Abstract
γ-Aminobutyric acid type A receptors (GABARs) have long been implicated in mediating ethanol (EtOH) actions, but so far most of the reported recombinant GABAR combinations have shown EtOH responses only at fairly high concentrations (≥60 mM). We show that GABARs containing the δ-subunit, which are highly sensitive to γ-aminobutyric acid, slowly inactivating, and thought to be located outside of synapses, are enhanced by EtOH at concentrations that are reached with moderate, social EtOH consumption. Reproducible ethanol enhancements occur at 3 mM, a concentration six times lower than the legal blood-alcohol intoxication (driving) limit in most states (0.08% wt/vol or 17.4 mM). GABARs responsive to these low EtOH concentrations require the GABAR δ-subunit, which is thought to be associated exclusively with α4- and α6-subunits in vivo, and the β3-subunit, which has recently been shown to be essential for the in vivo anesthetic actions of etomidate and propofol. GABARs containing β2-instead of β3-subunits in α4βδ- and α6βδ-receptor combinations are almost 10 times less sensitive to EtOH, with threshold enhancement at 30 mM. GABARs containing γ2-instead of δ-subunits with α4β and α6β are three times less sensitive to EtOH, with threshold responses at 100 mM, a concentration not usually reached with social EtOH consumption. These combined findings suggest that “extrasynaptic” δ-subunit-containing GABARs, but not their “synaptic” γ-subunit-containing counterparts, are primary targets for EtOH.
Despite the fact that ethanol (EtOH) is the most widely used psychoactive agent, its actions on brain functions are poorly understood. Several types of receptors and channels have been shown to be functionally altered by EtOH, which include N-methyl-d-aspartate (1) and non-N-methyl-d-aspartate glutamate receptors (2, 3), serotonin (4), glycine (5, 6), and GABARs (7, 8), and G protein-coupled inwardly rectifying K+ channels (9, 10). With a few exceptions (3, 8-12), EtOH effects on these targets are seen only at fairly high concentrations (≥60 mM).
The GABAR, the major inhibitory neurotransmitter receptor, has been a long-time focus for studies on EtOH and anesthetic actions. For example, it has been shown that EtOH at low intoxicating concentrations was able to enhance Cl- flux in synaptoneurosomes (13, 14) and cultured neurons (15). However, electrophysiological studies of GABARs in single neurons and recombinant receptors showed current enhancement only at fairly high concentrations (>50 mM) of EtOH (5, 7, 16), which now appears to be due to the fact that these studies focused on synaptic and/or γ-subunit-containing receptors. It is thought that replacement of the γ-subunit in the GABAR 2α-2β-1γ pentameric complex by the δ-subunit changes not only the localization of the receptor from mainly postsynaptic to extrasynaptic, but also leads to up to a 50-fold increase in γ-aminobutyric acid (GABA) affinity and slower desensitization (17-19). These functional properties are consistent with αβδ GABARs, which are activated by ambient extracellular GABA concentrations (thought to be on the order of 0.5-1 μM; ref. 20). The tonic currents flowing through these channels contrast with synaptic αβγ GABARs, which open only briefly (≈10 ms) in response to near-saturating amounts (≥1 mM peak concentration) of GABA released into the synaptic cleft.
Even though δ-subunits can be forced to form receptors with all α- and β-subunits tested in recombinant systems (17, 18), in vivo they appear to associate virtually exclusively with α4- (21) and α6-subunits (22). The α6-subunit protein is expressed only in cerebellar granule cells, whereas α4-subunits have a more wide-spread distribution and are expressed (with decreasing abundance) in the thalamus, the dentate gyrus, the striatum, the outer layers of the cortex, and at lower levels in other brain areas like the hippocampus. In cerebellar granule cells, the δ-subunit together with the α6-subunit is exclusively extrasynaptic (23); and, in neurons expressing the α4/δ combination, an extrasynaptic location also seems likely (24). Consistent with its exclusive association with α4- and α6-subunits, the distribution of the δ-subunit revealed by immunostaining has a striking resemblance with α4-immunoreactivity in mouse brain (25), except for the cerebellar granule cell layer where the closely related α6-subunit replaces α4.
In chronic intermittent EtOH-treated rats, a model for human alcohol-withdrawal syndrome, δ-subunit protein levels decrease in the hippocampus, whereas α4- and γ2-proteins increase. The changes in synaptic benzodiazepine pharmacology suggest that α4-replaces α1-subunits in synaptic αβγ-receptors in chronic intermittent EtOH-treated rats (26). A comparison of α4β3δ and α4β3γ2 GABARs studied in expression systems revealed that the δ-subunit-containing receptors showed greater enhancement by etomidate, pentobarbital, propofol, and steroids [tetrahydrodeoxycorticosterone (THDOC) and alphaxalone] than those containing the γ2-subunit (18, 19, 27, 28). In knock-in mice, the in vivo actions of etomidate and propofol were almost completely abolished by a point mutation (N256M) in the GABAR β3-subunit, which demonstrates that GABARs containing the β3-subunit mediate the in vivo effects of these general anesthetics (29). In contrast, mice containing the etomidate-insensitive N265S mutation in the β2-subunit lose sedative but not the anesthetic effects of etomidate (30).
Here we show that recombinant α4β3δ and α6β3δ GABARs are uniquely sensitive to ethanol, with a dose-response relationship mirroring the well known effects of alcohol consumption on the human brain. Surprisingly, ethanol was much more effective on β3-than on β2-containing α4βδ- and α6βδ-receptors, which demonstrates that the incorporation of the GABAR β2- or β3-subunits can lead to functionally distinct receptors in recombinant expression systems. In fact, the EtOH sensitivity was increased only 3-fold by replacing the γ2-with the δ-subunit, whereas an almost 10-fold increase was observed by replacement of β2 with β3 in the αβδ GABARs. These findings lead us to propose that extrasynaptic α4β3δ- or α6β3δ-subunit-containing GABARs are primary targets for EtOH. This hypothesis probably applies also for other GABAR-specific general anesthetics (etomidate, propofol, and steroid anesthetics). The finding that these subtypes of GABAR are likely targets of EtOH action is consistent with their anatomical distribution in brain regions that mediate EtOH effects on behavior, such as the cerebellum (motor coordination), the hippocampal formation (amnesic effects), and the thalamus (sleep-promoting and possibly anesthetic effects).
