before virus challenge (3). In that study, the titers of plasma neutralizing antibody in different monkeys at the time of virus inoculation ranged from 1:3 to 1:123; these levels were found to be inversely related to the establishment of a subsequent infection after a SHIV challenge. The protective neutralization titer in the plasma needed to prevent infection of 99% of animals inoculated with 75 TCID50 of virus was calculated to be 1:38.
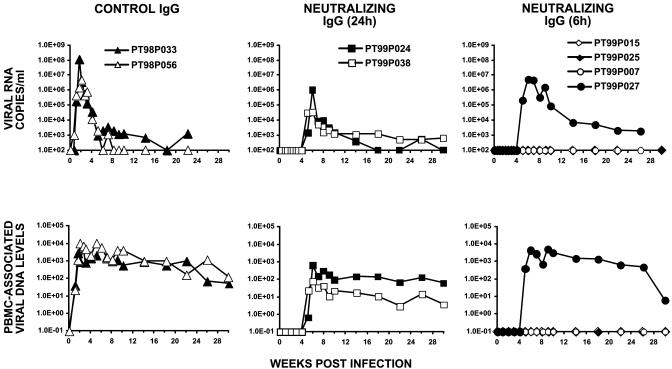
Based on our previous experience with neutralizing IgG from chimpanzee 4750, amounts (150 mg/kg) of IgG calculated to achieve plasma titers ≥1:38 within 24 h after administration were transferred to pig-tailed macaques. As control, two monkeys (PT98P033 and PT98P056) were recipients of IgG from HIV-1-uninfected chimpanzees. Both of these animals became viremic during the first week after SHIV inoculation, as monitored by RT and DNA PCR of plasma and PBMC lysates, respectively (Fig. 1 Left).
Fig. 1.
Plasma viral RNA levels and PBMC-associated viral DNA loads in pig-tailed macaques challenged with SHIVDH12 after the administration of neutralizing IgG at 6 or 24 h after virus inoculation. The number of SHIVDH12 RNA copies in the plasma or PBMC-associated viral DNA was determined over a 4- to 7-month period by quantitative RT-PCR or DNA PCR, respectively. Control animals PT98P033 and PT98P056 received IgG prepared from an HIV-1-uninfected chimpanzee.
In the first postexposure/passive transfer experiment, two monkeys (99P024 and 99P038) received neutralizing IgG 24 h after i.v. challenge (75 TCID50) of SHIVDH12. As shown in Fig. 1 Center, progeny virus production was undetectable in both animals until week 5 postinoculation, at which point plasma viremia and PBMC-associated SHIV DNA became measurable. Infectious virus was first recovered from these two monkeys by cocultivation of their PBMCs at week 6 but not at week 3 postinoculation (Table 1). Peak plasma viremia was delayed until weeks 6 to 7 in both animals and was considerably lower than that measured in control macaques PT98P033 and PT98P056. Furthermore, the mean postpeak viremia at week 22 postinfection was 35 copies of viral DNA per 105 PBMCs in the two recipients of neutralizing IgG compared with 570 copies of viral DNA per 105 PBMCs for the control monkeys, as measured by DNA PCR.
Table 1. Virus isolation from PBMCs and lymph node cells of passively immunized macaques.
| Virus isolation
|
|||
|---|---|---|---|
| PBMC
|
|||
| Animals | Week 3 | Week 6 | Lymph node |
| PT99P024 (24 h) | No | Yes | ND |
| PT99P038 (24 h) | No | Yes | ND |
| PT99P027 (6 h) | No | Yes | Yes (10 weeks PI) |
| PT99P007 (6 h) | No | No | No (32 weeks PI) |
| PT99P015 (6 h) | ND | No | No (32 weeks PI) |
| PT99P025 (6 h) | ND | No | No (10 weeks PI) |
ND, not done; PI, postinfection.
