Abstract
Epidemiological studies have suggested inverse associations between allergic diseases and malignancies. As a proof of concept for the capability of immunoglobulin E (IgE) to destruct tumor cells, several experimental strategies have evolved to specifically target this antibody class towards relevant tumor antigens. It could be demonstrated that IgE antibodies specific to overexpressed tumor antigens have been superior to any other immunoglobulin class with respect to antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP) reactions. In an alternative approach, IgE nonspecifically attached to tumor cells proved to be a powerful adjuvant establishing tumor-specific immune memory. Active Th2 immunity could also be achieved by applying an oral immunization regimen using mimotopes, i.e. epitope mimics of tumor antigens. The induced IgE antibodies could be cross-linked by live tumor cells leading to tumoricidic mediator release. Thus, IgE antibodies may not only act in natural tumor surveillance, but could possibly also be exploited for tumor control in active and passive immunotherapy settings. Thereby, eosinophils, mast cells and macrophages can be armed with the cytophilic IgE and become potent anti-tumor effectors, able to trace viable tumor cells in the tissues. It is strongly suggested that the evolving new field AllergoOncology will give new insights into the role of IgE-mediated allergy in malignancies, possibly opening new avenues for tumor therapy.
Keywords: AllergoOncology, cancer, eosinophils, IgE, tumoricidic
Before their identification as immunoglobulin E (IgE) antibodies (1–4), research on ‘reagins’ was conducted during five decades (5–7). Since this era, allergology and parasitology have been traditionally considered as a whole and this notion helped in evolving a lively picture of the IgE-mediated immune response. It is, however, more or less neglected today that the association of allergic diseases and tumor occurrence has been discussed already very early (8, 9). In the 1950s, precise experiments investigated ‘allergic responses’ towards tumor transplants (10). Consequently, the observed immunological phenomena were even termed tumor allergy (11). The discussion went on asking about the biological relevance of tumor allergy for tumor progression (12, 13), until a negative association between allergy and cancer was announced for the first time (14). Later, the IgE levels and atopy reactions in the skin of cancer patients were examined (15, 16), finally rendering knowledge that the prevalence of atopy was decreased in cancer patients (17). Passive anaphylaxis or weekly injections of histamine and serotonin inhibited tumor growth in a transplant mouse model, pointing towards a possible role of anaphylactic reactions in tumor immunity. Interestingly, an immunohistochemical study on the distribution of immunoglobulin classes in head and neck cancer revealed IgE antibodies to be the most abundant class, fixed in the cancer tissues on dispersed macrophage-like cells (18). This work suggests that IgE may have a natural surveillance function in malignancies. Advancements in immunology and molecular biology enable us today to go one step further and exploit this knowledge for developing IgE-based targeted cancer therapies.
This review aims at giving a comprehensive overview on recent epidemiological and experimental evidence for the occurrence of Th2-type immune mechanisms in tumor disease. Moreover, also targeted therapies with IgE antibodies and vaccination strategies will be discussed as a novel perspective to combat cancer.
IgE antibodies: prime target unknown
IgE is an evolutionary conserved member of the Ig family with the highest determined affinity to receptors (19–22) and antigens among all antibody classes (23). The titer of IgE is very low (nano- to micrograms per milliliter range) in plasma of normal healthy individuals and of normal laboratory mouse strains, but IgE is most prominent in epithelia and mucosa where it is bound to specific receptors on very potent effector cells like eosinophil or basophil granulocytes and mast cells. This suggests that IgE plays a role in local (rather than systemic) immune defence mechanisms. In these days, IgE is best known for its strong, unwanted effector functions, in the form of allergic reactions (19). However, the prime target for IgE is still unknown. From an evolutionary point of view, IgE is conserved and can be found in all mammalia, including monotremata (24). It therefore originated at least 160 million years ago, possibly even more than 300 million years ago (25), from a gene duplication of IgY, in which the anaphylactic and opsonic activities of IgY were separated, giving rise to IgE and IgG, respectively (26). Apparently, in an evolutionary sense, anaphylactic defence mechanisms are needed. The division of anaphylactic and opsonic activities in separate genes allowed principally a tighter and more specific control of both immune mechanisms.
In the recent past, five B-cell specific control mechanisms have been described that indicate a tight control of the IgE response, in agreement with the arguments shown, and that are different from the opsonic type of response.
IgE has the shortest free serum half-life (t½) of all immunoglobulins averaging 12 h in mice (27) and 1–5 days in humans (28, 29), limiting the danger of a systemic anaphylactic reaction. After production serum IgE is rapidly bound by the high-affinity FcεRI on the surface of effector cells like mast cells and basophils where it acquires a long half-life time (weeks to months).
Several studies with mice deficient of, or overexpressing the low-affinity IgE receptor CD23, clearly demonstrated CD23’s role as a major negative feedback regulator of IgE production. Yu et al. (30) showed that disruption of the CD23 gene led to increased specific IgE levels after immunization with 2,4-dinitrophenyl-ovalbumin (DNP-OVA), while IgG1 levels were twice as high, specific IgE levels were 6–12 times higher in these CD23−/− mice.
Two mIgE knock-out mice (31) underlined the key function of the IgE receptor in the regulation of IgE expression in vivo and by analyzing the phenotypes of the mice strains it could clearly be demonstrated that the antigen receptor is the only device for an effective antigen presentation (32, 33). In the first strain, the intracellular domain of IgE was removed except for three amino acids (Lys, Val, Lys; KVKΔtail line). The cytoplasmic domain of IgE in these mice is the same as that of mIgM and mIgD. In the second line, both the intracellular and transmembrane domains of IgE (ΔM1M2 line) are lacking (Fig. 1). In ΔM1M2 mice serum IgE is reduced to less than 10% of normal mice, while KVKΔ tail mice show a reduction of 50%, reflecting a serious impairment of the IgE-mediated immune response. Upon stimulation of isolated spleen cells of wild type, ΔM1M2 and KVKΔtail mice with LPS and IL4 in vitro, concentrations of IgE and IgG1 in the culture supernatants were comparable in wild-type and mutant mice. These results imply that the reduced IgE titers found in both mutant lines are solely a reflection of the loss of biological activities associated with the transmembrane and cytoplasmic domains of IgE.
The process of alternative polyadenylation restricts surface IgE expression and thus influences further serum IgE production. mRNA for the membrane form of both the murine and human epsilon (ε) heavy chain is poorly expressed, compared with the mRNA for the secreted form in activated, mIgE-bearing B cells (32, 34).
Finally, also the establishment of humoral memory is limited for IgE responses in vivo. IgE plasmablasts have an intrinsic, lower chance to contribute to the long-lived plasma cell pool and thus to humoral immunologic memory than IgG1 plasmablasts. Apparently, an IgE immune response is in all stages of the response negatively regulated. The IgE antibodies may have strong effector functions, but the IgE response is slow and limited in developing memory responses.
Figure 1.

In the immunoglobulin E (IgE)KVKΔtail mouse strain, the intracellular domain of IgE was removed except for the three conserved amino acids (Lys, Val, Lys). Thus, the cytoplasmic domain of IgE in these mice is identical to the cytoplasmic tail expressed by mIgM and mIgD. In the IgEΔM1M2 line, both the intracellular and transmembrane domains of IgE are lacking. The cytoplasmic tails of the membrane immunoglobulins have the capacity to bind proteins, committing a signal transduction pathway which is independent of the well-known Ig-α/Ig-β pathways. The green domains represent Ig heavy chains, red domains indicate Ig light chains, white domain indicates transmembrane domain, clewed domain indicates intracellular tail and pink and brown domains indicate Ig coat proteins Ig-α/Ig-β.
Therefore, the IgE antigen receptor itself plays a major role in the decision of quantity and quality of serum IgE antibodies and besides, is probably the most powerful surface receptor, influencing developmental processes of the cell. It was suggested that the signaling cascade underlies a permanent stochastic fluctuation, which induces no cellular response, but is important for maintenance of cell viability [reviewed in (35)]. Taken together, all these observations point towards the existence of mechanisms to restrain potentially dangerous, but apparently necessary, serum IgE titers.
Epidemiological association of IgE and malignancies
Hints from clinical observations triggered numerous epidemiological studies to examine the potential association between a history of IgE-mediated allergy and cancer. These studies, and important methodological considerations, were summarized in recent review articles (36, 37). Although the results are not entirely clear, there is some limited evidence to suggest a possible inverse relation.
