Abstract
The nitrile stretching vibration is increasingly used as a sensitive infrared probe of local protein environments. However, site-specific incorporation of a nitrile moiety into proteins is difficult. Here we show that various aromatic nitriles can be easily incorporated into peptides and proteins via either thiol alkylation or arylation reaction.
The C≡N (nitrile) stretching vibration has recently emerged as a valuable infrared (IR) probe of the conformation and local environment of biological molecules 1–26 due to its sensitivity to various factors,13,14,17 such as local electric field and hydrogen bonding interactions.27 For example, it has been used to probe peptide insertion into membranes,6 protein-ligand interactions,25 and the dehydration status of an antimicrobial peptide encapsulated in reverse micelles.8 For chemically synthesizable peptides, site-specific incorporation of a nitrile group is readily achievable through the use of nitrile-derivatized non-natural amino acids, such as cyanoalanine (AlaCN) andp-cyanophenylalanine (PheCN). For proteins that cannot be chemically synthesized, however, selective incorporation of a nitrile moiety is rather difficult. Currently, only the chemical method developed by Boxer and coworkers is available, which directly converts a cysteine thiol into a thiocyanate.7 Additionally, it has been shown that PheCN can be incorporated into proteins by using an orthogonal tRNA-synthetase pair,28,29 but the techniques involved are time-intensive and available to only a handful of laboratories worldwide. Thus, it would be quite helpful to develop both an alternative and easier method for selective incorporation of different nitrile moieties into proteins. Here, we show that cysteine alkylation and arylation reactions under mild conditions can be used for such a purpose.
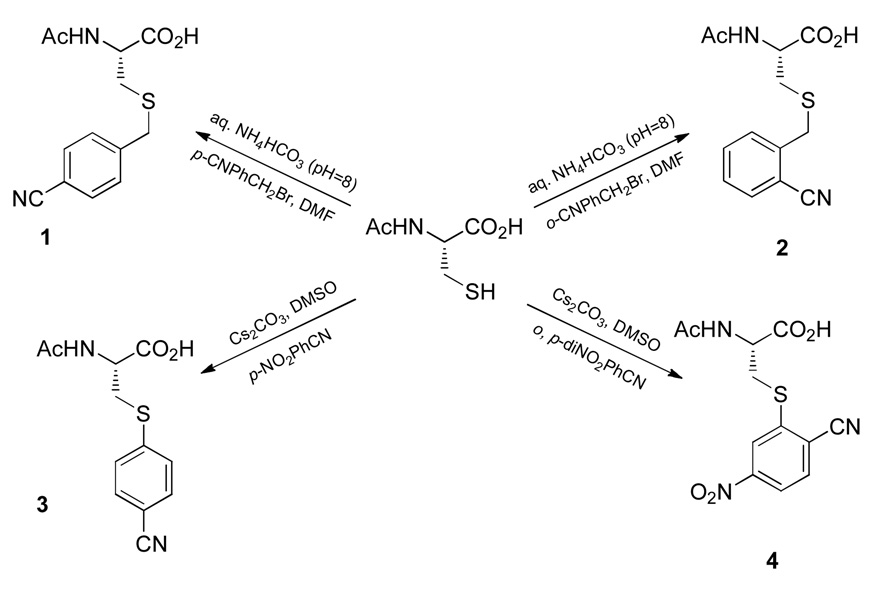
We tested the feasibility of the proposed method on four cyanobenzyl derivatives (Scheme 1), based on the consideration that the oscillator strength and stark tuning rate of aromatic nitriles are normally larger than those of alkyl nitriles.3,4 As shown (Scheme 1), these model probes can be quite easily attached to the cysteine sidechain via either thiol alkylation or arylation.30–32 Similar to that of PheCN,3 the C≡N stretching frequency of these nitrile derivatives in water is found to be in the range of 2233 – 2241 cm−1 (Table 1 and Figure S1 in Supporting Information), with the exact value depending on the molecular structure (e.g., the band of the benzylic derivatives shows an approximately 3 cm−1 shift to higher energy as compared to that of the aryl derivatives). Since any interactions that decrease/increase the electron density of the C≡N bond will result in an increase/decrease in the C≡N stretching vibrational frequency of nitriles,33 these results can be understood qualitatively in the context of the effect of an activating substituent on the cyanobenzyl ring (i.e., sulfur versus methylene and para versus ortho position with respect to the nitrile group). Furthermore, in comparison with those obtained in water, the C≡N stretching bands of these probes in tetrahydrofuran (THF) show a 7–8 cm−1 shift toward lower frequency and also a concomitant decrease in the bandwidth by approximately a factor of two (Table 1), demonstrating the potential utility of these aromatic nitriles as local environmental probes.
