Abstract
In female rodents, sleep and activity levels fluctuate over the estrous cycle. When estradiol (E2) levels are highest, sleep is reduced whereas locomotion is increased. The preoptic area (POA) is a key site for estrogenic regulation of these functions. However, molecular mechanisms by which E2 acts to reduce sleep and increase activity are unclear. Recently, we demonstrated a twofold reduction in lipocalin-type prostaglandin D synthase (L-PGDS) transcript levels, after E2 treatment, in the ventrolateral POA (VLPO), a putative sleep-active nucleus. Catalytic activity of L-PGDS produces PGD2, an endogenous somnogen. Thus, we hypothesized that decreases in PGD2 in the VLPO may contribute to the generalized arousal mediated by estrogens. To test this, we infused (i) antisense oligonucleotides (oligos), containing locked nucleic acid moieties (an improved technology), targeted to L-PGDS mRNA, (ii) scrambled sequence control oligos, or (iii) saline vehicle into the VLPO of ovariectomized female mice treated with E2 or oil. Arousal states and activity levels were assessed in response to a series of sensory stimuli (vestibular, olfactory, and somatosensory). The vestibular stimulus, which was administered first, resulted in the strongest responses and elicited significantly different responses among the groups: all groups in the E2 cohort demonstrated increases in overall home cage activity and duration of that activity compared with the oil-treated control groups. As predicted from E2 suppression of L-PGDS transcript levels, the responses of the locked nucleic acid antisense oligo-treated animals from the oil cohort did not differ from the E2-treated groups, such that they also demonstrated increases in activity and duration of activity compared with their controls. Thus, reducing L-PGDS in the VLPO of oil-treated females mimicked the effect of E2 on activity and arousal and represents a unique molecular pathway through which E2 may modulate these functions.
Keywords: antisense, genomics, sleep, locomotion, neuroendocrine
Activity levels and sleep homeostasis fluctuate over the estrous cycle in female rodents. Sleep is typically reduced and motor activity is increased during the proestrous dark period when estradiol (E2) levels are highest (1, 2). It is well established that estrogens are responsible for increases in activity in rats (3–7) and, more recently, Morgan et al. (8) have extended this finding to mice. E2-treated females demonstrated increased running wheel activity as well as general home cage activity that included rearing, grooming, and burying. These data indicate that E2 has an arousing effect allowing for increased locomotor behaviors, a component of estrogen-facilitated courtship responses. Cell groups in the preoptic area (POA) have been implicated in the regulation of sleep (reviewed in ref. 9) and the estrogenic mediation of locomotion (10). What molecular mechanisms may underlie E2 effects on activity and sleep?
Our high-density oligonucleotide (oglio) microarray screens have been useful in assessing changes in gene transcripts in neuroendocrine systems and in the discovery of unanticipated genomic alterations (11). One such finding is the estrogenic regulation of the lipocalin-type prostaglandin D synthase (L-PGDS) gene (12). L-PGDS is a CNS specific, nonneuronal enzyme that catalyzes the conversion of prostaglandin H2 to prostaglandin D2 (PGD2), the major prostanoid produced in the CNS of rodents and humans. As a neuromodulator, PGD2 modulates a variety of CNS actions such as pain (13, 14), odor responses (15), and body temperature regulation (16); however, it is best known as a potent endogenous somnogen acting at the level of the POA (reviewed in ref. 17). We recently demonstrated a highly specific regulation within the POA; the reduction of L-PGDS expression in the presence of E2 was limited to the ventrolateral POA (VLPO) cell group and caudal periventricular region (12). This is significant because the VLPO has been identified as a putative sleep center in the POA (18). Moreover, the VLPO receives inputs from key arousal and locomotor centers (19). In light of the fact that in the VLPO, E2 suppresses the transcript levels of L-PGDS, we hypothesize that a decrease in PGD2 may contribute to the estrogenic effects on general arousal and locomotion. To test this possibility, we infused locked nucleic acid antisense (LNA-AS) oligos (20) complementary to L-PGDS, or LNA-scrambled oligo sequences, or saline vehicle into the VLPO of adult mice either in the presence or absence of estradiol. We found that the reduction of L-PGDS protein by LNA-AS oligos in the absence of estrogens resulted in an increased arousal state that did not differ from that of the E2-treated groups.
Materials and Methods
Animals and Treatment. Swiss–Webster mice ovariectomized by the supplier (Taconic Farms) were 9- to 12-wk-old at time of arrival. They were maintained on a normal 12:12 h light/dark cycle (lights on at 9 a.m.), and food and water were available ad libitum. All acquisition, handling, and other animal procedures were within the National Institutes of Health guidelines. Five days after arrival, animals were surgically implanted with a treatment capsule (silastic tubing dimension 1.98-mm inner diameter × 3.18 mm outer diameter × 2 cm in length; Dow-Corning) containing either 17-β-estradiol (E2, 25 μg in 0.07 ml of sesame oil; n = 24) or 0.07 ml of sesame oil vehicle (n = 24). This dose was used based on previous work from our laboratory that demonstrated that the 25-μg dose of E2 (entering s.c. over 5 wk) resulted in E2 levels equivalent to those of proestrus. Moreover, this dose was most effective in eliciting changes in locomotor and home cage activity (8, 21). Animals were anesthetized with inhaled isofluorane, and surgery was performed to s.c. place the capsules just below the nape of the neck.