In perhaps the largest study of its kind, over 1.1 million US adults, for whom self-reported physician-diagnosed asthma or hay fever status at baseline was known, were followed-up for a period of 18 years (38). Results suggested a significant inverse association between a history of both asthma and hay fever, possibly the most relevant indicator of allergic status examined here, and all cancer mortality [relative risk (RR) = 0.88, 95% confidence interval (CI) 0.83–0.93]. The risk of mortality from several site-specific cancers was also reduced, with that for colorectal cancer significant (RR = 0.76, 95% CI 0.64–0.91). In a separate analysis of never smokers, results were similar, although they attenuated slightly and were no longer significant. Results from other smaller prospective studies were mixed (39–42), including some who used skin-prick testing to define allergic status (43–45). Conversely, one study recently followed 70 136 patients for whom data on total serum IgE levels were known and 57 815 patients for whom data on allergen-specific IgE levels were known (46). No association with cancer incidence was reported for either measure.
Case-control studies using self-reported allergy history information have also suggested several potential inverse associations between allergic status and site-specific cancers including pancreatic (47) and glioma (48) with the magnitude of the effect ranging from approximately 30–40% reductions in risk. Inverse associations were also reported in studies of childhood leukaemia (49–51) or myeloma of adults (52). Case-control studies, measuring allergen-specific IgE in cases following cancer diagnosis, have reported mixed findings. Wiemels et al. (53) reported inverse associations between glioma and total IgE levels [odds ratio (OR) elevated total IgE = 0.37, 95% CI 0.22–0.64) and allergen-specific IgE levels, particularly food IgE (OR = 0.12, 95% CI 0.04–0.41). Melbye et al. (54), in a large multi-center study, reported a significant inverse association between allergen-specific IgE and non-Hodgkin’s lymphoma (NHL; OR = 0.68, 95% CI 0.58–0.80). However, upon further analysis, NHL dissemination in cases was found to be inversely associated with specific IgE, and in a second, prospective, study, an inverse association was found only immediately prior to NHL diagnosis, leading the authors to conclude that inverse associations reported in previous studies were likely due to the suppression of the allergic response in NHL cases. Positive associations were reported between allergen-specific IgE and both prostate (55, 56) and breast cancer (57).
The following discussion will focus on the elucidation of the immunological mechanistic principles potentially underlying the epidemiological observations.
IgE and its receptors: interaction with intratumoral effector cells
If IgE antibodies (Abs) were directed against tumor-associated antigens (TAA) they could mediate the cell-to-cell association between tumor and effector cells, possibly resulting in antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP). For these reactions, the high- and low-affinity receptors, FcεRI and CD23 (19, 20), on effector cells being on site in the tumor tissue are crucial. The affinity of IgE for FcεRI is by two to five orders of magnitude higher than that of IgG for their receptors, making IgE the only antibody class being strongly retained by effector cells in the absence of antigen. Thus, IgE engaged to FcεRI in tissues could be more effective in anti-tumor responses than IgG and its receptors. FcεRI-expressing monocytes exerted primarily ADCC towards tumor cells. Upon upregulation of FcεRI by preincubation with IgE specific for the ovarian tumor antigen folate receptor, an increase in ADCC was observed (58–60).
Recent strategies, aiming at enhancing the anti-tumor responses of T cells, exploited the high affinty of the IgE–FcεRI complex by transfecting T lymphocytes with a chimeric molecule comprising the extracellular domain of FcεRI with the cytoplasmic domains of CD28 and T cell receptor zeta chains (61). FcεRI-expressing human T cells engaged by tumor-specific IgE monoclonal antibody (mAb) secreted cytokines, proliferated and mediated cytotoxic functions also in vivo following antigen ligation.
CD23, the low-affinity IgE receptor, exists in two forms, CD23a and CD23b, differing at the cytoplasmic N-terminus which contains different signaling motifs that determine their functions (62). The expression of CD23b by interleukin (IL)-4, e.g. derived from natural killer cells in breast cancer tissues, is induced on various cells, notably mast cells, basophils and monocytes (63, 64). By the way, IL-4, like IL-13, is also an important switch factor for IgE production, the latter being also directly derived from cancer cells (65). Engagement of CD23b by IgE–antigen complexes promotes monocyte/macrophage activation, induces nitric oxide synthase (iNOS) and generates pro-inflammatory cytokines (66, 67). CD23b mediates IgE ADCP (63) and may thereby, besides its control function for IgE production (30), mediate tumor cell death. This function has been confirmed as a CD23–IgE complex-driven mechanism of engaging monocytes in ADCP of ovarian tumor cells upregulated by IL-4 (59).
IgE antibodies can thus engage both cell surface IgE receptors, FcεRI and CD23, and activate several lines of effector cells against tumor cells in vitro and in vivo. Indeed, solid tumors are associated with inflammatory responses involving the infiltration by not only B and T lymphocytes, neutrophils and natural killer cells, but also mast cells, macrophages and eosinophils expressing the IgE receptors (68).
It has been shown that mast cells infiltrate the invasive fronts of tumor lesions where their degranulation promotes the remodeling of tissue architecture, angiogenesis, tumor cell growth and metastasis (69). The location of mast cells away from the core of solid tumors, loss of local tissue architecture and production of angiogenic factors [vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), IL-8, tumor necrosis factor (TNF)α, matrix metalloproteinase (MMP)-9], may play a role in their inability to target tumor cells. Mast cells have even been described to be a negative prognostic marker in Merkel cell carcinoma (70). Moreover, there is genetic evidence for a role of mast cells for tumor expansion in a pancreatic islet cell tumor model (71). On the other hand, the intensity of mast cell activation/response in the absence of antigenic stimuli may also contribute to tumor cell death (69, 72), e.g. by releasing preformed proapoptotic TNFα upon triggering (73, 74), as well as histamine, which acts to promote or inhibit tumor growth dependent on the type of histamine receptor expressed (75).
Tumor-associated macrophages (TAM) are found in virtually all types of tumors and can comprise more than 50% of the total tumor mass (76). Blood monocytes are recruited to the tumor sites by chemokines and cytokines released by tumor cells and neighboring endothelial cells. They can be stimulated to either kill tumor cells and release angiostatic compounds, or, like mast cells, promote tumor growth and metastasis by producing angiogenic factors and MMP (77). Immunohistochemical studies in breast cancer confirm the presence of CD23 on the surface of TAM and the expression of iNOS (78, 79). Cytotoxic mediators such as NO are produced by monocytes/macrophages in cancer tissues at levels below those required for tumor cell cytotoxicity, so that the balance of NO production has been suggested to be tipped in favor of tumor growth (80, 81). In contrast, recent studies report that NO is necessary to mediate the antiangiogenic effect of TNF via its receptor TNFR2 in the tumor (74). It has been suggested that these potential cytotoxic effects could be enhanced to activate TAM against tumor cells. Activation signals by tumor antigen-specific IgE may ‘awake’ TAM to this end.
Like macrophages, also other antigen-presenting cells such as B lymphocytes, Langerhans cells and dendritic cells present in tumor infiltrates express CD23 and/or FcεRI and can be activated by locally secreted lymphokines as well as tumor antigen-specific IgE. All these events can initiate IgE antibody-dependent antigen presentation to autologous T cells and induce active immunity (82–86). Therefore, although the mechanisms by which IgE antibodies exert their anti-tumor effects against cancer cells are only starting to emerge, it seems clear that all the ingredients necessary for an IgE-mediated response are in place in human malignant disease.