Scheme 1.
TABLE 1.
Band position (ν), full width at half maximum (Δν), and the estimated molar extinction coefficient (ε) of the C≡N stretching vibration of various probes in H2O and THF.
| Probe | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| ν(cm−1, H2O) | 2236.6 | 2240.3 | 2233.7 | 2237.9 |
| Δν(cm−1, H2O) | 11.8 | 14.4 | 11.2 | 16.4 |
| ν(cm−1, THF) | 2228.5 | 2232.3 | 2226.8 | 2229.6 |
| Δν(cm−1, THF) | 7.4 | 7.8 | 6.1 | 7.1 |
| ε (M−1cm−1) | 210 ± 60 | 22 ± 10 | 240 ± 60 | 24 ± 10 |
Moreover, it is interesting to note that in aqueous solution the C≡N stretching bandwidths of 2 and 4 are noticeably larger than those of 1 and 3. This finding is consistent with the study of Waegele et al.,34 which showed that the C≡N stretching vibration of an aromatic nitrile can be influenced by direct interactions between the nitrile group and solvent molecules and also indirectly by solvation status of the aromatic ring. In other words, the larger bandwidth of 2 and 4 arises most likely from their asymmetric molecular shape (with respect to the nitrile group), which leads to a more heterogeneous solvation of the respective aromatic ring and hence a broader vibrational transition.
Considering the fact that the synthesis of 1 and 2 involves much milder conditions (e.g., the reaction solution contains only a small amount of organic solvent and similar conditions have been used in chemical modification of proteins35) than those used in the synthesis of 3 and 4 and that the extinction coefficient of the C≡N stretching vibration of 1 and 3 is about an order of magnitude larger than that of 2 and 4 (Figure S1 in Supporting Information), only probe 1 is used in the subsequent proof-of-principle tests involving peptides and proteins.
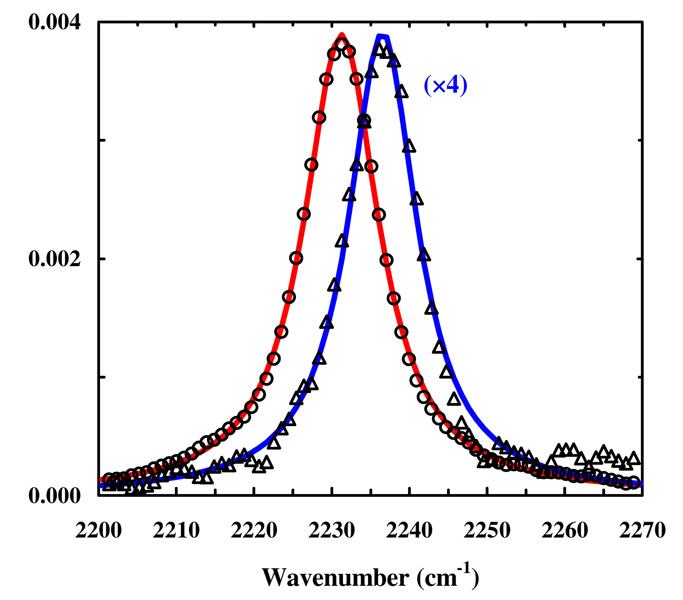
First, the method of cysteine alkylation is applied to two cysteine mutants of mastoparan-X (MpX), W3C and A8C. These mutants are chosen because upon association with calmodulin (CaM) the sidechains of Trp3 and Ala8 of MpX are known to situate inside the peptide-protein binding groove and, as a result, become less solvent-exposed.36 It is found that both peptides are efficiently labeled by p-cyanobenzyl bromide with >80% yield (determined by LC-MS) under the conditions specified in Scheme 1 (the corresponding nitrile-containing peptides are referred to hereafter as W3C-CN and A8C-CN). The FTIR spectra of W3C-CN (Figure 1) and A8C-CN (Figure S2 in supporting Information) also support the site-specific incorporation of probe 1 into these peptides, as their C≡N stretching bands in aqueous solution are centered at ~2236.5 cm−1 but shift to lower wavenumbers upon binding to CaM, as expected. In addition, the nitrile bandwidth of the free peptide is found to be slightly narrower than that of the peptide-CaM complex, due likely to the fact that both bound and unbound peptides exist in the complex solution. A similar finding was also observed in a previous study.3
FIGURE 1.