Because passive immunoprophylaxis at 24 h had failed to prevent the establishment of the SHIVDH12 infection, four additional monkeys were treated 6 h after virus challenge with the same amount of neutralizing IgG. In contrast to the first experiment, transfer of the neutralizing IgG at 6 h postinoculation conferred durable sterilizing protection in three of the four SHIV-challenged macaques (Fig. 1 Right and Table 1). The production of progeny virus was suppressed for more than 4 weeks in the fourth animal. RT and DNA PCR analyses and attempted virus isolations from lymph node samples, collected from the three protected animals at week 10 or 32 postinfection, revealed no evidence of SHIV infection. These three animals remain virus-free 50 weeks after virus challenge.
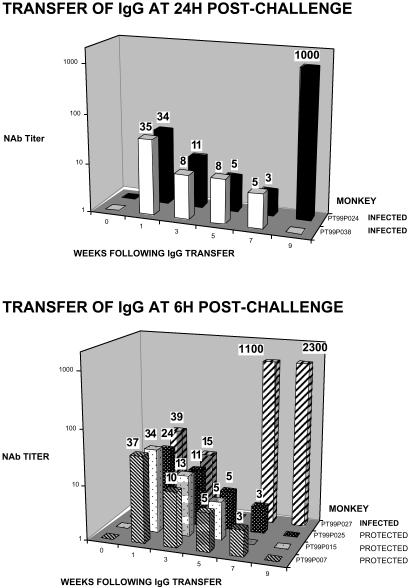
The Decay Properties of Passively Transferred Neutralizing IgG. As noted above, each monkey received an amount of IgG (150 mg/kg) calculated to achieve a plasma neutralization titer of 1:38, which would be sufficient to protect >99% of animals i.v. inoculated with 75 TCID50 of SHIVDH12 if present before virus infection (3). Despite its systemic dissemination into diverse body compartments, we previously reported that the concentrations of passively transferred IgG actually present in the plasma closely approximates that calculated on the basis of animal weight for this compartment (3). Nonetheless, anti-SHIV neutralization assays were performed on plasma samples collected at various times from the six IgG recipients to verify that the expected levels of activity were, in fact, present and to monitor the decay of the transferred IgG in vivo. As shown in Fig. 2, the titers of NAb at week 1 postchallenge were 1:24 to 1:39 in the plasma of the six macaques and, based on their decay rates, were well within the range needed to prevent the establishment of a virus infection at the time of SHIV inoculation 6 days earlier. Over the first 5 weeks, the plasma neutralization titers appeared to decay at similar rates in all six animals with a half-life of ≈2–3 weeks. During this period, the levels of NAbs in the infected versus protected monkeys were not discernibly different. The sudden appearance of high titers of plasma neutralizing activity at weeks 7 and 9 in monkeys PT99P027 and PT99P024, respectively, most likely reflects the immunogenicity of the high virus loads in animals experiencing “breakthrough” infections.
Fig. 2.
End-point titrations of plasma anti-SHIV NAbs at various times after virus challenge. Virus neutralizing activity in plasma was evaluated in quadruplicate cultures of MT-4 cells after a 1-h incubation of virus (75 TCID50) with 3-fold serial dilutions of plasma as described (5, 9). The number above each bar indicates the calculated NAb titer.
No NAb Escape Mutants Emerge After Passive Immunization in Animals Experiencing Breakthrough Infections. It was of interest to determine whether the virus that emerged in the two macaques receiving IgG 24 h postchallenge and in the single IgG recipient 6 h postchallenge had become resistant to the passively transferred neutralizing IgG. Virus was therefore isolated from animals PT99P024, PT99P027, and PT99P038 at week 6 postinoculation by cocultivation of their PBMCs with PBMCs from naïve monkeys, and stocks of each were titered in MT-4 cells and used in neutralization assays. The neutralization sensitivities of the recovered SHIVs to the passively transferred chimpanzee neutralizing IgG (starting concentration of 19.4 mg/ml) were determined by using 75 TCID50 of each virus in standard end-point dilution assays. Because this assay measures complete virus neutralization, significant amounts of resistant SHIV variants would have had to emerge to affect the measured neutralization titer. As shown in Table 2, the sensitivities of the recovered SHIVs were not appreciably different from one another or from the original SHIVDH12 used for the challenge. We therefore conclude that the virus recovered from break-through animals was not neutralization-resistant and most likely arose as a result of declining titers of NAbs in vivo.