Seven days after capsule implantation, animals were evenly divided into three groups and given, in the VLPO, a microinjection of LNA-AS oligos, LNA-scrambled oligo sequences (containing the same base composition of the LNA-AS oligos), or saline vehicle (0.9% sterile solution) on 2 consecutive days. The VLPO is a nucleus located near the ventral surface of the brain at the level of the bregma and 0.75-mm lateral from the midline (22). In brief, animals were anesthetized with Nembutal (0.8 mg/10 g) and placed in a stereotaxic apparatus fitted with a 5-μl Hamilton 22-gauge syringe. Injections were made bilaterally at a site ≈0.75-mm lateral to the bregma. From the dura surface, the needle was slowly lowered 5.2 mm into the brain. After the microinjections, the animals were individually housed, allowed 1 day for recovery, and then tested for general arousal followed by female sex behavior. After the end of the behavioral testing, animals were killed, and the brains removed and cut on a freezing cyrostat into 15-μm-thick sections. The sections were Nissl-stained and analyzed for the accuracy of the microinjections. An injection was considered successful if at least one of the needle marks was within 30 μm of the VLPO. The behavioral records for the animals with failed injections were removed from the study.
LNA Oligos. AS oligos are powerful tools for manipulating gene expression and, thus, elucidating the biological functions of the target gene. However, a classical moiety of AS oligos, phosphorothioates, despite their increased resistance to nucleases, tend to have low binding capacity to complementary nucleic acids and high nonspecific interactions with proteins resulting in toxic side effects (23, 24). Recently, a new generation nucleic acid analog, LNA (25) has been shown to have (i) high-affinity for the complementary nucleic acids, (ii) a high degree of resistance to nuclease activity, and (iii) the capacity to activate RNase H, an important AS mechanism (20, 26). LNA are nucleic acid analogs containing a methylene bridge between the 2′-oxygen and the 4′-carbon of the of the ribofuranose ring. This bridge restricts the flexibility of the monomer and locks the structure into a rigid bicyclic N-type conformation that confers exceptional biostability and low to zero cellular toxicity (for review see refs. 20 and 27).
We used AS gene inhibition of L-PGDS by LNA-DNA chimeras complementary to the 5′-untranslated region and the region surrounding the start codon of mRNA. The LNA-oligo (LNA/DNA/LNA) gapmers were obtained from Proligo (Boulder, CO). The sequences of the LNA-AS oligo to L-PGDS were 5′-AAGagcagccatTTG-3′ and 5′-AACcatccacagCAT-3′ (LNA monomers represented by uppercase letters and DNA monomers by lowercase letters). The scrambled sequences for the control ONs were 5′-GACagcgttcatAGA-3′ and 5′-CGAcatccaaccCAT-3′. Underlining indicates the AS sequence to the start codon that was also incorporated into the scrambled control oligo. The two AS LNA/DNA gapmers were combined in equimolar concentrations to equal 1 μg/μl of DNA in a saline vehicle. The same procedure was performed for the scrambled sequences. Infusions were made bilaterally, in a volume of 1 μl over a 120-s period by using a microsyringe pump and controller (World Precision Instruments, Sarasota, FL). The injection needle was left in situ for another 90 s. Control animals received the same volume and concentration of LNA-scrambled oligos or saline vehicle alone.
Western Blotting. The efficiency of the L-PGDS AS oligo was investigated by Western blot analysis. Ovariectomized Swiss–Webster mice received stereotaxic injections into the VLPO of either L-PGDS LNA-AS oligo (n = 5), scrambled oligo sequence (n = 6), or saline vehicle (n = 3) treatment as described and were killed 96 h after the second injection. The brains were quickly removed and placed on an ice-cold platform. A coronal slab containing the VLPO was cut from the each brain. The bilateral VLPO nuclei were dissected, snap frozen in tubes chilled on dry ice, and stored at -70°C until being homogenized. Protein (2 μg) from the three treatments was loaded into separate lanes in a 10% precast SDS/polyacrylamide gel (Invitrogen). Electrophoresis and blotting was performed as described (28). A goat polycolonal Ab against L-PGDS (dilution 1:100; Santa Cruz Biotechnology) was used for detection of the protein. Abs to the housekeeping gene, GAPDH (Chemicon), were used as a normalizing control. The phototope chemiluminesence system (New England Biolabs) was used for detection of the protein recognized by the antisera. The blots were exposed to Hyperfilm-ECL (Amersham Biosciences) for varying exposure times. The autoradiographic films were scanned and analyzed by using the gel-imaging macro program associated with National Institutes of Health IMAGE software running on a G3 Power Macintosh computer. The density of the immunoreactive band for L-PGDS was normalized to the density of the corresponding band for GAPDH and the data expressed as a ratio of L-PGDS optical density to GAPDH optical density. All measurements were in the linear range of the system.
