Abstract
Here we report structural dynamics of equine myoglobin (Mb) in response to the CO photodissociation visualized by picosecond time-resolved X-ray solution scattering. The data clearly reveals new structural dynamics that occurs in the timescale of ~360 picoseconds (ps) and ~9 nanoseconds (ns), which have not been clearly detected in previous studies.
Myoglobin (Mb) is a heme protein that carries small-molecule ligands such as O2, CO and NO in muscles, and can be considered as a subunit of hemoglobin, a paradigm protein for the study of allostery. Due to its small size, availability and photosensitivity of the heme-ligand bond, Mb has served as a prototypical model system for studying protein structural dynamics. Accordingly, structural dynamics of Mb have been intensively studied with various spectroscopic1-8 and structural9-16 tools. The ligands form covalent bonds with Fe2+ of the heme group and can be photolyzed by visible light on sub-picosecond time scale.2-3 Upon the CO photolysis of MbCO, a small portion of the dissociated CO ligands geminately rebind to the heme, while the remainder travels to various pockets that can accommodate the ligand and eventually escapes the protein matrix to the solvent. On a longer time scale, the vacant heme recovers the ligand via non-geminate recombination.
To directly track the structural changes associated with the ligand migration and rebinding and capture structurally distinct intermediates, we used pump-probe time-resolved X-ray solution scattering technique, where the time-dependent scattering of short X-ray pulses from a synchrotron are used to interrogate the structural dynamics of a liquid solution sample that is pumped with optical laser pulses in a pump-probe manner. Time-resolved X-ray solution scattering17-19 together with time-resolved X-ray crystallography20, X-ray absorption spectroscopy21 and electron diffraction21 can provide direct structural information, and thus complements time-resolved optical spectroscopy in the analysis of solution-phase reaction mechanisms. Recently time-resolved solution scattering technique has been applied to follow conformational changes in proteins with nanosecond22-24 and picosecond25 time resolution. Here, we show its application to another type of protein, Mb from equine heart, with picosecond time resolution.
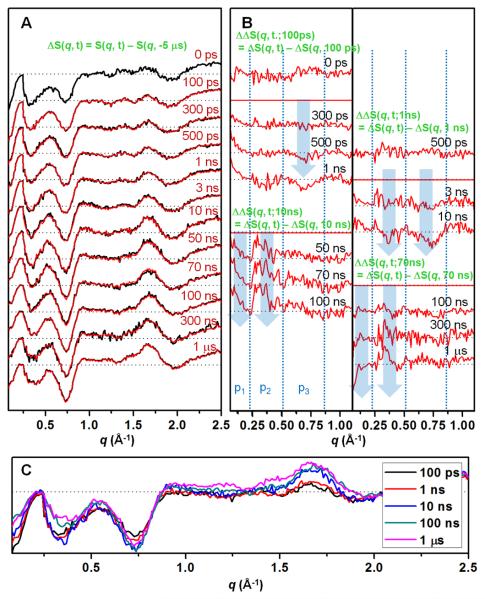
Time-resolved X-ray solution scattering data were measured at 14IDB beamline of Advanced Photon Source. The usual experimental protocol22, 25 was followed. Specifically, equine heart MbCO solution (8 mM, pH 7.0, 0.1 M sodium phosphate) filled in a quartz capillary was excited by a ~30 ps-long laser pulse at 532 nm to initiate the CO photodissociation, and a ~100-ps-long single X-ray pulse was sent to the sample at a well-defined time delay with respect to the arrival of the laser pulse. The scattered X-rays were recorded in a CCD detector as a function of the time delay between the laser and X-ray pulses. Figure 1A shows the difference X-ray scattering data measured for a wide time range spanning from 100 ps to 1 μs. The difference scattering curves, ΔS(q, t), were obtained by subtracting the scattering curve measured at −5 μs from the curves at various (positive) time delays, i.e., ΔS(q, t) = S(q, t) − S(q, −5 μs). Oscillatory features are clear in the difference curves at all time delays. Most of the features in the difference signal develop within 100 ps, but some features still evolve afterwards. To magnify the subtle changes, double difference curves (ΔΔS(q, t; tref) = ΔS(q, t) − ΔS(q, tref)) were obtained between the difference curves at two different time delays using various reference time points (100 ps, 1 ns, 10 ns and 70 ns). For example, ΔΔS(q, t; 100 ps) highlights the change occurring between t and 100 ps time delays. Close inspection of the time-resolved X-ray scattering data reveals four distinct transitions well separated in time scales. The first one occurs at 300 – 500 ps, the second at 1 – 10 ns, the third at 10 – 100 ns, the fourth at 100 ns – 1 μs. Difference curves at representative time delays for each time region are compared in Figure 1C.