Eosinophils: exquisite effectors in anti-tumor immunity
Eosinophils are today considered as multifunctional leukocytes, involved in inflammatory processes, tissue remodeling as well as in modulation of innate and adaptive immunity (87). Typically, differentiation and function of eosinophils is regulated by the CD4 Th2 cytokines IL-3, IL-5 and granulocyte macrophage (GM)-colony-stimulating factor (CSF), and may be regarded as typical cells in type 2 immunity (88). Eosinophils express membrane receptors involved in adaptive immune responses like Fc receptors for IgG, IgA or IgE, class II major histocompatability complex (MHC) or co-stimulatory molecules including CD86 and CD28 that allow interactions with lymphocytes and antigen-presenting cells (89). Triggering eosinophils by engagement of receptors for cytokines, immunoglobulins or complement lead to the secretion of numerous cytokines [IL-2, -4, -5, -10, -12, -13, -16, -18, (transforming growth factor (TGF)-α/β], chemokines (RANTES, eotaxin) and lipid mediators (platelet-activating factor, leukotriene C4). Moreover, release of Th2 or Th1 immunoregulatory cytokines and cationic proteins can be specific and dependent upon the nature of stimulus (90, 91). In their specific granules, eosinophils store great quantities of cytokines and cationic proteins, known to be highly cytotoxic mediators [reviewed in (92)]. They can also generate reactive oxygen species (ROS). This high cytotoxic potential can lead to deleterious effects in inflammatory and allergic processes, and eosinophil numbers have also been documented to be elevated in peripheral blood and/or infiltrate the tissues in some malignant disorders (93, 94). This eosinophilia is named TATE for ‘tumor-associated tissue eosinophilia’. High infiltrating eosinophil counts were associated with a significantly better prognosis in esophageal squamous cell carcinoma, gastric cancer, head and neck cancer and colorectal carcinoma (95–99). Activated, they may moreover prevent the metastasis potential of prostate cancer (100, 101). Although TATE may have favorable prognostic value, little is known however on the exact role played by eosinophils in anti-tumor responses.
Recent in vitro (102) and in vivo (103, 104) studies have suggested a potential tumoricidal activity of eosinophils. For the evaluation of the tumoricidal activity of human eosinophils two tumor cell lines, a T lymphoma and a colorectal adenocarcinoma, Jurkat and Colo-205 respectively, were used. Several in vitro arguments allow postulating that human eosinophils purified from the peripheral blood of different eosinophilic donors are able to use effectively their cytotoxic potential towards these human tumor cell lines by inducing their apoptosis in different ways (Capron et al., unpublished observations). Indeed, eosinophils from allergic donors are more efficient, and eosinophils from patients with HES (hyper eosinophilic syndrome) less efficient than eosinophils from normal donors. The heterogeneity of eosinophil-mediated tumor cytotoxicity according to eosinophil donors led to the suggestion that allergic patients are more efficient towards tumor development with a potential tumor sensing role of IgE. Experiments in the Capron-lab are in progress to define the respective cytotoxic properties of specific eosinophil cationic proteins vs tumoricidal molecules shared with other effector cells, such as granzymes and perforin. Besides IgE-dependent tumor cell cytotoxicity of eosinophils (59), there is evidence for IgE antibody-independent killing mechanisms.
IgE for passive immunotherapy of cancer patients?
In the late 1980s, experiments comparing the capacity of various mouse/human chimeric antibodies of different classes and subclasses to elicit ADCC and complement-dependent cytotoxicity (CDC), found that IgG1 was more effective than the other IgG subclasses tested suggesting its superior tumoricidal effect (105). In contrast to IgG, human IgE is incapable of directing complement activation against the targeted tumors (106). The use of recombinant DNA technology has then allowed the construction of recombinant IgG antibodies that can recognize TAA and confer protection against cancer cells by passive immunotherapy. The success of these efforts has resulted in FDA-approved therapeutics such as rituximab (Rituxan®) and trastuzumab (Herceptin®) both with human IgG1 constant regions and variable regions targeting CD20 (B-cell lymphoma TAA) or HER2/neu (breast and ovarian cancer TAA), respectively (107).
Coupled with the logical concern over IgE-induced type I hypersensitivity, these results have demotivated most researchers from further pursuing IgE for passive immunotherapy. However, the use of IgE for the passive immunotherapy of cancer would offer several advantages over conventional IgG-based approaches, including its high- and low-affinity receptors present on a broad spectrum of effector cells, its capacity for antigen uptake and presentation leading to a secondary immune response. In contrast to IgE where serum concentrations compose only 0.02% of the total antibody population, IgG constitutes up to 85%, suggesting that a larger pool of endogenous IgG competitors for cell surface receptors could reduce the ADCC efficacy of a therapeutic IgG dose (108). From this fact and its cytophilicity it may be expected that lower passive doses of IgE antibody preparations than necessary for IgG will be sufficient to achieve therapeutic effects at the targeted tumor.
The first studies using IgE mAbs for the passive immunotherapy of tumors were performed in the early 1990s (109). Using two IgE-producing hybridomas, Nagi et al. (109) generated murine IgE antibodies targeting a glycoprotein (gp36) of the mouse mammary tumor virus (MMTV). Intraperitoneal injections of the monoclonal IgE were given at 4-day intervals to CH3/HeJ mice to treat a syngeneic subcutaneously injected MMTV-secreting mammary carcinoma (H2712). After 6 or 8 weeks of treatment, the monoclonal IgE therapy was capable of preventing subcutaneous tumor development in 50% of the animals treated, but did not protect mice exposed to MMTV-negative mammary carcinoma cells (MA16/c). However, this study did not offer side-by-side comparison of IgE and IgG for the treatments of tumors. It was not until the late 1990s that studies by Kershaw et al. (110) investigated both the IgE- and IgG1-mediated growth inhibition of solid tumors using the human colorectal carcinoma (COLO 205) cell line. A murine IgE (30.6) targeting an antigenic determinant on the colorectal carcinoma cells was shown to transiently inhibit the growth of COLO 205 cells injected subcutaneously into SCID mice while both a mouse/human chimeric IgG1 and IgE containing the (30.6) variable region and corresponding human constant regions showed no effect. The immune response against the COLO 205 tumors directed by the murine IgE offered a robust yet transient reduction of tumor growth using a dose of 1 μg per mouse of IgE in contrast to the optimum dose of 4 × 250 μg per mouse required for IgG1. This effect may be attributed to the superior affinity of IgE to its receptor FcεRI and to the usage of highly tumoricidic effector cells. In fact, the lack of an anti-tumor effect observed with the mouse/human chimeric IgE containing human constant regions is not surprising as human IgE does not cross-react with murine FcεRI.
The potency of IgE interactions with effector cells and their receptors in tumor cell killing has been demonstrated using chimeric antibodies (MOv18 IgE and MOv18 IgG1) against an ovarian tumor-specific antigen, folate binding protein, expressed in 80% of ovarian cancers. In two mouse xenograft models of human ovarian carcinoma, treatment of the mice with IgE, combined with human peripheral blood mononuclear cells (PBMC), had a longer-lasting effect in restricting tumor growth than the same treatment with IgG1 (105). In the second model, grown orthotopically in nude mice, MOv18 IgE with human PBMC, gave significantly greater protection than PBMC alone, while MOv18 IgG1 with human PBMC offered no survival advantage (111). Immunohistochemical studies of tumor sections showed the infiltration of human monocytes/macrophages into tumor lesions, associated with tumor necrosis and increased survival.
Although a number of studies have demonstrated the potential of IgE in the passive immunotherapy of tumors, most attempts have been riddled with limitations including several incompatibilities between the mouse and human immune systems in the domain of IgE-mediated immunity (112). The complete potential of IgE-mediated therapy that would be expected in humans has not been achieved in many studies possibly because the expression of FcεRI in mice is limited to mast cells and basophils, whereas in humans it is also expressed in monocytes, macrophages, eosinophils, Langerhans cells and dendritic cells. Moreover, the experiments described thus far using mouse/human chimeric IgE for the treatment of tumor xenografts in immune-suppressed mice suffered from a limited supply of exogenous human PBMC; better results may be potentially achieved in patients that have a fully active immune system and a constant supply of effector cells.
Some of the incompatibilities challenging IgE-mediated immunity models have been solved with the development of FcεRI alpha chain transgenic mice. One of these mice carries both, a human and a murine FcεRI alpha chain (113, 114), while in the other the murine FcεRI alpha chain is replaced by the human FcεRI alpha chain (113, 114). The latter transgenic mouse exhibits the cell-specific pattern of expression of the human alpha chain as well as the correct structure of the receptor for each cell type. This unique model can be potentially used in future IgE immunotherapy studies in which a true IgE-directed effector cell response could be mounted against a syngeneic tumor model in vivo. Treatment of tumors using a mouse/human chimeric IgE should generate a response in mice closer to that of a human IgE response, but would still be limited by the risk of a mouse anti-human hypersensitivity response, preventing sequential administrations of chimeric IgE. These and other improvements of transgenic models may soon provide a fully functional in vivo readout system to test the efficacy of IgE against various types of cancers in murine models.