The C≡N stretching bands of W3C-CN obtained in the presence (open circles) and absence (open triangles) of CaM (50 mM HEPES buffer, pH 7.4, 30 mM CaCl2). For the former case, the concentrations of W3C-CN and CaM were estimated to be 1–2 mM. Lines are respective fits of these data to a Lorentzian function with the following parameters: for W3C-CN ν = 2236.5 cm−1 and Δν = 10.7 cm−1, for W3C-CN/CaM ν = 2231.2 cm−1 and Δν = 11.7 cm−1.
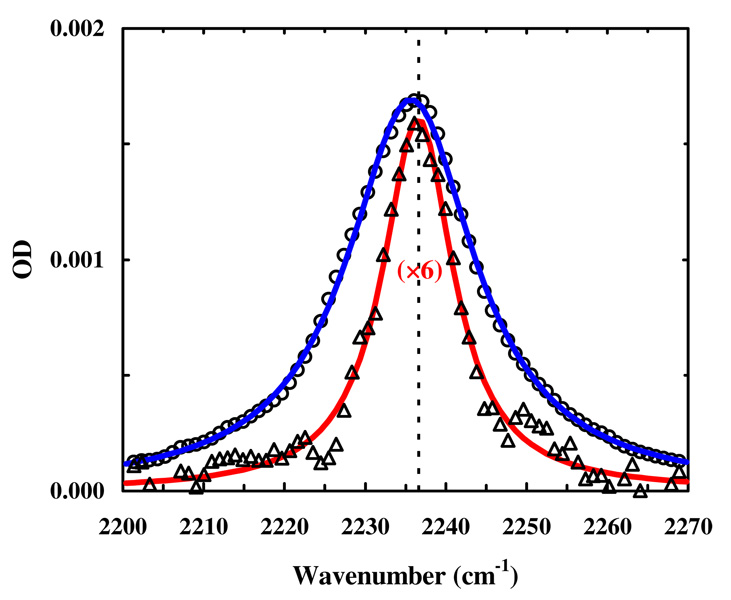
Second, the applicability of the cysteine alkylation reaction to proteins is tested by applying it to human calmodulin-like protein CALM3, which contains a unique cysteine residue (Supporting Information). As shown (Figure 2), the IR spectrum of the reaction product confirms the successful incorporation of probe 1 into the protein of interest and the corresponding yield was estimated to be >50%. More importantly, the far-UV CD spectrum of the nitrile-labeled CALM3 is almost identical to that of the parent protein (Figure S3 in Supporting Information), indicating that the labeling reaction does not change the structural integrity of the protein in question. The latter notion is further corroborated by the dependence of the bandwidth of the C≡N stretching vibration on Ca2+. As indicated (Figure 2), upon addition of Ca2+ the bandwidth of the IR transition is decreased from ~19 cm−1 to ~11 cm−1, a phenomenon expected to occur as Ca2+ is known to rigidify the calcium-binding domain37,38 where the labeled-cysteine is located. In addition, the peak position of this nitrile band (ν = 2236.0 cm−1) does not change upon addition of Ca2+, which is consistent with the fact that the labeled cysteine residue is exposed to solvent in CaM structures obtained in both the absence and presence of Ca2+.38
FIGURE 2.