Methods
cRNA. Rat GABAR subunit cDNAs were obtained from A. Tobin (University of California, Los Angeles; α1), H. Luddens (University of Mainz, Mainz, Germany; α4), R. Macdonald (Vanderbilt University, Nashville, TN; α6, δ), D. Pritchett (University of Pennsylvania, Philadelphia; β2), L. Mahan (National Institutes of Health, Bethesda; β3), and D. S. Weiss (University of Alabama, Birmingham; γ2L and γ2S). The ORFs and part of the 3′ UTRs of the rat α4- and δ-subunits were amplified by PCR and cloned into a vector containing the 5′ UTR of the Shaker potassium channel. The coding regions of all GABAR cDNA clones used in this study were verified by fluorescent (BigDye3, Sigma-Aldrich) sequencing. mRNA was transcribed from linearized template plasmids by using the mMESSAGE mMACHINE kits (Ambion, Austin, TX). We purified cRNA transcripts by LiCl precipitation and analyzed transcript quality and concentration by photometry and gel electrophoresis. Oocytes were injected with 0.4 ng of α- and β-subunit cRNA and 2 ng (in some cases, even 4 ng) of δ- or γ2-cRNA. This 5- or 10-fold excess of δ- and γ-cRNA over α and β was used to avoid “contamination” by functional α/β-subunit receptors. Currents were measured 3-4 days after oocyte injection for γ-subunit-containing receptors, whereas oocytes injected with δ-subunit-containing receptors, because of their apparently low expression levels, had their currents measured 7-8 days after injection.
Electrophysiology. We measured GABAR currents in oocytes with an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA) in the two-electrode voltage-clamp configuration. Electrodes were filled with 3 M KCl and had resistances between 0.5 and 1.5 MΩ, when measured dipped in the bath solution. The oocyte chamber was continuously perfused with ND96 bath solution (composition, 96 mM NaCl/2 mM KCl/1.8 mM CaCl2/1 mM MgCl2/5 mM Hepes, pH 7.5) with drugs and treatments mentioned. Solution exchanges were triggered with a programmable valve bank switching a three-way solenoid valve, and bath-volume exchange times were in the range of 1-3 s. Currents were measured at a holding potential (VH) of -80 mV (unless indicated otherwise), where GABA applications evoke an inward current in oocytes. Currents and voltages were recorded on a two-channel chart recorder (Soltec 1242, Sun Valley, CA). To minimize voltage-clamp errors, only current responses of <1 μA were considered for analysis. Chart recordings were digitized by using graph digitization software (N. Rodionov). GABA, EtOH, and the steroid THDOC (5β-pregnane-3α,21-diol-20-one) were obtained from Sigma. THDOC was dissolved in DMSO (10 mM stock solution). Etomidate was the clinical formulation from Bedford Laboratories (Bedford, OH). The curve fits for the GABA dose-responses were generated by the nonlinear sigmoidal dose-response equation I/Imax = 1/[1 + [EC50/(GABA)n], where EC50 is the concentration of drug eliciting a half-maximal response, n is the Hill coefficient, Imax is the maximum current, and I is the GABA-evoked current. Values for EC50 and percent EtOH enhancements were calculated for individual cells and combined to give means with 95% confidence intervals.
Results
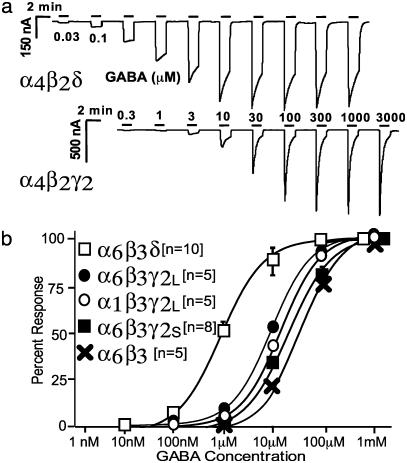
Expression and Functional Properties of α4β3δ or α6β3δ Receptors in Xenopus Oocytes. Both GABAR α4- and δ-subunits have been reported to be difficult to express in recombinant systems (27, 31). In most published studies, the α4-subunit and, in some cases, also the δ-subunit cDNAs (19) were used as chimeras in which the signal sequences and the 5′ UTRs were replaced by the signal sequence and the 5′ UTRs of the bovine α1 GABAR (19, 27, 28, 31). To prevent expression problems due to inhibitory 5′ UTRs, we amplified the coding regions of α4- and δ-subunits by PCR and cloned them into an expression vector, thereby replacing upstream noncoding parts of these clones. Rat α4- or α6-subunits were expressed together with rat β2- or β3- and δ- or γ2S (or γ2L)-subunits in Xenopus oocytes. The functional properties of δ-subunit-containing receptors confirmed previous findings obtained with recombinant receptors in eukaryotic cells (17-19, 27) that δ-subunit-containing recombinant receptors were >20 times more sensitive to GABA (than corresponding γ2S-subunit-containing receptors) and showed slower desensitization. These properties are consistent with their proposed function as extrasynaptic receptors mediating tonic inhibition (20). Recordings that illustrate the functional differences between receptors containing δ-subunits versus those containing γ2S-subunits are shown in Fig. 1a. GABA dose-response curves of αβγ and αβδ and binary αβ GABARs are shown in Fig. 1b. No significant differences occurred in the GABA dose-response curves when α4 was replaced by α6 or when β2 was replaced by β3 in αβγ2 (EC50 ≈ 18 μM), αβδ (EC50 ≈ 0.57 μM), or αβ (EC50 ≈ 22 μM) subunit combinations. The fact that receptors containing α4-subunits with a modified 5′-UTR expressed current levels similar to those containing α6-subunits suggests that the 5′ untranslated region in the “native” α4-subunit is responsible for the reported poor expression in recombinant expression systems rather than inefficient processing of the native α4-subunit protein.