IgE as adjuvant in tumor vaccination
Considering that the activation of the antigen–IgE–FcεRI axis profoundly affects the immune system both in its cellular orchestration and programming, resulting in a potent inflammatory state, an attempt of using IgE as a potent adjuvant in anti-tumor vaccination has been undertaken. In an initial study, the capability of targeting mouse IgE on tumor cells and the consecutive effect on tumor vaccination was assessed in C57BL/6 mice using a T cell lymphoma line and an adenocarcinoma cell line (115). Targeting of tumor cells was obtained exploiting biotin–avidin bridges both in vitro, prior to cell administration, or in vivo by the three-step targeting method (116). The use of biotin–avidin bridges eliminated the need for IgE tumor specificity, however biotinylated IgG anti-tumor antigen monoclonals were required to achieve selective IgE targeting on tumor cells. Immunization protocols were implemented in which irradiated tumor cells (loaded with IgE or IgG) were administered, followed by tumor cell challenge. Vaccinated mice exhibited a strong tumor protection in the IgE-targeted group, indicating that an IgE-specific adjuvant effect was established. Conversely, nonimmunized mice or mice immunized with IgG-targeted cells presented comparably fast tumor growth and death. As IgE was absent during tumor cell challenge, the protective effect should be ascribed to the immune system stimulation upon the IgE-targeted cell vaccination. Indeed, both eosinophils and T cells (CD4+ and CD8+) proved to be crucial for the anti-tumor immunity, as their depletion led to abolishment of tumor protection also in the IgE-treated mice. In addition, dendritic cells loaded with tumor cell fragments were found exclusively on draining lymph nodes of IgE-treated animals (Siccardi et al., unpublished). This represents a strong evidence for tumor antigen-loaded dendritic cell migration from the tumor site to peripheral lymph nodes, an important piece in the IgE-driven T cell immune memory picture.
Based on these evidences, the anti-tumor IgE adjuvanticity approach is being progressed trying to address three major issues: (i) improvement of the vaccination strategy; (ii) understanding IgE receptors involvement (FcεRI and CD23); and (iii) humanization of the reagents to move towards clinical applications.
In view of the possibility to engineer modified vaccinia virus Ankara (MVA) to produce recombinant anti-tumor factors, MVA additive/synergic effect in combination with IgE tumor cell loading was tested in mice immunization studies with encouraging results; in addition, several IgE-targeting protocols have been tested, with tumor cell haptenization and surface biotinylation as the most promising ones (Nigro et al., in preparation). In perspective, MVA being widely recognized as a safe and promising tool for human therapy (117), its use in IgE-based anti-tumor vaccination goes in the right direction for future clinical trials. Use of recombinant MVA to expressed membrane tumor antigens on the surface of (IgE-loaded) infected tumor cells should then induce an enhanced anti-tumor immunity. Most importantly, MVA could be engineered to express membrane IgE (mIgE) on the surface of tumor cells, exploiting the reported capability by mIgE to activate FcεRI (118). This strategy would simplify the vaccination protocol of MVA and IgE by fusing them into a single mIgE-expressing MVA. Moreover, knowledge on the IgE–FcεRI binding features (119) allowed the production of a truncated heavy chain mIgE (cleaved at the joint between the Cε2 and the Cε3 domain) that could represent the ultimate IgE version for anti-tumor vaccination: guaranteeing FcεRI activation, preventing soluble IgE circulation and excluding antigen binding. Testing FcεRI α-chain knock out BALB/c mice (120) in the MVA infection/IgE-loading tumor cell vaccination system provided strong evidence on FcεRI importance for the IgE-orchestrated adjuvanticity (unpublished observation). However, keeping in mind the differences in FcεRI expression between mouse and human cell types, a humanized FcεRI α-chain mouse (113) is presently being investigated.
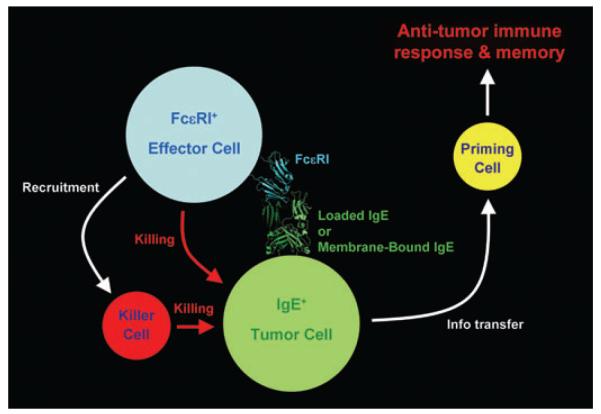
Overall, the mechanism behind IgE-driven tumor vaccination appears to imply IgE–FcεRI recognition, in a tumor cell–FcεRI+ cell scenario. Following, it is highly likely that tumor cell killing occurs, either directly by the FcεRI+-activated cells (mast cells/basophils) and/or by the on-site recruitment of specialized killer cells such as eosinophils. Cell killing releases tumor cell debris containing tumor-specific antigenic determinant available for dendritic cells that in turn transfer the information to peripheral immune districts for the establishment and consolidation of a tumor-specific immune memory (Fig. 2).
Figure 2.
Schematics of the mechanisms behind immunoglobulin E (IgE) adjuvanticity in anti-tumor vaccination. The IgE–FcεRI interaction is represented by the crystal structure of the FcεRI-α chain–IgE Cε3Cε4 complex.
From allergy to oncology: how to make a tumor antigen an allergen
From the discussion shown, it is clear that IgE antibodies targeted or fixed to tumor antigens – in contrast to overall elevated IgE levels – cause a marked effect on tumor development and growth. However, all these strategies used passive applications of IgE. A combination of knowledge in active cancer immunotherapy on the one hand, and in basic allergy mechanisms on the other, prompted recently the development of a vaccine that would induce tumor-specific IgE in vivo. Two strategies were combined: first, an epitope-specific vaccination against the tumor antigen HER-2, rendering antibodies with similar biological properties as the monoclonal antibody trastuzumab (121). Second, an oral immunization regimen discovered in food allergy research, resulting in IgE induction (122–125).
HER-2 is a member of the epidermal growth factor receptor (EGFR, also know as ErbB) family. This receptor is overexpressed in approximately 30% of breast cancer patients and confers a detrimental prognosis in the course of early as well as advanced breast cancer (126). The monoclonal antibody trastuzumab (Herceptin®) targets this molecule, and beneficially influences progression of early and advanced HER-2 overexpressing tumors. In active cancer immunotherapy studies, trastuzumab was used to generate an epitope-specific vaccine targeting HER-2. Peptide mimics (so-called mimotopes) were generated from the antibody-binding site. These mimotopes induced trastuzumab-like IgG antibodies in mice upon intraperitoneal immunization. Importantly, actively induced antibodies upon mimotope vaccination induce antibodies with similar biologic anti-tumor features as the original antibody itself (121, 127, 128).
Second, food proteins can effectively lead to IgE formation and sensitization when they persist the gastric passage undegraded (129). Gastric digestion is impaired in conditions of hypoacidity, as e.g. under anti-ulcer treatment. Consequently, an oral immunization regimen was developed that leads to induction of a Th2 bias in mice, namely high levels of food-protein-specific IgG1 and IgE antibodies, eosinophilia and hypersensitivity of skin and mucosa, when antigens are fed under concomitant gastric acid suppression (125, 130).
When the oral immunization regimen was adapted for the mimotope vaccine construct, it could indeed demonstrate that feedings of trastuzumab mimotopes under anti-acidic conditions induced HER-2-specific IgE antibodies in BALB/c mice. Apparently, this was the first documented active induction of tumor-specific IgE in vivo. The antibodies proved to be functional in an allergologic mediator release assay, where mimotope-induced IgE was found to react specifically with HER-2 overexpressing breast cancer cells. In an assay evaluating the ADCC-mediating potential of the induced anti-HER-2 IgE antibodies, they were shown to be effective in mediating lysis of these breast cancer cells. The degree of IgE–ADCC mediated by the sera correlated with the level of HER-2-specific IgE detected by the mediator release assay. These experiments show that it is possible to turn a tumor antigen into an allergen (Fig. 3).