Generalized Arousal Assay. The arousal state of each animal was measured by monitoring its activity immediately after the administration of sensory stimuli. Basal activity was kept low, and interfering responses that would cause large variability were virtually eliminated by assaying individually housed animals in their home cages, during the light phase of the cycle when typically they sleep. Moreover, assaying animals in their home cage allowed a precise measurement of activity in the presumed absence of fear. After each stimulus, (i) the duration of activity, (ii) horizontal activity (HACTV; included eating and grooming), and (iii) total distanced traveled in the home cage were monitored automatically with infrared beams and stored by using Digiscan analyzer and DIGISCAN software (RXYZCM, AccuScan Instruments, Columbus, OH). All arousal tests were conducted under incidental white light, starting ≈2 h after lights went on. In succession, a vestibular, then an olfactory, and finally a tactile stimulus were administered to the animal only when it was motionless and asleep. “Sleep” was denoted when all of the following criteria were met: (i) no registered movement for at least 3 min, (ii) the animal exhibited a stereotypical couched posture with its head tucked into its ventral side, and (iii) the animal's eyes were closed. After each stimulus, the activity was measured until the animal returned to the sleep state described above. The vestibular stimulus consisted of a vertical movement of the animal's home cage and the cage's placement into the apparatus. The olfactory stimulus was a 5-s introduction of a cotton swab soaked in pure almond extract held over the animal's home cage. The placement of this stimulus was consistent throughout the experiment. The tactile stimulus was a small puff of air administered from a compressed air can and directed over the back of the sleeping animal. The animals received 2 consecutive days of testing, and the treatment group was unknown to the experimenter administering the stimuli. For each individual animal, data from the consecutive days were averaged and the results from the duration of response, total HACTV, and total distance, were used to assess the animal's sensory arousal state.
Open Field Behavior Test. During the dark phase of the first day of arousal testing, mice were tested for 10 min in an open field apparatus (42 × 42 × 30 cm, AccuScan Instruments). At the start of the test, each mouse was gently placed in the lower-left corner with its head facing the wall. Animals were permitted to ambulate freely, and activity was monitored with infrared beams. Data were analyzed and stored by using a Digiscan analyzer and DIGISCAN software.
Female Sex Behavior. During the dark phase after the second day of arousal testing, female sex behavior was analyzed. To prevent the animals for achieving a maximum lordosis quotient and possibly masking the effects of changes in PGD2 on lordosis, we did not treat the animals with progesterone. Females were tested with a sexually active male in the male's home cage. The test lasted for 15 min or until the female had received 10 mounts. Lordosis responses of the female to mounts by the stud male were recorded.
Statistical Analysis. In the behavioral assays (arousal, open field, and female sex behavior), comparisons across the six treatment groups were analyzed by a two-way ANOVA with hormonal status and AS treatment as factors. The comparison of the optical density of immunoreactive bands from Western blots of L-PGDS protein expression was analyzed by a one-way ANOVA. All ANOVAs were followed by Newman–Keuls post hoc test to determine significance between groups. All statistical tests were conducted by using GB-STAT (Dynamic Microsystems, Silver Spring, MD) on a Macintosh computer.
Results
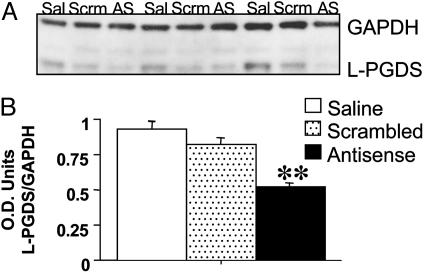
Western Blot Analysis of L-PGDS Protein Levels After AS Administration. Recent BLAST searches of the GenBank database indicated no significant homology of the L-PGDS LNA-AS oligo to any known mRNA sequences from the mouse. Moreover, the LNA-scrambled oligo control sequence was not homologous with any known mRNA sequences from the mouse. In the current study, intraparenchymal administration of LNA-AS oligos against L-PGDS mRNA into the VLPO, markedly reduced the in vivo level of the targeted proteins compared to control tissue as determined by Western blot analysis of protein homogenates (Fig. 1 A and B; ANOVA; P < 0.006). The L-PGDS Ab recognized a band at ≈33 kDa. To control for amount of total protein present, the optical density of the L-PGDS immunoreactive band was standardized to the GAPDH-immunoreactive band (51 kDa) from the same sample. Compared with the saline vehicle (n = 5) and LNA-scrambled oligo control (n = 6) treated animals, the optical density ratios demonstrated a significant decrease of ≈44% and 36% in the amount of L-PGDS protein in the lysates from AS-treated animals (n = 6), respectively.
Fig. 1.
AS LNA-oligos, specific for L-PGDS mRNA reduce the target protein in the VLPO. (A) Representative electrophoretic lanes from an immunoblot of VLPO tissue from ovariectomized adult female Swiss–Webster mice treated with saline vehicle (Sal), LNA-scrambled (Scrm), or AS LNA to L-PGDS (AS). A total of 2 μg of total protein was loaded onto each lane. The blot was probed with a rabbit polyclonal Ab to L-PGDS, which recognized the appropriatesized bands and with a mouse monoclonal to GAPDH. (B) Quantification of L-PGDS immunoreactive bands after treatment with saline vehicle, scrambled LNA, or LNA-AS. Data are represented as a ratio of the optical density (O.D.) of L-PGDS-immunoreactive band to the GAPDH-immunoreactive band. The mean optical density of L-PGDS protein-immunoreactive bands was reduced in the LNA-AS-treated animals compared with the saline vehicle and scrambled LNA control-treated animals. (**, P < 0.01.)