Fig. 1.
Comparison of synaptic (αβγ) and extrasynaptic (αβδ) GABARs. (a) Comparison of GABAR currents expressed in Xenopus oocytes. GABARs composed of α4β2δ-subunits show a slower desensitization and are activated at much lower concentrations of GABA than those formed by α4β2γ2S.(b) GABA dose-responses on GABAR subunit combinations containing either α4- or α6-subunits, with β2- or β3-subunits, with and without γ2S-, γ2L-, or δ-subunits. The plots shown are for α6β3δ, α6β3γ2L, α1β3γ2L, α6β3γ2S, and α6β3. GABARs containing α4-in place of α6-, and β2-instead of β3-subunits are virtually identical (see Table 1 for EC50 values). The αβ-subunits produce small-current levels and have a fairly low potency for GABA (EC50 ≈ 22 μM GABA). γ-Subunit-containing receptors respond to lower concentrations of GABA (γ2S,EC50 ≈ 18 μM GABA; α1β3γ2L,EC50 ≈ 10 μM GABA; α6β3γ2L, ≈ 8 μM GABA). δ-containing receptors are the most GABA-sensitive with just-detectable responses evident at concentrations as low as 30 nM GABA (EC50 ≈ 0.57 μM GABA).
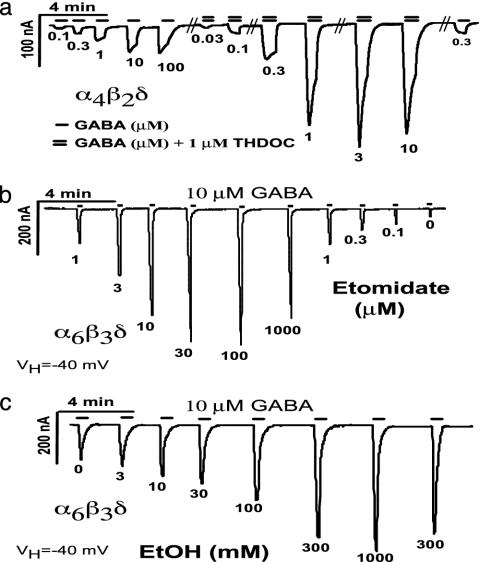
GABARs Containing the δ-Subunit at Saturating GABA Concentrations: GABA as a Partial Agonist. In all receptor combinations tested, GABA-evoked peak currents from γ2S-containing receptors were much larger than those from corresponding δ-subunit-containing receptors when measured at the same time after injection. This finding held true despite our efforts to improve expression by modifying the 5′ untranslated region in the δ-subunit cDNA. However, smaller currents are not due to the fewer channels expressed, because coapplication of a high concentration (1 μM) of the neurosteroid THDOC together with GABA increased current levels in α4β2δ-receptors about 5-fold at all GABA concentrations tested (Fig. 2), consistent with the reported effects of THDOC on α6β3δ (18) and α4β3δ (19). Because the efficacy of etomidate is much greater at α4β3δ versus α4β3γ2 GABARs (19), we tested whether etomidate would increase peak current responses on α6β3δ GABARs in oocytes. Etomidate (from 0.1 to 1000 μM) coapplied with a close to saturating concentration of GABA (10 μM or EC95; see Fig. 1a) led to a dramatic dose-dependent enhancement of peak current responses (Fig. 2b). At the most effective concentration of etomidate (100 μM), peak current increases about 20-fold. This result is probably an underestimation because of the fairly slow perfusion around the oocyte, which tends to obscure peak current responses at desensitizing receptors. This finding is the likely explanation for the lower peak current responses with 10 μMGABA + 1,000 μM etomidate (Fig. 2b) and for the decrease in peak currents 10 μM GABA + 1 μM THDOC (Fig. 2a). This dramatic enhancement of peak GABA currents (even at saturating GABA concentrations) by etomidate and THDOC suggests that the low-current levels we observe in δ-subunit-containing receptors are caused by GABA being a partial agonist on αβδ GABAR, rather than by a problem with expression (i.e., the number of functional channels formed).
Fig. 2.
δ-Subunit-containing GABARs as targets for neuroactive steroids, the general anesthetic etomidate, and ethanol. (a) GABA dose-response curve of α4β2δ GABAR alone and in the presence of 1 μM THDOC shows an up to 5-fold increase in peak currents with 1 μM THDOC even at saturating GABA concentrations. (b) Dose-response curve of the general anesthetic etomidate on α6β3δ GABAR current. Etomidate was coapplied with almost saturating amounts of GABA (10 μM = EC95). (c) EtOH coapplication with 10 μM (EC95) GABA causes an increase in current levels in α6β3δ GABARs as low as 3 mM and triples the peak currents at 1 M. Shown are single traces with full dose-response curves that demonstrate that GABA is only a partial agonist at δ-subunit-containing receptors. Similar results have been obtained with application of selected concentrations of THDOC, etomidate, and EtOH and with other δ-subunit-containing GABARs. Peaks marked with “0” show current responses to 10 μM GABA without etomidate or EtOH. Application of 1 μM THDOC, 300 μM etomidate, or 1 M EtOH alone does not evoke significant currents in α6β3δ-expressing oocytes (not shown).
α4β3δ and α6β3δ GABARs Show Threshold EtOH Enhancement at Concentrations Reached During Moderate Social Ethanol Consumption. Because EtOH is often classified as an anesthetic (32), and GABARs have long been implicated in mediating EtOH effects (7), we tested EtOH on δ-subunit-containing GABARs under the same conditions as etomidate (coapplication of EtOH with 10 μM GABA). With the α6β3δ GABARs, we saw just-detectable increases at 3 mM and a 2-fold increase in peak currents at 1 M EtOH (Fig. 2c). In the range from 0 to 300 nM etomidate, the amount of peak current enhancement coincides with that seen with 0-300 mM EtOH at α6β3δ-subunit-containing GABARs (compare Fig. 2b with Fig. 2c).