Figure 3.
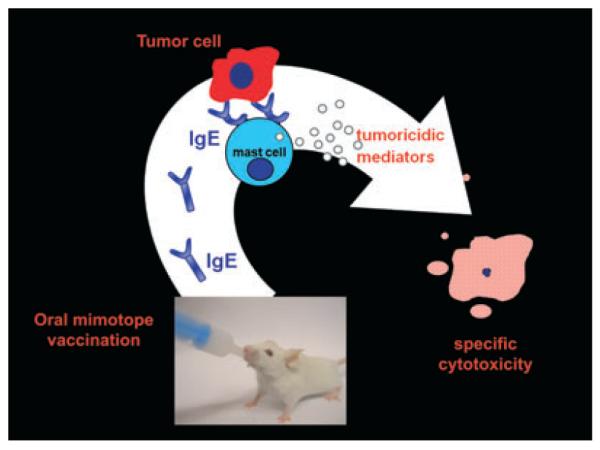
The principle of food allergy induction exploited for oral cancer vaccination. When BALB/c mice were fed with mimotopes for the important cancer antigen HER-2 under concomitant anti-ulcer therapy, they developed immunoglobulin E (IgE) specific for HER-2. The induced IgE was able to bind to FcεRI-positive RBL effector cells. Upon consecutive challenge with HER-2-positive SKBR-3 breast cancer cells, mediators were released which acted tumoricidic on the target cells. The triggering was specific, as non-HER-2-expressing control cancer cells (A432) were not able to lead to mediator release.
In the context of eliciting IgE towards self-antigens, it is self-evident that safety issues are paramount, and the risk of allergic reactions has to be carefully ruled out. Similarly as on allergens, the optimal target antigen/epitope should be present on the tumor cell surface in a sufficient density to cause cross-linking of IgE antibodies bound to the effector cells (131). On the other hand, the target antigen should either not be shed into the blood-stream, or its soluble form should not form aggregates that could lead to undue effector cell activation and systemic anaphylaxis. These issues will have to be thoroughly monitored in future in vivo trials in the setting of active as well as passive administrations of anti-tumor IgE antibodies.
Taken together, it is feasible to induce IgE anti-tumor antibodies by active immunization and it can be hypothesized that for suitable antigens, this approach could be developed into an easily applicable oral vaccination.
Conclusions
IgE antibodies favor the recognition of conformationally intact antigens with a dense epitope display. Intact, viable tumor cells fulfill these requirements because they overexpress antigens. Via interaction with its receptors on numerous defence cells, IgE directs potent effector cells into tumor tissues with proven tumoricidic activity. Thus it can be hypothesized that IgE antibodies might physiologically survey malignant cells. These principles will hopefully in the future be exploited for vaccines and passive antibody therapies.
Acknowledgments
This work was supported by grant PA-18238-B13 of the Austrian Science Fund (FWF), by the Italian MURST (Cofin 2005), University of California Cancer Research Coordinating Committee (CRCC), Susan G. Komen Breast Cancer Foundation, Howard Hughes Medical Institute Gilliam Fellowship for Advanced Studies, and UCLA MBI Whitcome Fellowship.
References
- 1.Ishizaka K, Ishizaka T. Physicochemical properties of reaginic antibody. 1. Association of reaginic activity with an immunoglobulin other than gammaA- or gammaG-globulin. J Allergy. 1966;37:169–185. doi: 10.1016/0021-8707(66)90091-8. [DOI] [PubMed] [Google Scholar]
- 2.Ishizaka K, Ishizaka T. Identification of gamma-E-antibodies as a carrier of reaginic activity. J Immunol. 1967;99:1187–1198. [PubMed] [Google Scholar]
- 3.Johansson SG. Raised levels of a new immunoglobulin class (IgND) in asthma. Lancet. 1967;2:951–953. doi: 10.1016/s0140-6736(67)90792-1. [DOI] [PubMed] [Google Scholar]
- 4.Johansson SG, Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology. 1967;13:381–394. [PMC free article] [PubMed] [Google Scholar]
- 5.Scherrer E. The distribution of reagins in the blood plasma. J Allergy. 1930;2:467. [Google Scholar]
- 6.Layton LL, Yamanaka E. Demonstration of human reagins to foods, cat dander, an insect, and ragweed and grass pollens. J Allergy. 1962;33:271–275. doi: 10.1016/0021-8707(62)90094-1. [DOI] [PubMed] [Google Scholar]
- 7.WEIR, Handbook of experimental immunology. Blackwell; Oxford-Edinburgh: 1967. Augustin RDemonstration of reagins in the serum of allergic subjects; p. 1076. [Google Scholar]
- 8.Martin EG. Predisposing factors and diagnosis of rectal cancer: a discussion of allergy. Ann Surg. 1935;102:56–61. [PMC free article] [PubMed] [Google Scholar]
- 9.Bienengraber A. Tumor metastasis in the light of allergology. Zentralbl Chir. 1952;77:1873–1881. [PubMed] [Google Scholar]
- 10.Molomut N, Spain DM, Kreisler L, Warshaw LJ. The effect of an allergic inflammatory response in the tumor bed on the fate of transplanted tumors in mice. Cancer Res. 1955;15:181–183. [PubMed] [Google Scholar]
- 11.Berdel W, Nass G, Wiedemann G. Mechanism of tumor allergy and its importance in tumor pathogenesis. Int Arch Allergy Appl Immunol. 1956;9:200–221. [PubMed] [Google Scholar]
- 12.Schlitter HE. Is there an allergy against malignant tumor tissue and what can it signify in regard to the defense of the body against cancer? Strahlentherapie. 1961;114:203–224. [PubMed] [Google Scholar]
- 13.McCormick DP, Ammann AJ, Ishizaka K, Miller DG, Hong R. A study of allergy in patients with malignant lymphoma and chronic lymphocytic leukemia. Cancer. 1971;27:93–99. doi: 10.1002/1097-0142(197101)27:1<93::aid-cncr2820270114>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Ure DM. Negative assoication between allergy and cancer. Scott Med J. 1969;14:51–54. doi: 10.1177/003693306901400203. [DOI] [PubMed] [Google Scholar]
- 15.Augustin R, Chandradasa KD. IgE levels and allergic skin reactions in cancer and non-cancer patients. Int Arch Allergy Appl Immunol. 1971;41:141–143. doi: 10.1159/000230505. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs D, Landon J, Houri M, Merrett TG. Circulating levels of immunoglobulin E in patients with cancer. Lancet. 1972;2:1059–1061. doi: 10.1016/s0140-6736(72)92341-0. [DOI] [PubMed] [Google Scholar]
- 17.Allegra J, Lipton A, Harvey H, Luderer J, Brenner D, Mortel R, et al. Decreased prevalence of immediate hypersensitivity (atopy) in a cancer population. Cancer Res. 1976;36:3225–3226. [PubMed] [Google Scholar]
- 18.Neuchrist C, Kornfehl J, Grasl M, Lassmann H, Kraft D, Ehrenberger K, et al. Distribution of immunoglobulins in squamous cell carcinoma of the head and neck. Int Arch Allergy Immunol. 1994;104:97–100. doi: 10.1159/000236714. [DOI] [PubMed] [Google Scholar]
- 19.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, et al. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Murphy RF, Agrawal DK. Decoding IgE Fc receptors. Immunol Res. 2007;37:1–16. doi: 10.1007/BF02686092. [DOI] [PubMed] [Google Scholar]
- 21.Keegan AD, Conrad DH. The receptor for the Fc region of IgE. Springer Semin Immunopathol. 1990;12:303–326. doi: 10.1007/BF00225321. [DOI] [PubMed] [Google Scholar]
- 22.Macglashan D., Jr. IgE and FcεRI regulation. Ann N Y Acad Sci. 2005;1050:73–88. doi: 10.1196/annals.1313.009. [DOI] [PubMed] [Google Scholar]
- 23.Hantusch B, Scholl I, Harwanegg C, Krieger S, Becker WM, Spitzauer S, et al. Affinity determinations of purified IgE and IgG antibodies against the major pollen allergens Phl p 5a and Bet v 1a: discrepancy between IgE and IgG binding strength. Immunol Lett. 2005;97:81–89. doi: 10.1016/j.imlet.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Vernersson M, Aveskogh M, Hellman L. Cloning of IgE from the echidna (Tachyglossus aculeatus) and a comparative analysis of epsilon chains from all three extant mammalian lineages. Dev Comp Immunol. 2004;28:61–75. doi: 10.1016/s0145-305x(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 26.Warr GW, Magor KE, Higgins DA. IgY: clues to the origins of modern antibodies. Immunol Today. 1995;16:392–398. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 27.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 28.Meno-Tetang GM, Lowe PJ. On the prediction of the human response: a recycled mechanistic pharmacokinetic/pharmacodynamic approach. Basic Clin Pharmacol Toxicol. 2005;96:182–192. doi: 10.1111/j.1742-7843.2005.pto960307.x. [DOI] [PubMed] [Google Scholar]