Table 2. SHIV sensitivity to neutralizing IgG.
| Virus | Calculated neutralization titer of chimpanzee IgG against 75 TCID50 of virus* |
|---|---|
| SHIVDH12 (inoculum) | 1:210 |
| Virus isolated from PT99P027 | 1:150 |
| Virus isolated from PT99P024 | 1:110 |
| Virus isolated from PT99P038 | 1:150 |
End-point virus neutralization assays were performed by using IgG from chimpanzee 4750 at a starting concentration of 19.4 mg/ml
Discussion
Two properties of effective prophylactic antiviral vaccines are now widely accepted: (i) they confer protection against the development of subsequent disease but rarely against infection; and (ii) they elicit the production of NAbs that remain at high levels for long periods of time. For many viral pathogens, acute infections or vaccination can induce levels of NAbs that are maintained for several years (e.g., polio, vaccinia, and measles) (14). This would require long-lived memory B lymphocytes and/or plasma cells. A variety of mechanisms have been proposed to explain the durability of both cell types, including the continuous reexposure to antigen, low-grade chronic infection, booster immunizations, and trapping of antigen/Ab complexes in follicular dendritic cells (15–17). Some effective vaccines elicit NAbs that are shorter-lived. In these cases, the titers of neutralizing activity increase markedly after exposure to cognate viral antigens. For example, in mice 300 days post-VSV vaccination, neutralizing Abs become detectable within 4 days after exposure to virus or antigen (15, 18). In vaccinated humans, NAbs to hepatitis B virus appear within 1–4 weeks of infection (19). In both cases, the delay in Ab recall fails to prevent infection. Nonetheless, subsequent virus-induced disease is effectively aborted.
Knowledge about the natural history of HIV-1 infections accrued over the past two decades clearly indicates that with the exception of a few long-term nonprogressors, virtually all untreated infected individuals will eventually develop immunodeficiency (20). Thus, an effective prophylactic vaccine against HIV-1 must prevent the establishment of infection; a delayed immune response that merely modulates the primary infection will likely fail to protect an exposed individual against disease. When extrapolated to the development of such a vaccine, the results obtained in the present study indicate that the titers of neutralizing Abs elicited by an effective HIV-1 vaccine must be at protective levels within 6–24 h after exposure to virus.
It has been argued that a partially effective prophylactic HIV-1 vaccine that lowers plasma viremia in an exposed individual might reduce the rate of virus transmission and, therefore, have a substantial impact on the ongoing AIDS epidemic (21). Thus, even though the two monkeys passively immunized with the neutralizing IgG 24 h after virus inoculation eventually became infected, their lower levels of postpeak viremia compared with that of control monkeys could translate into lower rates of virus transmission in endemic regions of the world.
A growing body of information is now available pertaining to humoral responses associated with HIV-1 infections and vaccination. HIV-1-infected persons continuously produce virus-specific Abs, detectable by ELISA or immunoblotting, through-out most of their clinical course. These Abs are useful for identifying infected individuals. The levels of autologous NAbs in HIV-1-infected persons, on the other hand, tend to be low and variable and are directed against antecedent rather than contemporary intrapatient viruses (22–25). These HIV-1 NAbs are limited in breadth, failing to suppress virus recovered from other infected persons (26). In a large fraction of patients, the autologous NAbs neither increase in titer nor suppress virus recovered from other infected persons.
The maintenance of high levels of even autologous anti-HIV-1 and primate lentiviral NAbs by vaccination has been more challenging. In a large clinical trial involving individuals vaccinated with recombinant HIV-1MN or HIV-1SF-2 glycoprotein Mr120 (gp120), the binding (ELISA) and NAbs elicited after several protein immunizations rapidly declined during the 6-month periods between individual protein boosts (27). In the SIV/SHIV systems, vaccinations of macaques with oligomeric gp140 (28), recombinant MVA (29), DNA (30), or recombinant vaccinia priming plus gp120 boosting (31) elicited virus-specific Abs with half-lives of only 2–4 weeks. This is similar to the decay of virus neutralizing activity observed in the present study after passive transfer of IgG to pig-tailed macaques (Fig. 2). Further-more, in studies of anamnestic humoral responses induced in macaques previously vaccinated with SIV or SHIV immunogens, several groups have reported that NAbs do not become detectable for 2–3 weeks after virus challenge (30, 32–34). As a consequence, infections were established in virtually all immunized animals in several recently reported vaccine studies involving SHIVs (32–37). Sterilizing protection was only observed in a few vaccinated monkeys with high levels of strain-specific NAbs, directed against the challenge virus, at the time of infection (31).