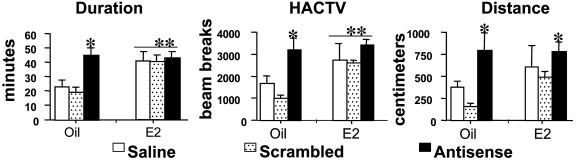
Behavioral Analysis of Generalized Arousal After Administration of Sensory Stimuli. For the behavioral analysis, of 48 animals microinjected, 35 animals received successful injections where the needle tracks were either in the VLPO or within 30 μm of the nucleus. The sample size for the groups ranged from n = 5 to 7 (vehicle-treated: saline n = 6, scrambled sequence n = 5, and AS n = 7; E2-treated: saline n = 5, scrambled sequence n = 5, and AS n = 7). Moreover, the areas surrounding the injection site appeared intact and with little structural damage (Fig. 2 A–C). Vestibular. In all treatment groups, the first (vestibular) stimulus, elicited the strongest overall response, as measured by the aforementioned parameters, compared to the measured responses elicited from the second (olfactory) and third (somatosensory) stimuli. The vestibular stimulus resulted in significantly prolonged activity for all E2-treated mice, regardless of their AS treatment [ANOVA; F(1,34) = 9.68, P < 0.005; Fig. 3]; and for the oil-treated AS group [ANOVA; F(2,34) = 4.60, P < 0.02; Fig. 3] compared with the oil-treated control groups (saline and scrambled). The increase in activity duration was more than twice as long as the oil-treated control groups, and it did not differ between the oil-treated AS group and E2-treated mice. Moreover, E2-treated mice, regardless of their AS treatment, [ANOVA; F(1,34) = 9.68, P < 0.005; Fig. 3] and the oil-treated AS group [ANOVA; F(2,34) = 4.60, P < 0.02; Fig. 3] exhibit more general activity (as measured by HACTV) compared with the oil-treated control groups (saline and scrambled). Finally, LNA-AS oligo administration to both oil- and E2-treated animals resulted in an increase in total distance traveled compared with the oil-treated saline and LNA-scrambled oligo controls [ANOVA; F(2,34) = 5.16, P < 0.01; Fig. 3].
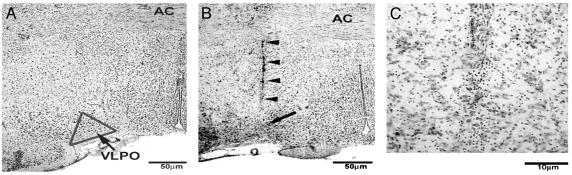
Fig. 2.
Histology of the injection site. Representative photomicrographs of Nissl-stained tissue from an untreated brain (A) and a brain microinjected with AS LNA-oligos to L-PGDS (B and C). (A) The VLPO is a triangle-shaped cluster of neurons along the base of the brain, just lateral to the optic chiasm. (B) A representative needle track from a successful microinjection. The arrowheads represent the tract, and the arrow demarcates the injection site. (Scale bars represent 50 μm.) (C) A high-power view of the injection site shown in B. Typically, the microinjection did little if any damage to the VLPO and surrounding structures. (Scale bar represents 10 μm.)
Fig. 3.
Analysis of generalized arousal. The vestibular stimulus elicited the strongest overall response and resulted in a significantly increased duration of general activity for both E2-treated mice (ANOVA; **, P < 0.01) and the oil-treated AS group (ANOVA; *, P < 0.05) compared with the oil-treated control groups (saline and scrambled). Also, E2-treated mice (**, P < 0.01) and the oil-treated AS group (*, P < 0.05) exhibited more general activity (as measured by HACTV) compared with the oil-treated control groups (saline and scrambled). Finally, AS administration to both oil- and E2-treated animals resulted in an increase in total distance traveled compared with the oil-treated saline and scrambled oligo controls. Neither the olfactory nor somatosensory stimulus elicited significantly different behaviors among the treatment groups.
Olfactory and tactile. Neither the olfactory or somatosensory stimulus elicited significantly different behaviors among the treatment groups (data not shown).
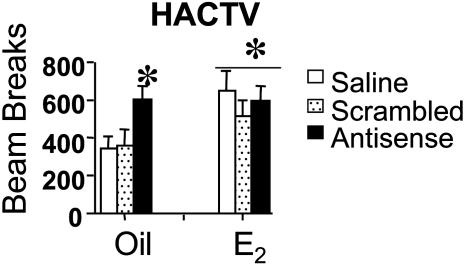
Analysis of Activity Levels After the Vestibular Stimulus. It is unclear whether the differences in home cage activity elicited by the vestibular stimulus were due to increased activity levels or due to the fact that the E2-treated and AS-infused oil-treated groups stayed awake longer. To address this question, we analyzed the activity levels within the first 5 min after stimulus administration (Fig. 4). In the first 5 min after the vestibular stimulus, E2-treated animals (regardless of AS treatment) and the oil-treated AS group [ANOVA; F(2,34) = 5.80, P < 0.02; Fig. 4] exhibited more general activity movements (as measured by HACTV) compared with the oil-treated control groups (saline and scrambled). There were no significant differences in the total distanced traveled [ANOVA; F(2,34) = 0.78, P = 0.47; data not shown].
Fig. 4.