- 29.Waldmann TA, Iio A, Ogawa M, McIntyre OR, Strober W. The metabolism of IgE. Studies in normal individuals and in a patient with IgE myeloma. J Immunol. 1976;117:1139–1144. [PubMed] [Google Scholar]
- 30.Yu P, Kosco-Vilbois M, Richards M, Kohler G, Lamers MC. Negative feedback regulation of IgE synthesis by murine CD23. Nature. 1994;369:753–756. doi: 10.1038/369753a0. [DOI] [PubMed] [Google Scholar]
- 31.Achatz G, Nitschke L, Lamers MC. Effect of transmembrane and cytoplasmic domains of IgE on the IgE response. Science. 1997;276:409–411. doi: 10.1126/science.276.5311.409. [DOI] [PubMed] [Google Scholar]
- 32.Achatz G, Lamers MC. In vivo analysis of the cytoplasmic domain of mIgE antibodies. Int Arch Allergy Immunol. 1997;113:142–145. doi: 10.1159/000237529. [DOI] [PubMed] [Google Scholar]
- 33.Luger E, Lamers M, Achatz-Straussberger G, Geisberger R, Infuhr D, Breitenbach M, et al. Somatic diversity of the immunoglobulin repertoire is controlled in an isotype-specific manner. Eur J Immunol. 2001;31:2319–2330. doi: 10.1002/1521-4141(200108)31:8<2319::aid-immu2319>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 34.Karnowski A, Achatz-Straussberger G, Klockenbusch C, Achatz G. Lamers MCInefficient processing of mRNA for the membrane form of IgE is a genetic mechanism to limit recruitment of IgE-secreting cells. Eur J Immunol. 2006;36:1917–1925. doi: 10.1002/eji.200535495. [DOI] [PubMed] [Google Scholar]
- 35.Geisberger R, Crameri R, Achatz G. Models of signal transduction through the B-cell antigen receptor. Immunology. 2003;110:401–410. doi: 10.1111/j.1365-2567.2003.01770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner MC, Chen Y, Krewski D, Ghadirian P. An overview of the association between allergy and cancer. Int J Cancer. 2006;118:3124–3132. doi: 10.1002/ijc.21752. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Diepgen TL. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies Allergy. 2005;60:1098–1111. doi: 10.1111/j.1398-9995.2005.00813.x. [DOI] [PubMed] [Google Scholar]
- 38.Turner MC, Chen Y, Krewski D, Ghadirian P, Thun MJ, Calle EE. Cancer mortality among US men and women with asthma and hay fever. Am J Epidemiol. 2005;162:212–221. doi: 10.1093/aje/kwi193. [DOI] [PubMed] [Google Scholar]
- 39.Kallen B, Gunnarskog J, Conradson TB. Cancer risk in asthmatic subjects selected from hospital discharge registry. Eur Respir J. 1993;6:694–697. [PubMed] [Google Scholar]
- 40.McWhorter WP. Allergy and risk of cancer. A prospective study using NHANESI follow-up data. Cancer. 1988;62:451–455. doi: 10.1002/1097-0142(19880715)62:2<451::aid-cncr2820620234>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 41.Mills PK, Beeson WL, Fraser GE, Phillips RL. Allergy and cancer: organ site-specific results from the Adventist Health Study. Am J Epidemiol. 1992;136:287–295. doi: 10.1093/oxfordjournals.aje.a116494. [DOI] [PubMed] [Google Scholar]
- 42.Vesterinen E, Pukkala E, Timonen T, Aromaa A. Cancer incidence among 78,000 asthmatic patients. Int J Epidemiol. 1993;22:976–982. doi: 10.1093/ije/22.6.976. [DOI] [PubMed] [Google Scholar]
- 43.Eriksson NE, Holmen A, Hogstedt B, Mikoczy Z, Hagmar L. A prospective study of cancer incidence in a cohort examined for allergy. Allergy. 1995;50:718–722. doi: 10.1111/j.1398-9995.1995.tb01212.x. [DOI] [PubMed] [Google Scholar]
- 44.Gergen PJ, Turkeltaub PC, Sempos CT. Is allergen skin test reactivity a predictor of mortality? Findings from a national cohort Clin Exp Allergy. 2000;30:1717–1723. doi: 10.1046/j.1365-2222.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- 45.Talbot-Smith A, Fritschi L, Divitini ML, Mallon DF, Knuiman MW. Allergy, atopy, and cancer: a prospective study of the 1981 Busselton cohort. Am J Epidemiol. 2003;157:606–612. doi: 10.1093/aje/kwg020. [DOI] [PubMed] [Google Scholar]
- 46.Lindelof B, Granath F, Tengvall-Linder M, Ekbom A. Allergy and cancer. Allergy. 2005;60:1116–1120. doi: 10.1111/j.1398-9995.2005.00808.x. [DOI] [PubMed] [Google Scholar]
- 47.Gandini S, Lowenfels AB, Jaffee EM, Armstrong TD. Maisonneuve PAllergies and the risk of pancreatic cancer: a meta-analysis with review of epidemiology and biological mechanisms. Cancer Epidemiol Biomarker Prev. 2005;14:1908–1916. doi: 10.1158/1055-9965.EPI-05-0119. [DOI] [PubMed] [Google Scholar]
- 48.Linos ERT, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99:1544–1550. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 49.Rosenbaum PF, Buck GM, Brecher ML. Allergy and infectious disease histories and the risk of childhood acute lymphoblastic leukaemia. Paediatr Perinat Epidemiol. 2005;19:152–164. doi: 10.1111/j.1365-3016.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 50.Schuz J, Morgan G, Bohler E, Kaatsch P, Michaelis J. Atopic disease and childhood acute lymphoblastic leukemia. Int J Cancer. 2003;105:255–260. doi: 10.1002/ijc.11054. [DOI] [PubMed] [Google Scholar]
- 51.Wen W, Shu XO, Linet MS, Neglia JP, Potter JD, Trigg ME, et al. Allergic disorders and the risk of childhood acute lymphoblastic leukemia (United States) Cancer Causes Control. 2000;11:303–307. doi: 10.1023/a:1008958724739. [DOI] [PubMed] [Google Scholar]
- 52.Matta GM, Battaglio S, Dibello C, Napoli P, Baldi C, Ciccone G, et al. Polyclonal immunoglobulin E levels are correlated with hemoglobin values and overall survival in patients with multiple myeloma. Clin Cancer Res. 2007;13:5348–5354. doi: 10.1158/1078-0432.CCR-06-2819. [DOI] [PubMed] [Google Scholar]
- 53.Wiemels JL, Wiencke JK, Patoka J, Moghadassi M, Chew T, McMillan A, et al. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer Res. 2004;64:8468–8473. doi: 10.1158/0008-5472.CAN-04-1706. [DOI] [PubMed] [Google Scholar]
- 54.Melbye M, Smedby KE, Lehtinen T, Rostgaard K, Glimelius B, Munksgaard L, et al. Atopy and risk of non-Hodgkin lymphoma. J Natl Cancer Inst. 2007;99:158–166. doi: 10.1093/jnci/djk019. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Diepgen TL. Atopic dermatitis and cancer risk. Br J Dermatol. 2006;154:205–210. doi: 10.1111/j.1365-2133.2005.07077.x. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Rothenbacher D, Low M, Stegmaier C, Brenner H, Diepgen TL. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. Int J Cancer. 2006;119:695–701. doi: 10.1002/ijc.21883. [DOI] [PubMed] [Google Scholar]
- 57.Petridou ET, Chavelas C, Dikalioti SK, Dessypris N, Terzidis A, Nikoulis DI, et al. Breast cancer risk in relation to most prevalent IgE specific antibodies: a case control study in Greece. Anticancer Res. 2007;27:1709–1713. [PubMed] [Google Scholar]
- 58.Bracher M, Gould HJ, Sutton BJ, Dombrowicz D, Karagiannis SN. Three-colour flow cytometric method to measure antibody-dependent tumour cell killing by cytotoxicity and phagocytosis. J Immunol Methods. 2007;323:160–171. doi: 10.1016/j.jim.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Karagiannis SN, Bracher MG, Hunt J, McCloskey N, Beavil RL, Beavil AJ, et al. IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol. 2007;179:2832–2843. doi: 10.4049/jimmunol.179.5.2832. [DOI] [PubMed] [Google Scholar]