Figure 1.
(A) Time-resolved X-ray solution scattering data for MbCO → Mb + CO in solution at representative time delays from 0 ps to 1 μs. The black curves are experimental difference curves (ΔS(q, t) = S(q, t) − S(q, −5 μs)) obtained by subtracting the scattering curve at −5 μs from the curves at other time delays. The red curves are theoretical data obtained from principal component analysis. Here the theoretical data is a linear sum of species-associated scattering curves obeying a simple sequential kinetic model. For the sake of presentation, the signal below q = 0.22 Å−1 were divided by 6. (B) To emphasize subtle changes at the low angle (q = 0.07 - 1.1 Å−1), double difference curves (ΔΔS(q, t; tref) = ΔS(q, t) − ΔS(q, tref)) obtained with various reference time points (100 ps, 1 ns, 10 ns and 70 ns). Each curve was scaled by a factor of 2 to magnify small changes. Transitions in 300 – 500 ps, 1 – 10 ns, 10 – 100 ns and 100 ns – 1 μs time ranges are clearly identified. Blue arrows are shown to guide q positions where major changes occur. In (A) and (B), the data corresponding to a common time delay are shown aligned at the same vertical position. (C) Difference curves at representative time delays are shown in higher magnification than in (A).
The data at 0 ps and 100 ps are identical within our experimental error if the data at 0 ps is doubled (as shown in Figure 1A) to compensate for the delayed signal buildup due to half overlap between the pump and probe pulses at 0 ps. The agreement between the two data indicates that significant degree of tertiary structural changes occurs faster than the time resolution of our experiment, resulting in the 0 ps signal with the same oscillatory features as in 100 ps signal. As time goes on, the negative peak around q = 0.7 Å−1 (p3 region) grows in two steps on the time scales of 300 – 500 ps and 1 – 10 ns. The negative peak around q = 0.35 Å−1 (p2 region) increases (i.e., becomes more negative) on 1 – 10 ns time scale and then decreases (i.e., becomes less negative) in 10 – 100 ns. These changes can be more clearly seen in the double difference curves. For example, ΔΔS(q, 10 ns; 1 ns) is negative whereas ΔΔS(q, 100 ns; 10 ns) is positive around q = 0.35 Å−1. On the time scale of 10 – 100 ns, the negative signal at low q (p1 region) decreases (i.e., becomes less negative) and thus ΔΔS(q, t; 10 ns) becomes more positive on this time scale. In 100 ns – 1 μs, the peak around q = 0.35 Å−1 broadens and the negative signal at low q (p1 region) increases (i.e., becomes more negative).