Activity levels after the vestibular stimulus. The activity levels during the first 5 min after the vestibular stimulus administration were analyzed. During this time, E2-treated animals, regardless of AS treatment and the oil-treated AS group (ANOVA; *, P < 0.05) exhibited more general activity movements (as measured by HACTV) compared with the oil-treated control groups (saline and scrambled). There were no significant differences in the total distanced traveled (P = 0.47).
Activity Levels During Dark Phase of the Daily Cycle (Open-Field Test). Regardless of hormonal status or oligo treatment, no differences were detected in general locomotor activity (horizontal movements and total distance traveled) among the groups when subjects were tested in an open-field apparatus during their dark-phase [HACTV: ANOVA, F(2,34) = 0.14, P = 0.87; distance: ANOVA F(2,34) = 0.31, P = 0.73]. Moreover, there were no significant differences in the amount of time the animal spent immobile [ANOVA, F(2,34) = 0.79, P = 0.46].
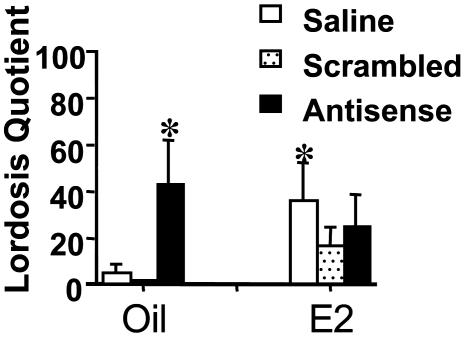
Female Sex Behavior. Two-way ANOVA analysis of lordosis behavior results revealed a significant effect of AS treatment [F(2,34) = 4.05, P < 0.03, Fig. 5]. Post hoc analysis demonstrated that the display of lordosis in response to the mounting of a stud male was significantly increased in E2-treated, saline-infused animals compared with the oil-treated saline and scrambled control animals. Moreover, infusion of the AS oligos to oil-treated animals significantly increased lordosis compared with the oil-treated controls (saline and scrambled), and surprisingly, this level of sex behavior was not significantly different from the E2-treated saline control. Curiously, the E2-treated AS and scrambled oligo groups were not significantly different from the saline controls.
Fig. 5.
Female sex behavior. Females were tested during the dark phase of the cycle under red light. Quantification of lordosis behavior revealed a significant effect of AS treatment (*, P < 0.05). Post hoc analysis demonstrated that the display of lordosis in response to the mounting of a stud male was significantly increased in E2-treated, saline-infused animals compared with the oil-treated saline and scrambled control animals. Moreover, infusion of the AS oligos to oil-treated animals significantly increased the display of lordosis compared to the oil-treated saline and scrambled controls. This level of behavior was not significantly different from the E2-treated saline controls.
Discussion
Previously, we demonstrated that E2 differentially regulates transcript levels of L-PGDS among several steroid-concentrating brain nuclei (12). Specifically, in the VLPO as well as the periventicular region, E2 suppresses the expression of L-PGDS mRNA by twofold compared with animals that lack circulating E2. Now we extend this finding to the functional significance for E2 suppression of L-PGDS. Because PGD2 is a potent somnogen, we were interested in testing whether reduction of the L-PGDS protein in oil-treated animals may mimic the effects mediated by E2 on such behaviors as arousal, general home cage activity, and sex behavior. Indeed, oil-treated animals micro-infused with AS oligos had a significant increase in the duration of activity as well as an increase in general home cage activity after the vestibular stimulus. This response was not different from the E2-treated animals. More interestingly, the reduction of L-PGDS by LNA-AS oligos in oil-treated females mimicked E2 effects on female sex behavior.
The VLPO is a bilateral collection of cells that has only recently been identified as a distinct nucleus that contains a cluster of sleep-active neurons (18, 29). When infused into the subarachnoid space just anterior to the POA, PGD2 is an effective somnogen (30). In rats, PGD2 infusion not only increases the firing rate of sleep-active POA neurons (31), but also induces Fos-immunoreactivity in the VLPO that is proportional to the production of sleep, suggesting that PGD2 may stimulate sleep-active VLPO neurons (32). These sleep-active cells are both galaninergic and γ-aminobutyric acidergic and send inhibitory projections to waking/arousal-related neurons in the tuberomammillary nucleus (33), the sole source of histaminergic innervation of the mammalian brain, the locus coeruleus, and the raphé nuclei (34). It is known that both galanin and γ-aminobutyric acid inhibit neurons in the tuberomammillary nucleus and locus coeruleus (35, 36), implying that the descending projection from the VLPO are inhibitory in nature (reviewed in ref. 37). Moreover, the VLPO receives reciprocal inputs from several monoaminergic systems that include the arousal-related histominergic projections from the tuberomammillary nucleus, noradenerigic inputs from the locus coeruleus, and serotininergic inputs from the midbrain raphé nuclei (19). VLPO neurons from acute hypothalamic slices are inhibited by noradrenaline and 5-hydroxytryptamine (38), and although histamine does not appear to elicit a response, tuberomammillary nucleus neurons also contain γ-aminobutyric acid and galanin, which may inhibit the VLPO (39). Saper et al. (37) have proposed that this reciprocity of projections is analogous to a “flip-flop” circuit in electrical engineering terms. Simply stated, when VLPO neurons are active during sleep and firing rapidly, they would inhibit the monoaminergic cell groups allowing for their own disinhibition and reinforced firing. Conversely, during wakefulness, monoaminergic neurons fire at a high rate, thus inhibiting the VLPO and resulting in the disinhibition of their own firing.