The C≡N stretching band of the nitrile-labeled human calmodulin-like protein (in 50 mM HEPES buffer, pH 7.4) in the absence (open circles) and presence (open triangles) of Ca2+ (30 mM). The protein concentrations were approximately 1–2 mM (blue) and 200 µM (red), respectively. The solid lines are respective fits of these data to a Lorentzian function and the thin dashed line indicates the peak position of the nitrile band of probe 1 in water.
In conclusion, we have demonstrated a post-translational method allowing site-specific incorporation of nitrile-based IR probes into peptides and proteins via cysteine alkylation or arylation. Because this method involves relatively routine and mild reaction conditions, we expect that it will find wide application in biophysical studies of proteins.
Supplementary Material
ACKNOWLEDGEMENT
We thank the NIH (GM-065978) and the NSF (DMR05-20020) for funding.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Materials, methods and FTIR spectra are available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Yoshikawa S, Okeeffe DH, Caughey WS. Investigations of cyanide as an infrared probe of hemeprotein ligand-binding sites. J. Biol. Chem. 1985;260:3518–3528. [PubMed] [Google Scholar]
- 2.Reddy KS, Yonetani T, Tsuneshige A, Chance B, Kushkuley B, Stavrov SS, Vanderkooi JM. Infrared spectroscopy of the cyanide complex of Iron(II) myoglobin and comparison with complexes of microperoxidase and hemoglobin. Biochemistry. 1996;35:5562–5570. doi: 10.1021/bi952596m. [DOI] [PubMed] [Google Scholar]
- 3.Getahun Z, Huang CY, Wang T, De Leon B, DeGrado WF, Gai F. Using nitrile-derivatized amino acids as infrared probes of local environment. J. Am. Chem. Soc. 2003;125:405–411. doi: 10.1021/ja0285262. [DOI] [PubMed] [Google Scholar]
- 4.Suydam IT, Boxer SG. Vibrational Stark effects calibrate the sensitivity of vibrational probes for electric fields in proteins. Biochemistry. 2003;42:12050–12055. doi: 10.1021/bi0352926. [DOI] [PubMed] [Google Scholar]
- 5.Huang CY, Wang T, Gai F. Temperature dependence of the CN stretching vibration of a nitrile-derivatized phenylalanine in water. Chem. Phys. Lett. 2003;371:731–738. [Google Scholar]
- 6.Tucker MJ, Getahun Z, Nanda V, DeGrado WF, Gai F. A new method for determining the local environment and orientation of individual side chains of membrane-binding peptides. J. Am. Chem. Soc. 2004;126:5078–5079. doi: 10.1021/ja032015d. [DOI] [PubMed] [Google Scholar]
- 7.Fafarman AT, Webb LJ, Chuang JI, Boxer SG. Site-specific conversion of cysteine thiols into thiocyanate creates an IR probe for electric fields in proteins. J. Am. Chem. Soc. 2006;128:13356–13357. doi: 10.1021/ja0650403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee S, Chowdhury P, DeGrado WF, Gai F. Site-specific hydration status of an amphipathic peptide in AOT reverse micelles. Langmuir. 2007;23:11174–11179. doi: 10.1021/la701686g. [DOI] [PubMed] [Google Scholar]
- 9.Maienschein-Cline MG, Londergan CH. The CN stretching band of aliphatic thiocyanate is sensitive to solvent dynamics and specific solvation. J. Phys. Chem. A. 2007;111:10020–10025. doi: 10.1021/jp0761158. [DOI] [PubMed] [Google Scholar]
- 10.Watson MD, Gai XS, Gillies AT, Brewer SH, Fenlon EE. A vibrational probe for local nucleic acid environments: 5-Cyano-2’-deoxyuridine. J. Phys. Chem. B. 2008;112:13188–13192. doi: 10.1021/jp8067238. [DOI] [PubMed] [Google Scholar]