To further characterize EtOH responses, we tested at a GABA concentration that produced 20% of peak currents (EC20) for each subunit combination (300 nM for αβδ, 10 μM for αβγ, and 30 μM for αβ GABAR combinations; see Fig. 1b). Measuring at a nonsaturating EC20 GABA concentration is reasonable for extrasynaptic GABARs, because they operate at usually non-saturating GABA concentrations.
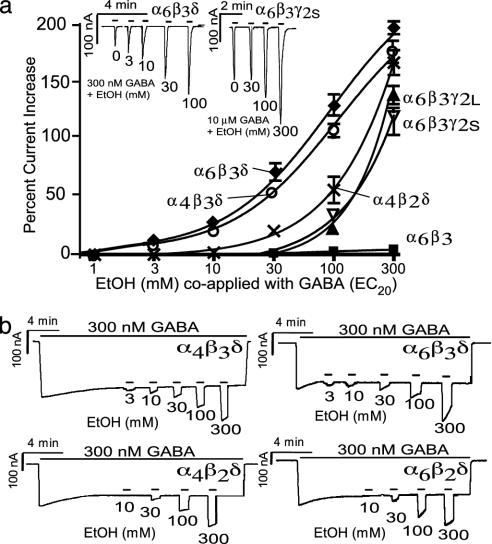
EtOH effects were recorded by using short ethanol coapplication (from 1 to 300 mM) with EC20 GABA concentrations to evoke a peak current, with recovery periods of at least 40 s between applications (Fig. 3 Inset). The peak responses were plotted as an increase over peak currents with EC20 GABA applications alone (Fig. 3a). GABARs composed of α6β3δ and α4β3δ showed the highest EtOH sensitivity and had a similar enhancement at low EtOH concentrations, whereas at concentrations of >10 mM EtOH, α6β3δ-receptors showed greater enhancement by EtOH than α4β3δ-receptors. Receptors containing the β2-subunit were much less sensitive than the corresponding β3-containing receptors at low EtOH concentrations [threshold responses at 30 mM (Fig. 3a)] but not at very high (300 mM) EtOH concentrations. Receptors containing the γ2-subunit showed significant EtOH activation starting at a concentration of 100 mM, and GABARs composed of only αβ-subunits were almost completely insensitive, even at 300 mM EtOH (see Table 1 for percent EtOH increases for each subunit combination).
Fig. 3.
Both the β3- and δ-subunits are required for high ethanol sensitivity. (a) EtOH current enhancement when coapplied with respective EC20 values of the various subunit combinations. αβδ-Subunits show the largest enhancement, β3-containing receptors being the most sensitive. γ-containing GABAR currents show significant potentiation only at 100 mM EtOH, and αβ-subunits seem to be completely insensitive. The plots shown are for α6β3δ (♦), α4β3δ (○), α4β2δ (×), α6β3γ2L (▴), α6β3γ2S (▿), and α6β3 (▪) (n = 13, 12, 15, 10, 9, and 5, respectively); the remaining combinations with δ, γ2S, or γ2L or neither were virtually indistinguishable from the subunits represented (see Table 2 for pooled EtOH-response values). (b) EtOH effects on tonically activated receptors. Replacement of β2-with β3-subunits in α4βδ or α6βδ GABARs leads to an almost 10-fold increase in EtOH sensitivity. EtOH response (from 3 to 300 mM) on α4β2δ-, α4β3δ-, α6β2δ-, and α6β3δ-containing GABARs activated by steady-state 300 nM GABA (≈EC30).
Table 1. GABA EC50 values for all subunits studied.
| Receptor | EC50, nM | n |
|---|---|---|
| α6β3δ | 0.67 ± 0.03 | 10 |
| α4β3δ | 0.62 ± 0.02 | 9 |
| α6β2δ | 0.75 ± 0.08 | 9 |
| α4β2δ | 0.5 ± 0.07 | 7 |
| α6β3γ2S | 18.8 ± 0.03 | 8 |
| α6β3γ2L | 7.6 ± 0.6 | 5 |
| α4β3γ2S | 16.6 ± 0.7 | 6 |
| α4β2γ2S | 17.7 ± 0.6 | 6 |
| α1β3γ2L | 10.3 ± 0.4 | 5 |
| α6β3 | 21.6 ± 1.8 | 5 |
| α4β3 | 22.5 ± 2.3 | 4 |
| α6β2 | 21.4 ± 1.1 | 4 |
| α4β2 | 22.0 ± 2.7 | 4 |
Values were calculated for each individual cell and represent the arithmetic mean ± SEM from a number (n) of various cells.
In addition to EtOH effects evaluated by GABA/EtOH coapplication, we used a protocol where we tried to mimic the physiological modus operandi of extrasynaptic GABA receptors (Fig. 3b). This protocol involved the prolonged application of 300 nM GABA (i.e., EC20) to the oocyte, where currents relaxed to the steady-state activation level to which increasing concentrations of EtOH (in 300 nM GABA) were applied (Fig. 3b). Similar to results seen in the GABA-EtOH coapplication protocol, we saw the threshold current enhancement at 30 mM EtOH with α4β2δ, whereas with the α6β3δ or α4β3δ combinations, the threshold response was observed at 3 mM, a concentration almost six times lower than the human blood-alcohol legal driving limit of 17.4 mM. Although the EtOH potency was similar under both measuring conditions, the absolute current enhancement by EtOH (efficacy) was about twice as large when GABA/EtOH was coapplied to nondesensitized receptors as in the EtOH application under steady-state conditions. This finding suggests that EtOH responsiveness may be diminished during the prolonged presence of GABA, possibly because of slowly populated states of the receptor with diminished EtOH sensitivity. It is interesting that EtOH responses in the presence of 300 nM GABA seem to have their own, only weakly dose-dependent, desensitization (see Fig. 3b).