Based on our previous and current findings, it could be proposed that E2 acts on this “flip-flop” circuit to weaken the “sleep” side of the switch, thus giving more relative strength and greater probability to an aroused state. Because (i) E2 dramatically decreases L-PGDS in the VLPO and nearby leptomeninges (12), and (ii) PGD2 activates “sleep” neurons in the VLPO (31, 32), E2 should decrease inhibitory inputs to key arousal centers. Indeed, our current data fit with this parsimonious explanation because the female mice lacking E2 that received microinjection of LNA-AS oligo exhibited an increase in the (i) duration and (ii) amount of home cage activity that mimics that of the animals with circulating E2, which is not additive with the E2 effect. Additionally, L-PGDS LNA-AS oligos may act on the underlying meninges, which also express high levels of L-PGDS.
It is becoming increasingly attractive, theoretically, to propose that estrogens affect generalized arousal states in experimental animals (21, 40–42). Hebb (43) defined “arousal” as a state that optimizes the processing of sensory stimuli. However, this by itself did not easily lend itself to quantitative measurements. Thus, we have proposed an operational definition: arousal is greater (i) responsiveness to sensory stimuli, (ii) motoric and general activity, and (iii) emotional reactivity (reviewed in refs. 41 and 42). The present data demonstrate that the E2-treated animals regardless of their oligo treatment were more responsiveness to the first (vestibular) sensory stimulus compared with the oil-treated controls such that the administration of the stimulus results in prolonged and increased home cage activity. The medial, superior, and lateral divisions of the vestibular nucleus send extensive projections to the medullary and pontine reticular formation, thus, providing an increased impetus for arousal when stimulated (44–47). The fact that the response of the AS oil-treated animals mimicked that of the E2 groups suggests that PGD2 plays a role in mediating the arousing effects of E2.
If estrogens heighten the generalized arousal state of female rodents might this affect specific natural behaviors or sexual motivation? For example, females receptive to mating display a heightened muscular tension throughout their body, rapid alternating movements and robust elevation of locomotor activities (reviewed in ref. 42). Prostaglandins, specifically PGE2, acting at the level of the POA have been implicated in the facilitation of sexual receptivity and lordosis behavior in female rats (48–51). Systemic administration or intradiencephalic implantation at the level of the AH-POA of PGE2 not only facilitates the display of female sex behavior in ovariectomized estrogen-primed rats but is also capable of inducing lordosis in ovariectomized rats in the absence of E2 priming (51). Indomethacin, a prostaglandin synthesis inhibitor, inhibits the sexual receptivity in rats when given before the start of estrogen priming (50).
Interestingly, in mammals, PGD2 and PGE2 have a number of opposing biological actions, such as (i) PGD2 induces sleep whereas PGE2 induces wakefulness, as mentioned above (52–54), (ii) PGD2 lowers body temperature (55) whereas PGE2 elevates it (16), and (iii) PGE2 stimulates secretion of luteinizing hormone-releasing hormone (56) whereas PGD2 suppresses release (57). In fact, when taken in context with PGE2 facilitation of lordosis, our present data, which suggests that reducing the synthesis of PGD2 with either E2 or AS oligos increased lordosis, fits with the contrasting nature of PGD2/PGE2.
Through E2 actions in the POA, cells in the ventromedial nucleus of the hypothalamus are disinhibited allowing for a higher probability of lordosis and mating. Here, we have demonstrated that a knockdown of L-PGDS in the POA and what we presume to be a subsequent decrease in PGD2 not only enhanced generalized arousal but also enhanced sexual arousal resulting in an increase in lordosis. Thus, it could be suggested that PGD2 is implicated in the POA inhibition of cells in the ventormedial nucleus. However, we found it surprising that (i) the AS oligos were no more effective in the presence of E2 than in the absence of E2 and (ii) that the E2-treated groups did not show a more robust response. The fact that the AS facilitated lordosis in the absence of E2, suggests that a decrease in PGD2 in the POA may directly release the ventromedial nucleus form cellular inhibition, which may involve PGE2, whereas in the presence of E2, other pathways may be activated leading to the attenuated response. In fact, Rodriguez-Sierra et al. (58) reported a similar phenomenon after vaginocervical stimulation, in rats. The resulting lordosis response persisted longer in the non-E2-treated rats than E2-treated rats.
Our previous finding that E2 so profoundly depressed L-PGDS mRNA expression in the VLPO, with timing analogous to the proestrous dark period, is consistent with the idea that preovulatory estrogen surges direct the suppression of the gene in intact cycling animals. During the proestrous dark period when E2 levels are highest, sleep is typically reduced and motor activity is increased (1, 2). In fact, the POA is a key site for the estrogenic regulation of activity and locomotion such that E2-treated females, in the absence of fear producing stimuli, demonstrated increased running wheel activity as well as general home cage activity that included rearing, grooming, and burying (8, 10, 21).
In summary, E2 apparently has an arousing effect allowing for increased locomotor behaviors, a component of estrogen-facilitated courtship responses. Our present data provide evidence that reduction of L-PGDS in the VLPO mimics the behaviors of E2. Thus, the E2-mediated decrease in PGD2 may contribute to the general arousal mediated by estrogens that leads to the increased locomotion evident in courtship behavior and, subsequently, to successful reproduction.