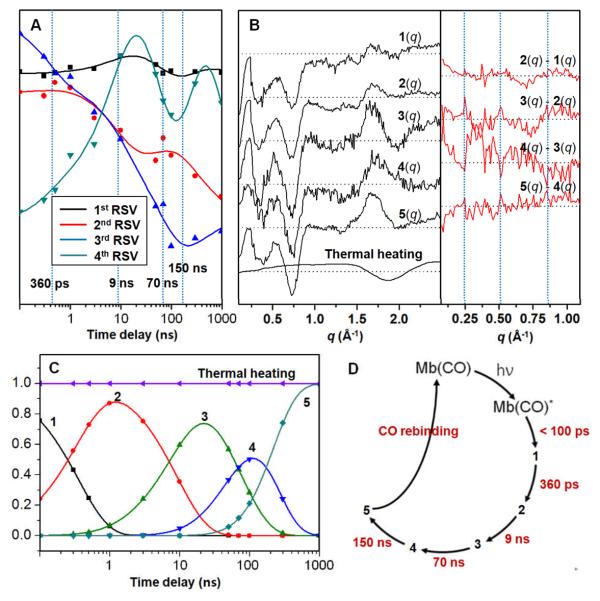
Quantitative analysis was also conducted by using singular value decomposition (SVD) and principal component analysis. We first applied singular value decomposition to the data. The first four principal time-dependent components (right singular vectors) can be simultaneously fitted with four exponentials of distinct time constants; 360 ps, 9 ns, 70 ns and 150 ns (Fig. 2A). We note that these time constants are consistent with the qualitative estimations outlined in Fig. 1. Using these time constants, we then applied principal component analysis using a simple sequential kinetic mechanism. From the analysis, by minimizing the discrepancy between theory and experiment, species-associated difference scattering curves corresponding to five intermediates (1 to 5) were extracted as shown in Fig. 2B. These species-associated scattering curves obey a sequential kinetic model with the time constants extracted from the SVD analysis and theoretical scattering curves at various time delays are well represented by a linear combination of those species-associated scattering curves as shown in Fig. 1. One caveat is that this does not necessarily mean the sequential kinetic model is the best one. The population changes of the reaction intermediates are shown in Fig. 2C and the photocycle of MbCO is summarized in Fig. 2D.
Figure 2.
(A) The first four principal time-dependent components from SVD analysis can be simultaneously fitted with a sum of four exponentials, yielding the following time constants; 360 ps, 9 ns, 70 ns, 150 ns. (B) (Left) Five species-associated difference scattering curves can be extracted via principal component analysis where the time constants from the SVD analysis were used. (Right) The double difference curves between temporally adjacent species are also shown in the same manner as in Fig. 1B, in order to bring out subtle changes at the low angle. (C) Population changes of reaction intermediates. (D) Reaction kinetics of MbCO photolysis.
The intermediates identified with distinct time constants from our analysis actually represent structurally distinct intermediates that are uniquely detected by X-ray scattering and thus give us information complimentary to optical spectroscopy. Structural analysis of the species-associated scattering curves will reveal detailed structural transitions such as time-dependent helix movements, and work is in progress in this regard.23 Here we simply compare the four time constants with those obtained from optical spectroscopy. The late two time scales (τ3→4 = 70 ns and τ4→5 = 150 ns) determined from our TR-WAXS study can be interpreted in the framework of the most well accepted four-state model1 (A→B→C→D→S), where A means the MbCO, B is the Mb with CO still in the heme pocket, C and D are the intermediate states where the CO is located some pockets and S means the Mb with free CO (in other words CO has completely escaped to the solvent). It is well established that B is populated on ultrafast time scale within 10 ps (τAB < 10 ps)26, much shorter than our TR-WAXS time resolution. Various spectroscopic measurements established τCD ~ 70 ns4 and τDS ~ 700 ns4, 27. In particular, the τ3→4 transition coincides with τCD ~ 70 ns decay component of the heterodyne-detected transient grating signal,4 which was assigned to transit time for ligand migration within Mb in the framework of the Also, τ4→5 = 150 ns transition is on the same order of magnitude with the τDS ~ 700 ns decay component in the transient grating signal.4, 7 This component was assigned to the escape of the photodissociated CO from the protein matrix to the solvent based on the fact that the escape of the CO ligand from the protein matrix induces a considerable volume change. It can be seen from Fig. 1 and Fig. 2B that the negative signal at low q values, which is known to be sensitive to the electron density of the protein, increases (i.e., becomes more negative) and this is consistent with the CO escape process. A very similar time constant (~180 ns) assigned to geminate rebinding of the CO molecule was observed from transient absorption spectroscopy28, but our 170 ns process should be related with CO escape since the decreased scattering intensity at low q region cannot be explained by the geminate recombination process alone.