Discussion
An Extrasynaptic GABAR Subunit Combination Is Highly Sensitive to Ethanol. EtOH has been shown to enhance γ-subunit-containing GABARs and glycine receptors in recombinant systems and in slice recordings to increase inhibitory postsynaptic potential/inhibitory postsynaptic current (IPSP/C) decay times. However, in most studies the concentrations needed (>40 mM) to show significant GABAR current enhancement are beyond the usual blood-ethanol concentrations reached during human alcohol consumption and would be potentially life-threatening (33). The median lethal blood-alcohol concentration in Finnish people was reported to be 0.33% or 72 mM (33). It seems unlikely, therefore, that synaptic receptors are primary EtOH responders, but they may contribute to EtOH toxicity at high concentrations. We confirm the relative EtOH insensitivity of γ-subunit-containing GABARs, which in our hands showed just-detectable EtOH enhancement at 100 mM but not at 30 mM, whereas GABARs containing the α4β2δ- or α6β2δ-subunits are about three times more sensitive. Most surprisingly, replacement of the β2-with the β3-subunit increased the EtOH sensitivity 10-fold, showing that β-subunit isoforms can lead to functional differences in recombinant GABARs. It is likely that the stimulation of GABA-dependent Cl- uptake into synaptoneurosomes by fairly low concentrations of EtOH in early studies (13) might have been due to the presence of extrasynaptic α6β3δ- or α4β3δ-subunit-containing receptors in these preparations and that the failure to see EtOH effects at such low concentrations in many preparations and laboratories since that time may be explained by research focusing on recombinant receptors containing γ-subunits and in vivo studies on synaptic GABARs. Apart from real (with the α4-subunit) or apparent difficulties (because GABA being only a partial agonist with low efficacy) to express δ-subunit-containing receptors in recombinant systems, δ-subunit-containing receptors may not have received attention because they make up only a fairly small fraction of GABARs in the mammalian brain, with each α4β3δ and α6β3δ estimated to contribute <5% to the total number of GABARs (34).
While this work was in progress, it was reported that α4β2δ-receptors expressed in Xenopus oocytes are enhanced by surprisingly low EtOH concentrations; threshold activation was reported to occur at 0.1 mM (11), a concentration 300 times lower than we observed with α6β2δ or α4β2δ GABARs. These workers also showed a bell-shaped dose-response with the EtOH effect declining at 10 mM, and report (in their supporting data) that α4β3δ-receptors, which were the most sensitive to EtOH in our hands, did not give functional expression in oocytes. In addition, the GABA-EC20 for their α4β2δ-receptors is almost 10 times lower than in our experiments and reported in the literature (19, 27). It remains to be determined what accounts for these discrepancies. One possibility is the differences in the cDNAs used, particularly the functionality of their α4-subunit.
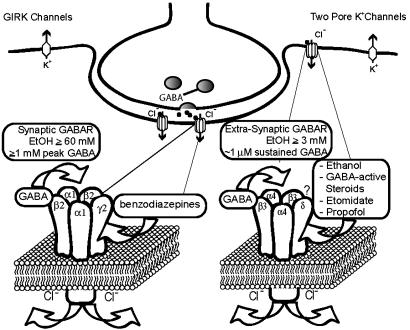
Tonic inhibitory K+ and Cl- Currents Modulated by Anesthetics and Ethanol: Mutant Mice and Compensatory Mechanisms. Extrasynaptic GABARs are high-affinity receptors activated by ambient GABA concentrations (thought to be in the range of 0.5-1 μM), and their activation usually leads to a decrease in neuronal excitability. Functionally, extrasynaptic GABA receptors are similar to the “background K+ channels,” which comprise two main families: the two pore K+ channels (TREK and TASK) and G protein-coupled inward rectifier K+ (GIRK) channels (see Fig. 4). These K+ channels are candidate targets for actions of volatile anesthetics (35, 36).
Fig. 4.
Synaptic versus extrasynaptic receptors. Synaptic receptors (shown is the most prevalent α1β2γ2 synaptic GABARs) respond to saturating GABA (>1 mM peak GABA concentrations) and show high efficacy but fairly low potency (45). In contrast, extrasynaptic receptors (composed of α4δ- or α6δ- and most likely β3-subunits) are activated by persistent and usually nonsaturating ambient GABA concentrations (0.5-1 μM), and, even at saturating GABA concentrations, are characterized by low-current levels (high-potency, low-efficacy receptors). We suggest a model where EtOH and other anesthetics lead to an increase in GABA efficacy (increase in open probability and/or possibly single-channel conductance), which leads to increased Cl- current. A massive increase in GABA-activated Cl- conductance by anesthetics could completely silence neurons expressing δ-subunit-containing GABARs, thereby producing anesthesia. The activation of extrasynaptic GABARs is functionally equivalent to activation of background K+ channels. G protein-coupled inwardly rectifying K+ (GIRK) channels have been shown to respond to fairly low concentrations of EtOH (3, 4) and may mediate EtOH analgesic actions (5). GIRK channels may also contribute to the anesthetic actions of volatile anesthetics (48, 49). The two-pore K+ channels, TASK1 and TREK, are likely targets for volatile anesthetics (35, 36, 50, 51). The functional similarity between extrasynaptic GABARs and two-pore K+ channels is supported by the finding that, in cerebellar granule cells, a background potassium channel (most likely TASK1), compensates for the loss of extrasynaptic GABARs in mice lacking the GABAR α6-subunit (37).
GABAR α6-subunit knock-out mice not only lack α6-but also δ-subunit protein in cerebellar granule cells (22), and lose “tonic” GABAR currents (37). Cerebellar granule cells compensate for this loss of extrasynaptic GABA receptors in α6(-/-) mice by increasing their background leak conductance by expressing K+ channels with properties characteristic for the two-pore-domain K+ channel TASK1 (37). The up-regulation of TASK1 and possibly other proteins may explain why α6(-/-) mice do not show motor deficits or changes in response to EtOH or other general anesthetics, including barbiturates (38). Although in β3-subunit knock-out mice a significant reduction occurred in etomidate sensitivity of loss of righting reflex (39), the fairly moderate reduction is surprising given that mice carrying a single knock-in point mutation in the β3-subunit (N256M, which makes β3-containing receptors insensitive to etomidate in vitro) essentially eliminates etomidate- and propofol-induced anesthesia (40).