Abbreviations: POA, preoptic area; oligo, oligonucleotide; VLPO, ventrolateral POA; E2, estradiol; L-PGDS, lipocalin-type prostaglandin D synthase; PGD2, prostaglandin D2; LNA, locked nucleic acid; AS, antisense; HACTV, horizontal activity.
References
- 1.Schwierin, B., Borbely, A. A. & Tobler, I. (1998) Brain Res. 811, 96-104. [DOI] [PubMed] [Google Scholar]
- 2.Fang, J. & Fishbein, W. (1996) Brain Res. 734, 275-285. [PubMed] [Google Scholar]
- 3.Wade, G. N. & Zucker, I. (1970) J. Comp. Physiol. Psychol. 72, 328-336. [DOI] [PubMed] [Google Scholar]
- 4.Gerall, A. A., Napoli, A. M. & Cooper, U. C. (1973) Physiol. Behav. 10, 225-229. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy, G. (1964) J. Physiol. 172, 383-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas, D. K., Storlien, L. H., Bellingham, W. P. & Gillette, K. (1986) Physiol. Behav. 36, 567-573. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz de Elvira, M. C., Persaud, R. & Coen, C. W. (1992) Physiol. Behav. 52, 277-284. [DOI] [PubMed] [Google Scholar]
- 8.Morgan, M. A. & Pfaff, D. W. (2002) Behav. Brain Res. 132, 85-93. [DOI] [PubMed] [Google Scholar]
- 9.McGinty, D. & Szymusiak, R. (2001) Sleep Med. Rev. 5, 323-342. [DOI] [PubMed] [Google Scholar]
- 10.Fahrbach, S. E., Meisel, R. L. & Pfaff, D. W. (1985) Physiol. Behav. 35, 985-992. [DOI] [PubMed] [Google Scholar]
- 11.Mong, J. A., Krebs, C. & Pfaff, D. W. (2002) Endocrinology 143, 2002-2006. [DOI] [PubMed] [Google Scholar]
- 12.Mong, J. A., Devidze, N., Frail, D. E., O'Connor, L. T., Samuel, M., Choleris, E., Ogawa, S. & Pfaff, D. W. (2003) Proc. Natl. Acad. Sci. USA 100, 318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eguchi, N., Minami, T., Shirafuji, N., Kanaoka, Y., Tanaka, T., Nagata, A., Yoshida, N., Urade, Y., Ito, S. & Hayaishi, O. (1999) Proc. Natl. Acad. Sci. USA 96, 726-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horiguchi, S., Ueno, R., Hyodo, M. & Hayaishi, O. (1986) Eur. J. Pharmacol. 122, 173-179. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe, Y., Mori, K., Imamura, K., Takagi, S. F. & Hayaishi, O. (1986) Brain Res. 378, 216-222. [DOI] [PubMed] [Google Scholar]
- 16.Ueno, R., Narumiya, S., Ogorochi, T., Nakayama, T., Ishikawa, Y. & Hayaishi, O. (1982) Proc. Natl. Acad. Sci. USA 79, 6093-6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urade, Y. & Hayaishi, O. (1999) Biochim. Biophys. Acta 1436, 606-615. [DOI] [PubMed] [Google Scholar]
- 18.Sherin, J. E., Shiromani, P. J., McCarley, R. W. & Saper, C. B. (1996) Science 271, 216-219. [DOI] [PubMed] [Google Scholar]
- 19.Chou, T. C., Bjorkum, A. A., Gaus, S. E., Lu, J., Scammell, T. E. & Saper, C. B. (2002) J. Neurosci. 22, 977-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahlestedt, C., Salmi, P., Good, L., Kela, J., Johnsson, T., Hokfelt, T., Broberger, C., Porreca, F., Lai, J., Ren, K., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan, M. A. & Pfaff, D. W. (2001) Horm. Behav. 40, 472-482. [DOI] [PubMed] [Google Scholar]
- 22.Franklin, K. B. J. & Paxinos, G. (1997) The Mouse Brain in Stereotaxic Coordinates (Academic, New York).