- 60.Karagiannis SN, Bracher MG, Beavil RL, Beavil AJ, Hunt J, McCloskey N, et al. Role of IgE receptors in IgE antibody-dependent cytotoxicity and phagocytosis of ovarian tumor cells by human monocytic cells. Cancer Immunol Immunother. 2008;57:247–263. doi: 10.1007/s00262-007-0371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teng MW, Kershaw MH, Jackson JT, Smyth MJ, Darcy PK. Adoptive transfer of chimeric FcεRI gene-modified human T cells for cancer immunotherapy. Hum Gene Ther. 2006;17:1134–1143. doi: 10.1089/hum.2006.17.1134. [DOI] [PubMed] [Google Scholar]
- 62.Yokota A, Kikutani H, Tanaka T, Sato R, Barsumian EL, Suemura M, et al. Two species of human Fcε receptor II (Fcε RII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell. 1988;55:611–618. doi: 10.1016/0092-8674(88)90219-x. [DOI] [PubMed] [Google Scholar]
- 63.Yokota A, Yukawa K, Yamamoto A, Sugiyama K, Suemura M, Tashiro Y, et al. Two forms of the low-affinity Fc receptor for IgE differentially mediate endocytosis and phagocytosis: identification of the critical cytoplasmic domains. Proc Natl Acad Sci USA. 1992;89:5030–5034. doi: 10.1073/pnas.89.11.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lorenzen J, Lewis CE, McCracken D, Horak E, Greenall M, McGee JO. Human tumour-associated NK cells secrete increased amounts of interferon-gamma and interleukin-4. Br J Cancer. 1991;64:457–462. doi: 10.1038/bjc.1991.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paul-Eugene N, Mossalayi D, Sarfati M, Yamaoka K, Aubry JP, Bonnefoy JY, et al. Evidence for a role of Fcε RII/CD23 in the IL-4-induced nitric oxide production by normal human mononuclear phagocytes. Cell Immunol. 1995;163:314–318. doi: 10.1006/cimm.1995.1132. [DOI] [PubMed] [Google Scholar]
- 67.Mossalayi MD, Paul-Eugene N, Ouaaz F, Arock M, Kolb JP, Kilchherr E, et al. Involvement of Fcε RII/CD23 and l-arginine-dependent pathway in IgE-mediated stimulation of human monocyte functions. Int Immunol. 1994;6:931–934. doi: 10.1093/intimm/6.7.931. [DOI] [PubMed] [Google Scholar]
- 68.Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis. 2002;19:247–258. doi: 10.1023/a:1015587423262. [DOI] [PubMed] [Google Scholar]
- 69.Ribatti D, Crivellato E, Roccaro AM, Ria R, Vacca A. Mast cell contribution to angiogenesis related to tumour progression. Clin Exp Allergy. 2004;34:1660–1664. doi: 10.1111/j.1365-2222.2004.02104.x. [DOI] [PubMed] [Google Scholar]
- 70.Beer T, Ng L, Murray K. Mast cells have prognostic value in Merkel cell carcinoma. Am J Dermatopathol. 2008;30:27–30. doi: 10.1097/DAD.0b013e31815c932a. [DOI] [PubMed] [Google Scholar]
- 71.Soucek L, Lawlor E, Soto D, Shchors K, Swigart L, Evan G. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 72.Crivellato E, Beltrami CA, Mallardi F, Ribatti D. The mast cell: an active participant or an innocent bystander? Histol Histopathol. 2004;19:259–270. doi: 10.14670/HH-19.259. [DOI] [PubMed] [Google Scholar]
- 73.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 74.Zhao X, Mohaupt M, Jiang J, Liu S, Li B, Qin Z. Tumor necrosis factor receptor 2-mediated tumor suppression is nitric oxide dependent and involves angiostasis. Cancer Res. 2007;67:4443–4450. doi: 10.1158/0008-5472.CAN-07-0185. [DOI] [PubMed] [Google Scholar]
- 75.Medina V, Croci M, Crescenti E, Mohamad N, Sanchez-Jimenez F, Massari N, et al. The role of histamine in human mammary carcinogenesis: H3 and H4 receptors as potential therapeutic targets for breast cancer treatment. Cancer Biol Ther. 2008;7:28–35. doi: 10.4161/cbt.7.1.5123. [DOI] [PubMed] [Google Scholar]
- 76.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 77.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 78.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomsen LL, Miles DW. Role of nitric oxide in tumour progression: lessons from human tumours. Cancer Metastasis Rev. 1998;17:107–118. doi: 10.1023/a:1005912906436. [DOI] [PubMed] [Google Scholar]
- 80.Xie K, Huang S, Dong Z, Juang SH, Gutman M, Xie QW, et al. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J Exp Med. 1995;181:1333–1343. doi: 10.1084/jem.181.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keller R, Geiges M. Keist Rl-arginine-dependent reactive nitrogen intermediates as mediators of tumor cell killing by activated macrophages. Cancer Res. 1990;50:1421–1425. [PubMed] [Google Scholar]
- 82.Luiten RM, Fleuren GJ, Warnaar SO, Litvinov SV. Target-specific activation of mast cells by immunoglobulin E reactive with a renal cell carcinoma-associated antigen. Lab Invest. 1996;74:467–475. [PubMed] [Google Scholar]
- 83.Luiten RM, Warnaar SO, Schuurman J, Pasmans SG, Latour S, Daeron M, et al. Chimeric immunoglobulin E reactive with tumor-associated antigen activates human FcεRI bearing cells. Hum Antibodies. 1997;8:169–180. [PubMed] [Google Scholar]
- 84.Sapino A, Cassoni P, Ferrero E, Bongiovanni M, Righi L, Fortunati N, et al. Estrogen receptor alpha is a novel marker expressed by follicular dendritic cells in lymph nodes and tumor-associated lymphoid infiltrates. Am J Pathol. 2003;163:1313–1320. doi: 10.1016/s0002-9440(10)63490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dadabayev AR, Sandel MH, Menon AG, Morreau H, Melief CJ, Offringa R, et al. Dendritic cells in colorectal cancer correlate with other tumor-infiltrating immune cells. Cancer Immunol Immunother. 2004;53:978–986. doi: 10.1007/s00262-004-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 87.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 88.Asquith KL, Ramshaw HS, Hansbro PM, Beagley KW, Lopez AF, Foster PS. The IL-3/IL-5/GM-CSF common receptor plays a pivotal role in the regulation of Th2 immunity and allergic airway inflammation. J Immunol. 2008;180:1199–1206. doi: 10.4049/jimmunol.180.2.1199. [DOI] [PubMed] [Google Scholar]
- 89.Woerly G, Roger N, Loiseau S, Dombrowicz D, Capron A, Capron M. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J Exp Med. 1999;190:487–495. doi: 10.1084/jem.190.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Woerly G, Roger N, Loiseau S, Capron M. Expression of Th1 and Th2 immunoregulatory cytokines by human eosinophils. Int Arch Allergy Immunol. 1999;118:95–97. doi: 10.1159/000024038. [DOI] [PubMed] [Google Scholar]
- 91.Woerly G, Lacy P, Younes AB, Roger N, Loiseau S, Moqbel R, et al. Human eosinophils express and release IL-13 following CD28-dependent activation. J Leukoc Biol. 2002;72:769–779. [PubMed] [Google Scholar]
- 92.Decot V, Capron M. Eosinophils: structure and functions. Presse Med. 2006;35:113–124. doi: 10.1016/s0755-4982(06)74534-1. [DOI] [PubMed] [Google Scholar]
- 93.Ionescu MA, Rivet J, Daneshpouy M, Briere J, Morel P, Janin A. In situ eosinophil activation in 26 primary cutaneous T-cell lymphomas with blood eosinophilia. J Am Acad Dermatol. 2005;52:32–39. doi: 10.1016/j.jaad.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 94.Munitz A, Levi-Schaffer F. Eosinophils: ‘new’ roles for ‘old’ cells. Allergy. 2004;59:268–275. doi: 10.1111/j.1398-9995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 95.Ishibashi S, Ohashi Y, Suzuki T, Miyazaki S, Moriya T, Satomi S, et al. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006;26:1419–1424. [PubMed] [Google Scholar]