Consistent with the notion that δ GABARs are important targets for anesthetics, mice globally lacking the GABAR δ-subunit show not only a reduction in sensitivity to neurosteroid anesthetics but also a reduction in etomidate sensitivity (41). δ(-/-) mice also show defects in their behavioral responses to ethanol, with reduced ethanol consumption, attenuated withdrawal from chronic ethanol exposure, and reduced seizure-protective effects of ethanol (42). However, these mice still have normal anxiolytic and hypothermic EtOH responses and develop both chronic and acute tolerance. Taken together, compensatory homeostatic mechanisms may mask EtOH effects in straight α6 and β3 knock-out mice and some of the EtOH effects in δ-subunit knock-out animals. Based on our findings, it may be interesting to reevaluate EtOH and anesthetic effects in these knock-out animals.
We found that the β3-subunit is required for high EtOH sensitivity of α6δ- or α4δ-subunit-containing GABARs and, therefore, if these receptors are important for in vivo EtOH effects, it would be expected that the β3-subunit may be assembled in extrasynaptic α6βδ or α4βδ GABARs preferentially over other β-subtypes. The fact that the GABAR β3(N256M) mutation in mice produces an almost complete loss of anesthetic etomidate's effects in vivo and almost perfectly mirrors the loss of etomidate effect of this mutation in recombinant systems is surprising because β2 (but not β1)-subunit-containing receptors would be expected to be sensitive to etomidate in vivo as well (29, 40, 43). In marked contrast to the β3(N256M) mice, the etomidate-insensitive β2(N265S) mutant, when introduced into knock-in mice, does not abolish anesthesia, but eliminates most of the sedative effects of etomidate (30). An exclusive association of extrasynaptic α4δ (and α6δ) with β3-subunits (but not β2- or β1-subunits) to form the etomidate-sensitive anesthetic GABARs would provide a plausible explanation for the almost complete loss of anesthetic etomidate effects in the β3(N256M) mice. The depth and duration of sedation by etomidate and other GABAR-specific anesthetics is augmented by their actions on synaptic αβ2γ-subunit-containing receptors (leading to increased IPSP/C decay times), and these effects are drastically reduced in the β2(N265S) mutant mice (30). Along those lines, GABARs composed of α1β2γ2-subunits are thought to represent ≈50% of all GABARs in mammalian brain, and, because GABAR β2- and β3-subunits are each estimated to constitute ≈50% of total β-subunits (the β1-subunit is a rare subunit) (34), most GABARs other than primarily synaptic α1β2γ2 GABARs must contain the β3-subunit. These GABARs would include synaptic α2- and α3-containing receptors and probably most extrasynaptic receptors.
GABA as a Partial Agonist: Ethanol and Anesthetics Increase Efficacy. Oocytes expressing δ-subunit-containing receptors have only low-current levels. Although we cannot exclude that single-channel conductance increases with EtOH, etomidate, and THDOC (44), the 20-fold increase of GABA current produced by etomidate at saturating GABA concentrations is probably due to a low open probability with GABA alone. This low open probability provides an explanation for the apparent “expression problems” of δ-subunit-containing receptors. Therefore, GABA has only poor efficacy at δ-subunit-containing receptors and might be considered a partial agonist. The dramatic increase in GABA peak currents by anesthetics suggests that they may convert the partial agonist GABA into a full agonist at this receptor subtype.
Although GABAR-specific anesthetics (particularly etomidate) are more efficacious on δ-subunit-containing receptors than on γ-subunit-containing receptors (19), they also activate γ2-containing (generally synaptic) GABARs [usually evaluated at nonsaturating (EC10 or EC50) GABA concentrations]. The fact that inhibitory postsynaptic currents (and potentials) rarely show peak increases in response to GABAR-enhancing agents (benzodiazepine and anesthetics), but rather a slowed IPSP/C decay, suggests that (i) synaptic GABARs are usually activated by saturating (≈1 mM) GABA concentrations in the synaptic cleft and (ii) GABA is a full agonist at these receptors and leads to a nearly full receptor activation, which leaves only little or no room for peak current increases (26, 45-47). However, the stabilization of receptors in the open state by anesthetics may lead to the observed slowing of IPSP/C decay due to slower closing rates and/or decreased desensitization rates. It is therefore likely that the sedative benzodiazepine-like effects of GABAR-specific anesthetics are mediated by effects on synaptic, γ GABARs (see Fig. 4).
In summary, we show that GABARs composed of α4β3δ- and α6β3δ-subunits, which others have shown to be located extra-synaptically, are activated by low concentrations of EtOH, and that the β3-subunit is required for effects of EtOH at these low concentrations. Previous studies apparently have not noted this potent action because δ-subunits were not studied in recombinant systems, and synaptic currents, generated by γ2-rather than δ-containing GABARs, have been emphasized in neuronal studies. Because the same receptors that we find to be sensitive to low concentrations of EtOH also show dramatic enhancement with general anesthetics, we propose and argue for a simple model (Fig. 4), where extrasynaptic receptors are the primary targets for EtOH, other GABAR-specific anesthetics, and steroids. Stell et al.‡ have shown that neurosteroids enhance tonic conductance generated by δ-containing GABARs. It will be important to determine whether EtOH (and other GABAR-specific anesthetics) enhance extrasynaptic currents at comparable concentrations in neurons expressing α4βδ- and α6βδ-receptors (but not in those neurons that do not) and if this action mediates physiological effects.