The results obtained in this study are not unlike that reported for hu-PBL-SCID mice that received IgG1b12 mAb i.p. at different times after i.p. challenge with HIV-1 (38). When examined at only a single time postchallenge (3 weeks), none of the mice receiving the neutralizing mAb up to 6 h after virus challenge exhibited evidence of infection. In this regard, it is worth noting that in the SHIV/macaque system described, the three monkeys that eventually became infected after postchallenge immunoprophylaxis were also virus-negative at week 3 (Fig. 1). In the hu-PBL-SCID system, engrafted human PBMCs are the only targets for HIV-1; no spreading infection to mouse cells occurs (39). Based on the experimental design described, the postexposure IgG1b12 mAb-mediated virus neutralization reported most likely occurs within the confines of the peritoneal cavity. It is therefore somewhat difficult to extrapolate results obtained from these mice to the SHIV/macaque model, in which an i.v. administered virus inoculum becomes systemically disseminated to multiple organs/body compartments for 6 h before the administration of the neutralizing IgG. In another postexposure immunoprophylaxis experiment involving the transfer of neutralizing Abs to non-human primates, a mixture of four neutralizing mAbs was administered to newborn rhesus monkeys at both 1 h and 8 days after an oral SHIV89.6P challenge (40). SHIV infection was blocked in two of four challenged/treated animals. The inability to achieve sterilizing protection after passive transfer at 1 h postchallenge in that study suggests that insufficient types/amounts of neutralizing Abs were administered. In our study, polyclonal NAbs administered 6 h after virus challenge blocked the establishment of infection in three of four challenged macaques. The failure to protect the fourth monkey (PT99P027) suggests that the level of NAbs achieved by passive transfer was near the threshold of protection.
The administration of NAbs effectively and durably eliminated infectious virus systemically in the three animals in which passive transfer of IgG at 6 h postchallenge conferred sterilizing protection. In monkeys experiencing breakthrough infections, progeny virions did not become detectable until week 5 post-inoculation. This is in marked contrast to naïve pig-tailed macaques in which plasma viremia becomes measurable within the first week of infection (Fig. 1). The delayed appearance of breakthrough virus in three of the IgG recipients suggested that virus production was suppressed but not completely blocked in these animals during the first 4 weeks of infection. This was consistent with the presence of plasma NAb titers ranging from 1:8 to 1:15 in the six passively immunized animals at week 3 postchallenge (Fig. 2). By week 5, however, the levels of anti-SHIV NAbs had declined even further. Between weeks 4 and 5, the levels of breakthrough SHIV production very likely exceeded the neutralization threshold for containment, and virus was able to disseminate systemically. When considered together with the finding that sterilizing protection was only achieved in monkeys receiving neutralizing IgG at 6 h and not 24 h postchallenge, these results suggest that NAbs mediate the complete control of SHIV infections in vivo at the time of or during the initial cycle of virus replication.
It is also not clear why the 75 TCID50 SHIV inoculum still remained sensitive to NAb 6 h postinfection. It is possible that a few cells did become infected within this period but were eliminated by antibody-dependent cellular cytotoxicity and/or complement-dependent cytotoxicity, as has been reported for the anti-HIV-1 human neutralizing mAbs b12 and 2G12 (41, 42). Alternatively, some of the virus that is rapidly cleared from the circulation (43, 44) may be sequestered at sites where it remains susceptible to NAbs [e.g., in association with dendritic cells (DCs) or endothelial cells] (45–48). At later times (>6 h postinoculation), the transfer of captured virions from DCs to CD4+ T cells in lymphoid tissues very likely initiates productive infection, thereby rendering the particles refractory to passively transferred NAbs.