The early two transitions (τ1→2 = 360 ps and τ2→3 = 9 ns) observed in this study have been articulated to a much lesser extent relative to the late two transitions (τ3→4 and τ4→5). The closest to τ1→2 is the ~300 ps decay component of the transient circular dichroism signal of the N band, which was assigned to conformational changes.6 However the later systematic CD study corrected this time scale to less than 100 ps.29 It seems that there has been no direct observation of the B→C process in the framework of the four-state model. According to some indirect estimation, τ BC is about 180 ps1. This time scale is close to the τ1→2 = 360 ps time scale recovered from our data, but whether the τ1→2 component can be assigned to the B→C process is not certain because this B→C process originally refers to the migration of CO rather than the structural transition of the protein matrix itself. The closest similarity to the τ2→3 = 9 ns transition is found in the time-resolved ultraviolet resonance Raman signal measured on the tryptophan and tyrosine Raman bands.5 In that work, it was suggested that the decay component on 1 – 2.5 ns time scale can be attributed to the motion of the A helix toward the displaced E helix.
Recently, CO migration kinetics in a different type of Mb (from sperm whale) was studied using the same technique,25 and the kinetics consisting of the exponentials of 74 ps, 2.7 ns, 220 ns and 5.7 μs time constants were reported. Surprisingly, the kinetics and the shape of the scattering patterns of equine heart Mb and sperm whale Mb turns out to be very different. The origin of the difference in structural dynamics between the two Mb systems will be further investigated.
We acknowledge BioCARS staff for discussions and experimental assistance. This work was supported by the Creative Research Initiatives (Center for Time-Resolved Diffraction) of MEST/NRF. Use of the BioCARS Sector 14 was supported by NIH (RR007707). Time-resolved set-up at Sector 14 was funded in part through a collaboration with Philip Anfinrud (NIH/NIDDK). We acknowledge the support from the WCU program.
Supplementary Material
Footnotes
Electronic supplementary information (ESI) available: data collection protocol, data processing and data analysis.
Notes and references
- 1.Beece D, Eisenstein L, Frauenfelder H, Good D, Marden MC, Reinisch L, Reynolds AH, Sorensen LB, Yue KT. Biochemistry. 1980;19:5147–5157. doi: 10.1021/bi00564a001. [DOI] [PubMed] [Google Scholar]
- 2.Franzen S, Bohn B, Poyart C, Martin JL. Biochemistry. 1995;34:1224–1237. doi: 10.1021/bi00004a016. [DOI] [PubMed] [Google Scholar]
- 3.Richard L, Genberg L, Deak J, Chiu H-L, Miller RJD. Biochemistry. 1992;31:10703–10715. doi: 10.1021/bi00159a010. [DOI] [PubMed] [Google Scholar]
- 4.Dadusc G, Ogilvie JP, Schulenberg P, Marvet U, Miller RJD. Proc. Natl. Acad. Sci. 2001;98:6110–6115. doi: 10.1073/pnas.101130298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sato A, Mizutani Y. Biochemistry. 2005;44:14709–14714. doi: 10.1021/bi051732c. [DOI] [PubMed] [Google Scholar]
- 6.Xie X, Simon JD. Biochemistry. 1991;30:3682–3692. doi: 10.1021/bi00229a013. [DOI] [PubMed] [Google Scholar]
- 7.Sakakura M, Yamaguchi S, Hitota N, Terazima M. J. Am. Chem. Soc. 2001;123:4286–4296. doi: 10.1021/ja9944655. [DOI] [PubMed] [Google Scholar]