- 23.Brown, D., Kang, S., Gryaznov, S., DeDionisio, L., Heidenreich, O., Sullivan, S., Xu, X. & Nerenberg, M. (1994) J. Biol. Chem. 269, 26801-26805. [PubMed] [Google Scholar]
- 24.Levin, A. (1999) Biochim. Biophys. Acta 1489, 69-84. [DOI] [PubMed] [Google Scholar]
- 25.Kumar, R., Singh, S. K., Koshkin, A. A., Rajwanshi, V. K., Meldgaard, M. & Wengel, J. (1998) Bioorg. Med. Chem. Lett. 8, 2219-2222. [DOI] [PubMed] [Google Scholar]
- 26.Kurreck, J., Wyszko, E., Gillen, C. & Erdmann, V. A. (2002) Nucleic Acids Res. 30, 1911-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braasch, D. A. & Corey, D. R. (2001) Chem. Biol. 8, 1-7. [DOI] [PubMed] [Google Scholar]
- 28.Mong, J. A., McCarthy, M. M. & Nunez, J. L. (2002) J. Neuroendocrinol. 14, 45-55. [DOI] [PubMed] [Google Scholar]
- 29.Szmusiak, R., N, A., Steininger, T. L. & McGinty, D. (1998) Brain Res. 803, 178-188. [DOI] [PubMed] [Google Scholar]
- 30.Matsumura, H., Nakajima, T., Osaka, T., Satoh, S., Kawase, K., Kubo, E., Kantha, S. S., Kasahara, K. & Hayaishi, O. (1994) Proc. Natl. Acad. Sci. USA 91, 11998-12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyama, Y. & Hayaishi, O. (1993) Brain Res. Bull. 33, 367-372. [DOI] [PubMed] [Google Scholar]
- 32.Scammell, T., Gerashchenko, D., Urade, Y., Onoe, H., Saper, C. & Hayaishi, O. (1998) Proc. Natl. Acad. Sci. USA 95, 7754-7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherin, J. E., Elmquist, J. K., Torrealba, F. & Saper, C. B. (1998) J. Neurosci. 18, 4705-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steininger, T. L., Gong, H., McGinty, D. & Szymusiak, R. (2001) J. Comp. Neurol. 429, 638-653. [PubMed] [Google Scholar]
- 35.Seutin, V., Verbanck, P., Massotte, L. & Dresse, A. (1989) Eur. J. Pharm. 164, 373-376. [DOI] [PubMed] [Google Scholar]
- 36.Yang, Q. Z. & Hatton, G. I. (1997) Brain Res. 773, 162-172. [DOI] [PubMed] [Google Scholar]
- 37.Saper, C. B., Chou, T. C. & Scammell, T. E. (2001) Trends Neurosci. 24, 726-731. [DOI] [PubMed] [Google Scholar]
- 38.Gallopin, T., Fort, P., Eggermann, E., Cauli, B., Luppi, P. H., Rossier, J., Audinat, E., Muhlethaler, M. & Serafin, M. (2000) Nature 404, 992-995. [DOI] [PubMed] [Google Scholar]
- 39.Airaksinen, M. S., Alanen, S., Szabat, E., Visser, T. J. & Panula, P. (1992) J. Comp. Neurol. 323, 103-116. [DOI] [PubMed] [Google Scholar]
- 40.Frohlich, J., Morgan, M., Ogawa, S., Burton, L. & Pfaff, D. (2001) Horm. Behav. 39, 39-47. [DOI] [PubMed] [Google Scholar]
- 41.Frohlich, J., Ogawa, S., Morgan, M., Burton, L. & Pfaff, D. (1999) Behav. Brain Res. 105, 5-27. [DOI] [PubMed] [Google Scholar]
- 42.Pfaff, D., Frohlich, J. & Morgan, M. (2002) Trends Neurosci. 25, 45-50. [DOI] [PubMed] [Google Scholar]
- 43.Hebb, D. O. (1962) in Brain Mechanism and Learning, ed. Delafresnaye, J. F. (Oxford, London).
- 44.Siegel, J. M. & McGinty, D. J. (1977) Science 196, 678-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson, B. W. & Abzug, C. (1975) J. Neurophysiol. 38, 1421-1435. [DOI] [PubMed] [Google Scholar]
- 46.Steinbacher, B. C., Jr. & Yates, B. J. (1996) Am. J. Physiol. 271, R1070-R1077. [DOI] [PubMed] [Google Scholar]
- 47.Matesz, C., Bacskai, T., Nagy, E., Halasi, G. & Kulik, A. (2002) Brain Res. Bull. 57, 313-315. [DOI] [PubMed] [Google Scholar]
- 48.Dudley, C. & Moss, R. L. (1976) J. Endocrinol. 71, 457-458. [DOI] [PubMed] [Google Scholar]
- 49.Hall, N. R. & Luttge, W. G. (1977) Brain Res. Bull. 2, 203-207. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Sierra, J. L. & Komisaruk, B. R. (1977) Horm. Behav. 9, 281-289. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Sierra, J. L. & Komisaruk, B. (1978) Prostaglandins 15, 513-524. [DOI] [PubMed] [Google Scholar]
- 52.Huang, Z.-L., Sato, Y., Mochizuki, T., Okada, T., Qu, W.-M., Yamatodani, A., Urade, Y. & Hayaishi, O. (2003) J. Neurosci. 23, 5975-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsumura, H., Honda, K., Choi, W., Inoue, S., Sakai, T. & Hayaishi, O. (1989) Proc. Natl. Acad. Sci. USA 86, 5666-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsumura, H., Honda, K., Goh, Y., Ueno, R., Sakai, T., Inoue, S. & Hayaishi, O. (1989) Brain Res. 481, 242-249. [DOI] [PubMed] [Google Scholar]
- 55.Milton, A. S. & Wendlandt, S. (1970) J. Physiol. 207, 76-77. [PubMed] [Google Scholar]
- 56.Harms, P. G., Ojeda, S. R. & McCann, S. M. (1973) Science 181, 760-761. [DOI] [PubMed] [Google Scholar]
- 57.Kinoshita, F., Nakai, Y., Katakami, H., Imura, H., Shimizu, T. & Hayaishi, O. (1982) Endocrinology 110, 2207-2209. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Sierra, J. F., Crowley, W. R. & Komisaruk, B. R. (1975) J. Comp. Physiol. Psych. 89, 79-85. [DOI] [PubMed] [Google Scholar]