In the experiments described in the current study, the sterilizing protection that was achieved at 6 h but not 24 h postin-oculation occurred in the absence of any prechallenge cell-mediated immunity. One could argue, therefore, that the presence of antiviral cellular immunity in a hypothetical vaccine might extend the 6- to 24-h time frame for preventing the establishment of infection by NAbs. However, several recent reports indicate that vaccine-primed anamnestic cellular/cytotoxic T lymphocyte responses against SIV or SHIV challenges are rarely demonstrable within the first week of infection (32, 37, 49–51). A delay of this magnitude makes it highly unlikely that secondary cellular immune responses, in combination with NAbs induced by prior vaccination, would significantly prolong the 6-h period during which NAbs alone confer sterilizing protection. Moreover, a recent report shows that when DNA vaccination is combined with the passive transfer of neutralizing mAbs, the cellular immunity induced did not increase the number of animals experiencing sterilizing protection (52).
It is also worth mentioning that postexposure immunoprophylaxis using broadly acting neutralizing mAbs might be useful as adjunct therapy to prevent the maternal–child transmission of HIV-1. Antiretroviral drugs must be given daily to breast fed infants for optimal protective effects. From the results shown, it is possible that the administration of neutralizing mAbs at the time of birth and monthly during the neonatal period, in combination with antiretroviral therapies, might provide sterilizing protection.
A major goal of current HIV-1 vaccine research has been the development of immunogens capable of inducing broadly reactive NAbs. More recently, this effort has focused on highly conserved epitopes associated with oligomeric forms of HIV-1 envelope glycoproteins (53–56). Even if an immunogen capable of inducing broadly reactive NAbs were at hand, available and safe vehicles for its delivery would probably not elicit a rapid and durable humoral response. In the simplest systems, activation of memory B cells and secretion of IgG after immunization with inert antigens takes at least 18–72 h (57–59). It is therefore highly unlikely that vaccine approaches currently in use would generate protective levels of NAbs within 6–24 h of exposure to HIV-1. An effective anti-HIV-1 vaccine may require the development of safe replicating or inducible vectors capable of expressing immunogens that will elicit high and sustained levels of broadly reactive NAbs.
Acknowledgments
We thank Lowrey Rhodes, Brent Morse, Sekou Savane, Frances Banks, and Wes Thornton for diligence and assistance in the care and maintenance of our animals, Ron Willey for critical comments during the preparation of this paper, and William F. Sutton for expert technical assistance in IgG preparation. This work was supported by Public Health Service Grant AI27577 (to N.L.H.) and the University of Washington Center for AIDS Research.
Abbreviations: NAb, neutralizing antibody; PBMC, peripheral blood mononuclear cell; RT, reverse transcriptase; SIV, simian immunodeficiency virus; SHIV, SIV/HIV chimeric virus; TCID50, tissue culture 50% infective dose.
References
- 1.Mascola, J. R., Lewis, M. G., Stiegler, G., Harris, D., VanCott, T. C., Hayes, D., Louder, M. K., Brown, C. R., Sapan, C. V., Frankel, S. S., et al. (1999) J. Virol. 73, 4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascola, J. R., Stiegler, G., VanCott, T. C., Katinger, H., Carpenter, C. B., Hanson, C. E., Beary, H., Hayes, D., Frankel, S. S., Birx, D. L. & Lewis, M. G. (2000) Nat. Med. 6, 207-210. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura, Y., Igarashi, T., Haigwood, N., Sadjadpour, R., Plishka, R. J., Buckler-White, A., Shibata, R. & Martin, M. A. (2002) J. Virol. 76, 2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parren, P. W. H. I., Marx, P. A., Hessell, A. J., Luckay, A., Harouse, J., Cheng-Mayer, C., Moore, J. P. & Burton, D. R. (2001) J. Virol. 75, 8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata, R., Igarashi, T., Haigwood, N., Buckler-White, A., Ogert, R., Ross, W., Willey, R., Cho, M. W. & Martin, M. A. (1999) Nat. Med. 5, 204-210. [DOI] [PubMed] [Google Scholar]
- 6.Shibata, R., Maldarelli, F., Siemon, C., Matano, T., Parta, M., Miller, G., Fredrickson, T. & Martin, M. A. (1997) J. Infect. Dis. 176, 362-373. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Care and Use of Laboratory Animals (1985) Guide for the Care and Use of Laboratory Animals (Natl. Inst. of Health, Bethesda), DHHS Publ. No. (NIH) 85-23, Revised Ed.