Table 2. Ethanol enhancement of GABAR.
| Receptors | n | 3 mM EtOH | 30 mM EtOH | 100 mM EtOH | 300 mM EtOH |
|---|---|---|---|---|---|
| α4β3δ and α6β3δ | 25 | 15.6 ± 0.8* | 74.8 ± 1.5* | 109.5 ± 1.9* | 199.0 ± 2.6 |
| α4β2δ and α6β2δ | 29 | 0 | 20.8 ± 2.7* | 57.1 ± 7.0* | 169.8 ± 5.1 |
| α1β2γ2L | 11 | 0 | 0 | 36.7 ± 3.4 | 150.1 ± 12.9 |
| α6β3γ2L | 10 | 0 | 0 | 35.7 ± 4.6 | 154.0 ± 11.0 |
| α4β2γ2s, α6β2γ2s, α4β3γ2s, and α6β3γ2s | 36 | 0 | 0 | 29.3 ± 2.3* | 134.4 ± 3.9* |
| α4β2, α6β2, α4β3, and α6β3 | 21 | 0 | 0 | 2.4 ± 0.4 | 3.6 ± 0.3 |
Values are increase of GABA EC20 peak responses in percent ± SD at the indicated EtOH concentrations with different subunit combination. Values for α4- and α6-receptors were pooled, and all those for β2- and β3-receptors in αβγ and αβ combinations. Asterisks indicate significant differences (P < 0.005); e.g., a significant difference occurs in EtOH enhancement between αβγ and αβ, but no significant difference (P > 0.05) was seen between αβ2δ and αβγ (Student's paired t test).
Acknowledgments
We thank Dr. Tom Otis for discussions and critical comments on the manuscript and Bob Silverman for expert technical help. This work was supported by National Institutes of Health Grants NS35985, AA07680, and GM58448.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: THDOC, tetrahydrodeoxycorticosterone; GABA, γ-aminobutyric acid; GABAR, GABA type A receptor; EtOH, ethanol; IPSP/C, inhibitory postsynaptic potential/inhibitory postsynaptic current.
Footnotes
Stell, B. M., Brickley, S., Farrant, M., Tang, C. Y. & Mody, I. (2002) Soc. Neurosci. Abstr. 148.7.
References
- 1.Lovinger, D. M., White, G. & Weight, F. F. (1989) Science 243, 1721-1724. [DOI] [PubMed] [Google Scholar]
- 2.Woodward, J. J. (2000) Crit. Rev. Neurobiol. 14, 69-89. [DOI] [PubMed] [Google Scholar]
- 3.Carta, M., Ariwodola, O. J., Weiner, J. L. & Valenzuela, C. F. (2003) Proc. Natl. Acad. Sci. USA 100, 6813-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovinger, D. M. & White, G. (1991) Mol. Pharmacol. 40, 263-270. [PubMed] [Google Scholar]
- 5.Mihic, S. J., Ye, Q., Wick, M. J., Koltchine, V. V., Krasowski, M. D., Finn, S. E., Mascia, M. P., Valenzuela, C. F., Hanson, K. K., Greenblatt, E. P., et al. (1997) Nature 389, 385-389. [DOI] [PubMed] [Google Scholar]
- 6.Lei, Q., Jones, M. B., Talley, E. M., Schrier, A. D., McIntire, W. E., Garrison, J. C. & Bayliss, D. A. (2000) Proc. Natl. Acad. Sci. USA 97, 9771-9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguayo, L. G., Peoples, R. W., Yeh, H. H. & Yevenes, G. E. (2002) Curr. Top. Med. Chem. 2, 869-885. [DOI] [PubMed] [Google Scholar]
- 8.Roberto, M., Madamba, S. G., Moore, S. D., Tallent, M. K. & Siggins, G. R. (2003) Proc. Natl. Acad. Sci. USA 100, 2053-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi, T., Ikeda, K., Kojima, H., Niki, H., Yano, R., Yoshioka, T. & Kumanishi, T. (1999) Nat. Neurosci. 2, 1091-1097. [DOI] [PubMed] [Google Scholar]
- 10.Lewohl, J. M., Wilson, W. R., Mayfield, R. D., Brozowski, S. J., Morrisett, R. A. & Harris, R. A. (1999) Nat. Neurosci. 2, 1084-1090. [DOI] [PubMed] [Google Scholar]
- 11.Sundstrom-Poromaa, I., Smith, D. H., Gong, Q. H., Sabado, T. N., Li, X., Light, A., Wiedmann, M., Williams, K. & Smith, S. S. (2002) Nat. Neurosci. 5, 721-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie, Z., Madamba, S. G. & Siggins, G. R. (2000) J. Pharmacol. Exp. Ther. 293, 654-661. [PubMed] [Google Scholar]
- 13.Suzdak, P. D., Schwartz, R. D., Skolnick, P. & Paul, S. M. (1986) Proc. Natl. Acad. Sci. USA 83, 4071-4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allan, A. M. & Harris, R. A. (1987) Recent Dev. Alcohol 5, 313-325. [DOI] [PubMed] [Google Scholar]
- 15.Mehta, A. K. & Ticku, M. K. (1988) J. Pharmacol. Exp. Ther. 246, 558-564. [PubMed] [Google Scholar]
- 16.Wan, F. J., Berton, F., Madamba, S. G., Francesconi, W. & Siggins, G. R. (1996) Proc. Natl. Acad. Sci. USA 93, 5049-5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena, N. C. & Macdonald, R. L. (1994) J. Neurosci. 14, 7077-7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wohlfarth, K. M., Bianchi, M. T. & Macdonald, R. L. (2002) J. Neurosci. 22, 1541-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown, N., Kerby, J., Bonnert, T. P., Whiting, P. J. & Wafford, K. A. (2002) Br. J. Pharmacol. 136, 965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mody, I. (2001) Neurochem. Res. 26, 907-913. [DOI] [PubMed] [Google Scholar]
- 21.Sur, C., Farrar, S. J., Kerby, J., Whiting, P. J., Atack, J. R. & McKernan, R. M. (1999) Mol. Pharmacol. 56, 110-115. [DOI] [PubMed] [Google Scholar]