- 8.Zhang LY, Wang LJ, Kao YT, Qiu WH, Yang Y, Okobiah O, Zhong D. Proc. Natl. Acad. Sci. 2007;104:18461–18466. doi: 10.1073/pnas.0707647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M, Nienhaus K, Pahl R, Krasselt A, Anderson S, Parak F, Nienhaus GU, Srajer V. Proc. Natl. Acad. Sci. 2005;102:11704–11709. doi: 10.1073/pnas.0504932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourgeois D, Vallone B, Schotte F, Arcovito A, Miele AE, Sciara G, Wulff M, Anfinrud P, Brunori M. Proc. Natl. Acad. Sci. 2003;100:8704–8709. doi: 10.1073/pnas.1430900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostermann A, Waschipky R, Parak FG, Nienhaus GU. Nature. 2000;404:205–208. doi: 10.1038/35004622. [DOI] [PubMed] [Google Scholar]
- 12.Srajer V, Teng T, Ursby T, Pradervand C, Ren Z, Adachi S, Schildkamp W, Bourgeois D, Wulff M, Moffat K. Science. 1996;274:1726–1729. doi: 10.1126/science.274.5293.1726. [DOI] [PubMed] [Google Scholar]
- 13.Schotte F, Lim M, Jackson TA, Smirnov AV, Soman J, Olson JS, Phillips GNJ, Wulff M, Anfinrud P. Science. 2003;300:1944–1947. doi: 10.1126/science.1078797. [DOI] [PubMed] [Google Scholar]
- 14.Quillin ML, Li T, Olson JS, Phillips GN, Jr., Dou Y, Ikeda-Saito M, Regan R, Carlson M, Gibson QHL. J. Mol. Biol. 1995;245:416–436. doi: 10.1006/jmbi.1994.0034. H. [DOI] [PubMed] [Google Scholar]
- 15.Aranda R, Levin EJ, Schotte F, Anfinrud PA, Phillips GN. Acta Cryst. D. 2006;62:776–783. doi: 10.1107/S0907444906017318. [DOI] [PubMed] [Google Scholar]
- 16.Chu K, Vojtechovsky J, McMahon BH, Sweet RM, Berendzen J, Schlichting I. Nature. 2000;403:921–923. doi: 10.1038/35002641. [DOI] [PubMed] [Google Scholar]
- 17.Haldrup T, Lemke HT, Haldrup K, Nielsen TN, Arms DA, Walko DA, Miceli A, Landahl EC, Dufresne EM, Nielsen MM. J. Synch. Rad. 2009;16:387–390. doi: 10.1107/S0909049509004658. [DOI] [PubMed] [Google Scholar]
- 18.Ihee H. Acc. Chem. Res. 2009;42:356–366. doi: 10.1021/ar800168v. [DOI] [PubMed] [Google Scholar]
- 19.Vincent J, Andersson M, Eklund M, Wohri AB, Odelius M, Malmerberg E, Kong QY, Wulff M, Neutze R, Davidsson J. J. Chem. Phys. 2009;130 doi: 10.1063/1.3111401. [DOI] [PubMed] [Google Scholar]
- 20.Moffat K. Chem. Rev. 2001;101:1569–1581. doi: 10.1021/cr990039q. [DOI] [PubMed] [Google Scholar]
- 21.Chergui M, Zewail AH. ChemPhysChem. 2009;10:28–43. doi: 10.1002/cphc.200800667. [DOI] [PubMed] [Google Scholar]
- 22.Cammarata M, Levantino M, Schotte F, Anfinrud PA, Ewald F, Choi J, Cupane A, Wulff M, Ihee H. Nat. Methods. 2008;5:881–886. doi: 10.1038/nmeth.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn S, Kim KH, Kim Y, Kim J, Ihee H. J. Phys. Chem. B. 2009;113:13131–13133. doi: 10.1021/jp906983v. [DOI] [PubMed] [Google Scholar]
- 24.Andersson M, Malmerberg E, Westenhoff S, Katona G, Cammarata M, Wohrl AB, Johansson LC, Ewald F, Eklund M, Wulff M, Davidsson J, Neutze R. Structure. 2009;17:1265–1275. doi: 10.1016/j.str.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Cho HS, Dashdorj N, Schotte F, Graber T, Henning R, Anfinrud P. P Natl Acad Sci USA. 2010;107:7281–7286. doi: 10.1073/pnas.1002951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richard L, Genberg L, Deak J, Chiu H-L, Miller RJD. Biochemistry. 1992:10703–10715. doi: 10.1021/bi00159a010. [DOI] [PubMed] [Google Scholar]
- 27.Sakakura M, Yamaguchi S, Hirota N, Terazima M. J. Am. Chem. Soc. 2001;123:4286–4294. doi: 10.1021/ja9944655. [DOI] [PubMed] [Google Scholar]
- 28.Henry ER, Sommer JH, Hofrichter J, Eaton WA. J Mol Biol. 1983;166:443–451. doi: 10.1016/s0022-2836(83)80094-1. [DOI] [PubMed] [Google Scholar]
- 29.Dartigalongue T, Niezborala C, Hache F. Phys. Chem. Chem. Phys. 2007;9:1611–1615. doi: 10.1039/b616173a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.