- 8.Haigwood, N. L., Watson, A., Sutton, W. F., McClure, J., Lewis, A., Ranchalis, J., Travis, B., Voss, G., Letvin, N. L., Hu, S.-L., Hirsch, V. M. & Johnson, P. R. (1996) Immunol. Lett. 51, 107-114. [DOI] [PubMed] [Google Scholar]
- 9.Willey, R. L., Shibata, R., Freed, E. O., Cho, M. W. & Martin, M. A. (1996) J. Virol. 70, 6431-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada, S., Koyanagi, Y. & Yamamoto, N. (1985) Science 229, 563-566. [DOI] [PubMed] [Google Scholar]
- 11.Willey, R. L., Smith, D. H., Lasky, L. A., Theodore, T. S., Earl, P. L., Moss, B., Capon, D. J. & Martin, M. A. (1988) J. Virol. 62, 139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed, L. J. & Muench, H. (1938) Am. J. Hyg. 27, 493-497. [Google Scholar]
- 13.Endo, Y., Igarashi, T., Nishimura, Y., Buckler, C., Buckler-White, A., Plishka, R., Dimitrov, D. S. & Martin, M. A. (2000) J. Virol. 74, 6935-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zinkernagel, R. M. (2003) Annu. Rev. Immunol. 21, 515-546. [DOI] [PubMed] [Google Scholar]
- 15.Ochsenbein, A. F., Pinschewer, D. D., Sierro, S., Horvath, E., Hengartner, H. & Zinkernagel, R. M. (2000) Proc. Natl. Acad. Sci. USA 97, 13263-13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. (1998) Immunity 8, 363-372. [DOI] [PubMed] [Google Scholar]
- 17.Zinkernagel, R. M. (2002) Curr. Opin. Immunol. 14, 523-536. [DOI] [PubMed] [Google Scholar]
- 18.Karrer, U., López-Macías, C., Oxenius, A., Odermatt, B., Bachmann, M. F., Kalinke, U., Bluethmann, H., Hengartner, H. & Zinkernagel, R. M. (2000) J. Immunol. 164, 768-778. [DOI] [PubMed] [Google Scholar]
- 19.West, D. J. & Calandra, G. B. (1996) Vaccine 14, 1019-1027. [DOI] [PubMed] [Google Scholar]
- 20.Pantaleo, G. & Fauci, A. S. (1995) Annu. Rev. Immunol. 13, 487-512. [DOI] [PubMed] [Google Scholar]
- 21.Anderson, R. M. & Garnett, G. P. (1996) Lancet 348, 1010-1013. [DOI] [PubMed] [Google Scholar]
- 22.Kostrikis, L. G., Cao, Y., Ngai, H., Moore, J. P. & Ho, D. D. (1996) J. Virol. 70, 445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moog, C., Fleury, H. J. A., Pellegrin, I., Kirn, A. & Aubertin, A. M. (1997) J. Virol. 71, 3734-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilgrim, A. K., Pantaleo, G., Cohen, O. J., Fink, L. M., Zhou, J. Y., Zhou, J. T., Bolognesi, D. P., Fauci, A. S. & Montefiori, D. C. (1997) J. Infect. Dis. 176, 924-932. [DOI] [PubMed] [Google Scholar]
- 25.Richman, D. D., Wrin, T., Little, S. J. & Petropoulos, C. J. (2003) Proc. Natl. Acad. Sci. USA 100, 4144-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews, T. J., Langlois, A. J., Robey, W. G., Chang, N. T., Gallo, R. C., Fischinger, P. J. & Bolognesi, D. P. (1986) Proc. Natl. Acad. Sci. USA 83, 9709-9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McElrath, M. J., Corey, L., Montefiori, D., Wolff, M., Schwartz, D., Keefer, M., Belshe, R., Graham, B. S., Matthews, T., Wright, P., et al. (2000) AIDS Res. Hum. Retroviruses 16, 907-919. [DOI] [PubMed] [Google Scholar]