- 22.Jones, A., Korpi, E. R., McKernan, R. M., Pelz, R., Nusser, Z., Makela, R., Mellor, J. R., Pollard, S., Bahn, S., Stephenson, F. A., et al. (1997) J. Neurosci. 17, 1350-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nusser, Z., Sieghart, W. & Somogyi, P. (1998) J. Neurosci. 18, 1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nusser, Z. & Mody, I. (2002) J. Neurophysiol. 87, 2624-2628. [DOI] [PubMed] [Google Scholar]
- 25.Peng, Z., Hauer, B., Mihalek, R. M., Homanics, G. E., Sieghart, W., Olsen, R. W. & Houser, C. R. (2002) J. Comp. Neurol. 446, 179-197. [DOI] [PubMed] [Google Scholar]
- 26.Cagetti, E., Liang, J., Spigelman, I. & Olsen, R. W. (2003) Mol. Pharmacol. 63, 53-64. [DOI] [PubMed] [Google Scholar]
- 27.Adkins, C. E., Pillai, G. V., Kerby, J., Bonnert, T. P., Haldon, C., McKernan, R. M., Gonzalez, J. E., Oades, K., Whiting, P. J. & Simpson, P. B. (2001) J. Biol. Chem. 276, 38934-38939. [DOI] [PubMed] [Google Scholar]
- 28.Belelli, D., Casula, A., Ling, A. & Lambert, J. J. (2002) Neuropharmacology 43, 651-661. [DOI] [PubMed] [Google Scholar]
- 29.Jurd, R., Arras, M., Lambert, S., Drexler, B., Siegwart, R., Crestani, F., Zaugg, M., Vogt, K. E., Ledermann, B., Antkowiak, B., et al. (2003) FASEB J. 17, 250-252. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds, D. S., Rosahl, T. W., Cirone, J., O'Meara, G. F., Haythornthwaite, A., Newman, R. J., Myers, J., Sur, C., Howell, O., Rutter, A. R., et al. (2003) J. Neurosci. 23, 8608-8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wafford, K. A., Thompson, S. A., Thomas, D., Sikela, J., Wilcox, A. S. & Whiting, P. J. (1996) Mol. Pharmacol. 50, 670-678. [PubMed] [Google Scholar]
- 32.Mihic, S. J., Whiting, P. J. & Harris, R. A. (1994) Eur. J. Pharmacol. 268, 209-214. [DOI] [PubMed] [Google Scholar]
- 33.Koski, A., Ojanpera, I. & Vuori, E. (2002) Alcohol Clin. Exp. Res. 26, 956-959. [DOI] [PubMed] [Google Scholar]
- 34.Whiting, P., Wafford, K. & McKernan, R. M. (2000) in GABA in the Nervous System: The View at Fifty Years, eds. Martin, D. L. & Olsen, R. W. (Lippincot Williams & Wilkins, Philadelphia), pp. 113-126.
- 35.Lesage, F. (2003) Neuropharmacology 44, 1-7. [DOI] [PubMed] [Google Scholar]
- 36.Talley, E. M., Sirois, J. E., Lei, Q. & Bayliss, D. A. (2003) Neuroscientist 9, 46-56. [DOI] [PubMed] [Google Scholar]
- 37.Brickley, S. G., Revilla, V., Cull-Candy, S. G., Wisden, W. & Farrant, M. (2001) Nature 409, 88-92. [DOI] [PubMed] [Google Scholar]
- 38.Homanics, G. E., Ferguson, C., Quinlan, J. J., Daggett, J., Snyder, K., Lagenaur, C., Mi, Z. P., Wang, X. H., Grayson, D. R. & Firestone, L. L. (1997) Mol. Pharmacol. 51, 588-596. [DOI] [PubMed] [Google Scholar]
- 39.Quinlan, J. J., Homanics, G. E. & Firestone, L. L. (1998) Anesthesiology 88, 775-780. [DOI] [PubMed] [Google Scholar]
- 40.Siegwart, R., Jurd, R. & Rudolph, U. (2002) J. Neurochem. 80, 140-148. [DOI] [PubMed] [Google Scholar]
- 41.Mihalek, R. M., Banerjee, P. K., Korpi, E. R., Quinlan, J. J., Firestone, L. L., Mi, Z. P., Lagenaur, C., Tretter, V., Sieghart, W., Anagnostaras, S. G., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 12905-12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mihalek, R. M., Bowers, B. J., Wehner, J. M., Kralic, J. E., VanDoren, M. J., Morrow, A. L. & Homanics, G. E. (2001) Alcohol Clin. Exp. Res. 25, 1708-1718. [PubMed] [Google Scholar]
- 43.Belelli, D., Lambert, J. J., Peters, J. A., Wafford, K. & Whiting, P. J. (1997) Proc. Natl. Acad. Sci. USA 94, 11031-11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eghbali, M., Birnir, B. & Gage, P. W. (2003) J. Physiol. 552, 13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mody, I., De Koninck, Y., Otis, T. S. & Soltesz, I. (1994) Trends Neurosci. 17, 517-525. [DOI] [PubMed] [Google Scholar]
- 46.Belelli, D., Muntoni, A. L., Merrywest, S. D., Gentet, L. J., Casula, A., Callachan, H., Madau, P., Gemmell, D. K., Hamilton, N. M., Lambert, J. J., et al. (2003) Neuropharmacology 45, 57-71. [DOI] [PubMed] [Google Scholar]
- 47.Manuel, N. A. & Davies, C. H. (1998) Br. J. Pharmacol. 125, 1529-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weigl, L. G. & Schreibmayer, W. (2001) Mol. Pharmacol. 60, 282-289. [DOI] [PubMed] [Google Scholar]
- 49.Yamakura, T., Lewohl, J. M. & Harris, R. A. (2001) Anesthesiology 95, 144-153. [DOI] [PubMed] [Google Scholar]
- 50.Sirois, J. E., Lei, Q., Talley, E. M., Lynch, C., III, & Bayliss, D. A. (2000) J. Neurosci. 20, 6347-6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franks, N. P. & Lieb, W. R. (1988) Nature 333, 662-664. [DOI] [PubMed] [Google Scholar]