- 11.Fang C, Bauman JD, Das K, Remorino A, Arnold E, Hochstrasser RM. Two-dimensional infrared spectra reveal relaxation of the nonnucleoside inhibitor TMC278 complexed with HIV-1 reverse transcriptase. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1472–1477. doi: 10.1073/pnas.0709320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindquist BA, Haws RT, Corcelli SA. Optimized quantum mechanics/molecular mechanics strategies for nitrile vibrational probes: Acetonitrile and para-tolunitrile in water and tetrahydrofuran. J. Phys. Chem. B. 2008;112:13991–14001. doi: 10.1021/jp804900u. [DOI] [PubMed] [Google Scholar]
- 13.Oh KI, Choi JH, Lee JH, Han JB, Lee H, Cho M. Nitrile and thiocyanate IR probes: molecular dynamics simulation studies. J. Chem. Phys. 2008;128:154504. doi: 10.1063/1.2904558. [DOI] [PubMed] [Google Scholar]
- 14.Lindquist BA, Furse KE, Corcelli SA. Nitrile groups as vibrational probes of biomolecular structure and dynamics: an overview. Phys. Chem. Chem. Phys. 2009;11:8119–8132. doi: 10.1039/b908588b. [DOI] [PubMed] [Google Scholar]
- 15.Taskent-Sezgin H, Chung J, Patsalo V, Miyake-Stoner SJ, Miller AM, Brewer SH, Mehl RA, Green DF, Raleigh DP, Carrico I. Interpretation of p-cyanophenylalanine fluorescence in proteins in terms of solvent exposure and contribution of side-chain quenchers: a combined fluorescence, IR and molecular dynamics study. Biochemistry. 2009;48:9040–9046. doi: 10.1021/bi900938z. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Strzalka J, Tronin A, Johansson JS, Blasie JK. Mechanism of interaction between the general anesthetic halothane and a model ion channel protein, II: fluorescence and vibrational spectroscopy using a cyanophenylalanine probe. Biophys. J. 2009;96:4176–4187. doi: 10.1016/j.bpj.2009.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boxer SG. Stark realities. J. Phys. Chem. B. 2009;113:2972–2983. doi: 10.1021/jp8067393. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh A, Remorino A, Tucker MJ, Hochstrasser RM. 2D IR photon echo spectroscopy reveals hydrogen bond dynamics of aromatic nitriles. Chem. Phys. Lett. 2009;469:325–330. doi: 10.1016/j.cplett.2008.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waegele MM, Tucker MJ, Gai F. 5-Cyanotryptophan as an infrared probe of local hydration status of proteins. Chem. Phys. Lett. 2009;478:249–253. doi: 10.1016/j.cplett.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aschaffenburg DJ, Moog RS. Probing hydrogen bonding environments: solvatochromatic effects on the CN vibration of benzonitrile. J. Phys. Chem. B. 2009;113:12736–12743. doi: 10.1021/jp905802a. [DOI] [PubMed] [Google Scholar]
- 21.Ha JH, Lee KK, Park KH, Choi JH, Jeon SJ, Cho M. Integrated and dispersed photon echo studies of nitrile stretching vibration of 4-cyanophenol in methanol. J. Chem. Phys. 2009;130:204509. doi: 10.1063/1.3140402. [DOI] [PubMed] [Google Scholar]
- 22.Marek P, Mukherjee S, Zanni MT, Raleigh DP. Residue-specific, real-time characterization of lag-phase species and fibril growth during amyloid formation: a combined fluorescence and IR study of pcyanophenylalanine analogs of islet amyloid polypeptide. J. Mol. Biol. 2010;400:878–888. doi: 10.1016/j.jmb.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon HA, Alfieri KN, Clark CAA, Londergan CH. Cyanylated cysteine: a covalently attached vibrational probe of protein-lipid contacts. J. Phys. Chem. Lett. 2010;1:850–855. doi: 10.1021/jz1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inouye H, Gleason KA, Zhang D, Decatur SM, Kirschner DA. Differential effects of Phe19 and Phe20 on fibril formation by amyloidogenic peptide A beta 16–22 (Ac-KLVFFAE-NH2) Proteins: Struct. Funct. Bioinf. 2010;78:2306–2321. doi: 10.1002/prot.22743. [DOI] [PubMed] [Google Scholar]