- 28.Earl, P. L., Sugiura, W., Montefiori, D. C., Broder, C. C., Lee, S. A., Wild, C., Lifson, J. & Moss, B. (2001) J. Virol. 75, 645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ourmanov, I., Brown, C. R., Moss, B., Carroll, M., Wyatt, L., Pletneva, L., Goldstein, S., Venzon, D. & Hirsch, V. M. (2000) J. Virol. 74, 2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doria-Rose, N. A., Ohlen, C., Polacino, P., Pierce, C. C., Hensel, M. T., Kuller, L., Mulvania, T., Anderson, D., Greenberg, P. D., Hu, S. L. & Haigwood, N. L. (2003) J. Virol. 77, 11563-11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho, M. W., Kim, Y. B., Lee, M. K., Gupta, K. C., Ross, W., Plishka, R., Buckler-White, A., Igarashi, T., Theodore, T., Byrum, R., et al. (2001) J. Virol. 75, 2224-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amara, R. R., Villinger, F., Altman, J. D., Lydy, S. L., O'Neil, S. P., Staprans, S. I., Montefiori, D. C., Xu, Y., Herndon, J. G., Wyatt, L. S., et al. (2001) Science 292, 69-74. [DOI] [PubMed] [Google Scholar]
- 33.Barouch, D. H., Santra, S., Schmitz, J. E., Kuroda, M. J., Fu, T.-M., Wagner, W., Bilska, M., Craiu, A., Zheng, X. X., Krivulka, G. R., et al. (2000) Science 290, 486-492. [DOI] [PubMed] [Google Scholar]
- 34.Willey, R. L., Byrum, R., Piatak, M., Kim, Y. B., Cho, M. W., Rossio, J. L., Jr., Bess, J., Jr., Igarashi, T., Endo, Y., Arthur, L. O., et al. (2003) J. Virol. 77, 1163-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barouch, D. H., Kunstman, J., Kuroda, M. J., Schmitz, J. E., Santra, S., Peyerl, F. W., Krivulka, G. R., Beaudry, K., Lifton, M. A., Gorgone, D. A., et al. (2002) Nature 415, 335-339. [DOI] [PubMed] [Google Scholar]
- 36.Rose, N. F., Marx, P. A., Luckay, A., Nixon, D. F., Moretto, W. J., Donahoe, S. M., Montefiori, D., Roberts, A., Buonocore, L. & Rose, J. K. (2001) Cell 106, 539-549. [DOI] [PubMed] [Google Scholar]
- 37.Shiver, J. W., Fu, T.-M., Chen, L., Casimiro, D. R., Davies, M.-E., Evans, R. K., Zhang, Z.-Q., Simon, A. J., Trigona, W. L., Dubey, S. A., et al. (2002) Nature 415, 331-335. [DOI] [PubMed] [Google Scholar]
- 38.Gauduin, M. C., Parren, P. W., Weir, R., Barbas, C. F., Burton, D. R. & Koup, R. A. (1997) Nat. Med. 3, 1389-1393. [DOI] [PubMed] [Google Scholar]
- 39.Mosier, D. E., Gulizia, R. J., Baird, S. M., Wilson, D. B., Spector, D. H. & Spector, S. A. (1991) Science 251, 791-794. [DOI] [PubMed] [Google Scholar]
- 40.Ferrantelli, F., Hofmann-Lehmann, R., Rasmussen, R. A., Wang, T., Xu, W., Li, P.-L., Montefiori, D. C., Cavacini, L. A., Katinger, H., Stiegler, G., et al. (2003) AIDS 17, 301-309. [DOI] [PubMed] [Google Scholar]
- 41.Hezareh, M., Hessell, A. J., Jensen, R. C., van de Winkel, J. G. J. & Parren, P. W. H. I. (2001) J. Virol. 75, 12161-12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trkola, A., Purtscher, M., Muster, T., Ballaun, C., Buchacher, A., Sullivan, N., Srinivasan, K., Sodroski, J., Moore, J. P. & Katinger, H. (1996) J. Virol. 70, 1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Igarashi, T., Brown, C., Azadegan, A., Haigwood, N., Dimitrov, D., Martin, M. A. & Shibata, R. (1999) Nat. Med. 5, 211-216. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, L., Dailey, P. J., He, T., Gettie, A., Bonhoeffer, S., Perelson, A. S. & Ho, D. D. (1999) J. Virol. 73, 855-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frankel, S. S., Steinman, R. M., Michael, N. L., Kim, S. R., Bhardwaj, N., Pope, M., Louder, M. K., Ehrenberg, P. K., Parren, P. W. H. I., Burton, D. R., et al. (1998) J. Virol. 72, 9788-9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geijtenbeek, T. B. H., Kwon, D. S., Torensma, R., van Vliet, S. J., van Duijnhoven, G. C. F., Middel, J., Cornelissen, I. L. M. H. A., Nottet, H. S. L. M., KewalRamani, V. N., Littman, D. R., et al. (2000) Cell 100, 587-597. [DOI] [PubMed] [Google Scholar]
- 47.Pohlmann, S., Soilleux, E. J., Baribaud, F., Leslie, G. J., Morris, L. S., Trowsdale, J., Lee, B., Coleman, N. & Doms, R. W. (2001) Proc. Natl. Acad. Sci. USA 98, 2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissman, D., Li, Y., Orenstein, J. M. & Fauci, A. S. (1995) J. Immunol. 155, 4111-4117. [PubMed] [Google Scholar]
- 49.Barouch, D. H., Santra, S., Kuroda, M. J., Schmitz, J. E., Plishka, R., Buckler-White, A., Gaitan, A. E., Zin, R., Nam, J.-H., Wyatt, L. S., et al. (2001) J. Virol. 75, 5151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santra, S., Barouch, D. H., Kuroda, M. J., Schmitz, J. E., Krivulka, G. R., Beaudry, K., Lord, C. I., Lifton, M. A., Wyatt, L. S., Moss, B., et al. (2002) J. Virol. 76, 6376-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seth, A., Ourmanov, I., Schmitz, J. E., Kuroda, M. J., Lifton, M. A., Nickerson, C. E., Wyatt, L., Carroll, M., Moss, B., Venzon, D., et al. (2000) J. Virol. 74, 2502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mascola, J. R., Lewis, M. G., VanCott, T. C., Stiegler, G., Katinger, H., Seaman, M., Beaudry, K., Barouch, D. H., Korioth-Schmitz, B., Krivulka, G., et al. (2003) J. Virol. 77, 10348-10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Binley, J. M., Sanders, R. W., Clas, B., Schuelke, N., Master, A., Guo, Y., Kajumo, F., Anselma, D. J., Maddon, P. J., Olson, W. C. & Moore, J. P. (2000) J. Virol. 74, 627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Earl, P. L., Broder, C. C., Long, D., Lee, S. A., Peterson, J., Chakrabarti, S., Doms, R. W. & Moss, B. (1994) J. Virol. 68, 3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farzan, M., Choe, H., Desjardins, E., Sun, Y., Kuhn, J., Cao, J., Archambault, D., Kolchinsky, P., Koch, M., Wyatt, R. & Sodroski, J. (1998) J. Virol. 72, 7620-7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang, X., Florin, L., Farzan, M., Kolchinsky, P., Kwong, P. D., Sodroski, J. & Wyatt, R. (2000) J. Virol. 74, 4746-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bar-Or, A., Oliveira, E. M., Anderson, D. E., Krieger, J. I., Duddy, M., O'Connor, K. C. & Hafler, D. A. (2001) J. Immunol. 167, 5669-5677. [DOI] [PubMed] [Google Scholar]
- 58.Tangye, S. G., Avery, D. T., Deenick, E. K. & Hodgkin, P. D. (2003) J. Immunol. 170, 686-694. [DOI] [PubMed] [Google Scholar]
- 59.Tangye, S. G., Avery, D. T. & Hodgkin, P. D. (2003) J. Immunol. 170, 261-269. [DOI] [PubMed] [Google Scholar]