- 96.Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88:1544–1548. [PubMed] [Google Scholar]
- 97.Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brunner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol. 1999;189:487–495. doi: 10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 98.Samoszuk M. Eosinophils and human cancer. Histol Histopathol. 1997;12:807–812. [PubMed] [Google Scholar]
- 99.Iwasaki K, Torisu M, Fujimura T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986;58:1321–1327. doi: 10.1002/1097-0142(19860915)58:6<1321::aid-cncr2820580623>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 100.Furbert-Harris PM, Parish-Gause D, Hunter KA, Vaughn TR, Howland C, Okomo-Awich J, et al. Activated eosinophils upregulate the metastasis suppressor molecule E-cadherin on prostate tumor cells. Cell Mol Biol (Noisy-le-grand) 2003;49:1009–1016. [PubMed] [Google Scholar]
- 101.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 102.Munitz A, Bachelet I, Fraenkel S, Katz G, Mandelboim O, Simon HU, et al. 2B4 (CD244) is expressed and functional on human eosinophils. J Immunol. 2005;174:110–118. doi: 10.4049/jimmunol.174.1.110. [DOI] [PubMed] [Google Scholar]
- 103.Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, et al. Pivotal advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79:1131–1139. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gould HJ, Mackay GA, Karagiannis SN, O’Toole CM, Marsh PJ, Daniel BE, et al. Comparison of IgE and IgG antibody-dependent cytotoxicity in vitro and in a SCID mouse xenograft model of ovarian carcinoma. Eur J Immunol. 1999;29:3527–3537. doi: 10.1002/(SICI)1521-4141(199911)29:11<3527::AID-IMMU3527>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 106.Murphy KM, Travers P, Walpost M. Janeway’s Immunobiology. 7th edn Garland Science Publishing; Oxford: 2008. [Google Scholar]
- 107.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 108.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 109.Nagy E, Berczi I, Sehon AH. Growth inhibition of murine mammary carcinoma by monoclonal IgE antibodies specific for the mammary tumor virus. Cancer Immunol Immunother. 1991;34:63–69. doi: 10.1007/BF01741326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kershaw MH, Darcy PK, Trapani JA, MacGregor D, Smyth MJ. Tumor-specific IgE-mediated inhibition of human colorectal carcinoma xenograft growth. Oncol Res. 1998;10:133–142. [PubMed] [Google Scholar]
- 111.Karagiannis SN, Wang Q, East N, Burke F, Riffard S, Bracher MG, et al. Activity of human monocytes in IgE antibody-dependent surveillance and killing of ovarian tumor cells. Eur J Immunol. 2003;33:1030–1040. doi: 10.1002/eji.200323185. [DOI] [PubMed] [Google Scholar]
- 112.Kinet JP. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 113.Dombrowicz D, Brini AT, Flamand V, Hicks E, Snouwaert JN, Kinet JP, et al. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157:1645–1651. [PubMed] [Google Scholar]
- 114.Fung-Leung WP, De Sousa-Hitzler J, Ishaque A, Zhou L, Pang J, Ngo K, et al. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions. J Exp Med. 1996;183:49–56. doi: 10.1084/jem.183.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reali E, Greiner JW, Corti A, Gould HJ, Bottazzoli F, Paganelli G, et al. IgEs targeted on tumor cells: therapeutic activity and potential in the design of tumor vaccines. Cancer Res. 2001;61:5517–5522. [PubMed] [Google Scholar]
- 116.Paganelli G, Magnani P, Zito F, Villa E, Sudati F, Lopalco L, et al. Three-step monoclonal antibody tumor targeting in carcinoembryonic antigen-positive patients. Cancer Res. 1991;51:5960–5966. [PubMed] [Google Scholar]
- 117.Drexler I, Staib C, Sutter G. Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential? Curr Opin Biotechnol. 2004;15:506–512. doi: 10.1016/j.copbio.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vangelista L, Soprana E, Cesco-Gaspere M, Mandiola P, Di Lullo G, Fucci RN, et al. Membrane IgE binds and activates FcεRI in an antigen-independent manner. J Immunol. 2005;174:5602–5611. doi: 10.4049/jimmunol.174.9.5602. [DOI] [PubMed] [Google Scholar]
- 119.Vangelista L. Current progress in the understanding of IgE-FcεRI interaction. Int Arch Allergy Immunol. 2003;131:222–233. doi: 10.1159/000072134. [DOI] [PubMed] [Google Scholar]
- 120.Dombrowicz D, Flamand V, Brigman KK, Koller BH, Kinet JP. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell. 1993;75:969–976. doi: 10.1016/0092-8674(93)90540-7. [DOI] [PubMed] [Google Scholar]
- 121.Riemer AB, Klinger M, Wagner S, Bernhaus A, Mazzucchelli L, Pehamberger H, et al. Generation of peptide mimics of the epitope recognized by trastuzumab on the oncogenic protein Her-2/neu. J Immunol. 2004;173:394–401. doi: 10.4049/jimmunol.173.1.394. [DOI] [PubMed] [Google Scholar]
- 122.Untersmayr E, Jensen-Jarolim E. The effect of gastric digestion on food allergy. Curr Opin Allergy Clin Immunol. 2006;6:214–219. doi: 10.1097/01.all.0000225163.06016.93. [DOI] [PubMed] [Google Scholar]
- 123.Untersmayr E, Bakos N, Scholl I, Kundi M, Roth-Walter F, Szalai K, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 2005;19:656–658. doi: 10.1096/fj.04-3170fje. [DOI] [PubMed] [Google Scholar]
- 124.Scholl I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, Boltz-Nitulescu G, et al. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005;81:154–160. doi: 10.1093/ajcn/81.1.154. [DOI] [PubMed] [Google Scholar]
- 125.Untersmayr E, Scholl I, Swoboda I, Beil WJ, Forster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112:616–623. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- 126.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 127.Riemer AB, Kurz H, Klinger M, Scheiner O, Zielinski CC, Jensen-Jarolim E. Vaccination with cetuximab mimotopes and biological properties of induced anti-epidermal growth factor receptor antibodies. J Natl Cancer Inst. 2005;97:1663–1670. doi: 10.1093/jnci/dji373. [DOI] [PubMed] [Google Scholar]
- 128.Bramswig KH, Knittelfelder R, Gruber S, Untersmayr E, Riemer AB, Szalai K, et al. Immunization with mimotopes prevents growth of carcinoembryonic antigen positive tumors in BALB/c mice. Clin Cancer Res. 2007;13:6501–6508. doi: 10.1158/1078-0432.CCR-07-0692. [DOI] [PubMed] [Google Scholar]
- 129.Riemer AB, Untersmayr E, Knittelfelder R, Duschl A, Pehamberger H, Zielinski CC, et al. Active induction of tumor-specific IgE antibodies by oral mimotope vaccination. Cancer Res. 2007;67:3406–3411. doi: 10.1158/0008-5472.CAN-06-3758. [DOI] [PubMed] [Google Scholar]
- 130.Untersmayr E, Ellinger A, Beil WJ, Jensen-Jarolim E. Eosinophils accumulate in the gastric mucosa of food-allergic mice. Int Arch Allergy Immunol. 2004;135:1–2. doi: 10.1159/000080035. [DOI] [PubMed] [Google Scholar]
- 131.Scholl I, Kalkura N, Shedziankova Y, Bergmann A, Verdino P, Knittelfelder R, et al. Dimerization of the major birch pollen allergen Bet v 1 is important for its in vivo IgE cross-linking potential in mice. J Immunol. 2005;175:6645–6650. doi: 10.4049/jimmunol.175.10.6645. [DOI] [PubMed] [Google Scholar]