- 25.Webb LJ, Boxer SG. Electrostatic fields near the active site of human aldose reducatase: 1. New inhibitors and vibrational stark effect measurements. Biochemistry. 2008;47:1588–1598. doi: 10.1021/bi701708u. [DOI] [PubMed] [Google Scholar]
- 26.Stafford AJ, Ensign DL, Webb LJ. Vibrational stark effect spectroscopy at the interface of Ras and Rap1A bound to the Ras binding domain of Ra1GDS reveals an electrostatic mechanism for protein-protein interaction. J. Phys. Chem. B. 2010 doi: 10.1021/jp106974e. DOI: 10.1021/jp106974e. [DOI] [PubMed] [Google Scholar]
- 27.Fafarman AT, Sigala PA, Herschlag D, Boxer SG. Decomposition of vibrational shifts of nitriles into electrostatic and hydrogen-bonding effects. J. Am. Chem. Soc. 2010;132:12811–12813. doi: 10.1021/ja104573b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultz KC, Supekova L, Ryu YH, Xie JM, Perera R, Schultz PG. A genetically encoded infrared probe. J. Am. Chem. Soc. 2006;128:13984–13985. doi: 10.1021/ja0636690. [DOI] [PubMed] [Google Scholar]
- 29.Miyake-Stoner SJ, Miller AM, Hammill JT, Peeler JC, Hess KR, Mehl RA, Brewer SH. Probing protein folding using site-specifically encoded unnatural amino acids as FRET donors with tryptophan. Biochemistry. 2010;48:5953–5962. doi: 10.1021/bi900426d. [DOI] [PubMed] [Google Scholar]
- 30.Kondoh A, Yorimitsu H, Oshima K. Nucleophilic aromatic substitution reaction of nitroarenes with alkyl- or arylthi o groups in dimethyl sulfoxide by means of cesium carbonate. Tetrahedron. 2006;62:2357–2360. [Google Scholar]
- 31.Sano K, Ikegami Y, Uesugi T. Initial intraorgan formation of mercapturic acid. Biol. Pharm. Bull. 2001;24:1324–1328. doi: 10.1248/bpb.24.1324. [DOI] [PubMed] [Google Scholar]
- 32.Timmerman P, Barderas R, Desmet J, Altschuh D, Shochat S, Hollestelle MJ, Hoppener JWM, Monasterio A, Casal JI, Meloen RH. A combinatorial approach for the design of complementarity-determining region-derived peptidomimetics with in Vitro anti-tumoral activity. J. Biol. Chem. 2009;284:34126–34134. doi: 10.1074/jbc.M109.041459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews SS, Boxer SG. Vibrational stark effects of nitriles I. Methods and experimental results. J. Phys. Chem. A. 2000;104:11853–11863. [Google Scholar]
- 34.Waegele MM, Gai F. Computational modeling of the nitrile stretching vibration of 5-cyanoindole in water. J. Phys. Chem. Lett. 2010;1:781–786. doi: 10.1021/jz900429z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MEB, Schumacher FF, Ryan CP, Tedaldi LM, Papaioannou D, Waksman G, Caddick S, Baker JR. Protein modification, bioconjugation, and disulfide bridging using bromomaleimides. J. Am. Chem. Soc. 2010;132:1960–1965. doi: 10.1021/ja908610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikura M, Clore GM, Gronenborn AM, Zhu G, Klee CB, Bax A. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science. 1992;256:632–638. doi: 10.1126/science.1585175. [DOI] [PubMed] [Google Scholar]
- 37.Urbauer JL, Short JH, Dow LK, Wand AJ. Structural analysis of a novel interaction by calmodulin – high-affinity binding of a peptide in the absence of calcium. Biochemistry. 1995;34:8099–8109. doi: 10.1021/bi00025a016. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Tanaka T, Ikura M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 1995;2:758–767. doi: 10.1038/nsb0995-758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.