Abstract
Immunological synapses (ISs) are formed at the T cell–antigen-presenting cell (APC) interface during antigen recognition, and play a central role in T-cell activation and in the delivery of effector functions. ISs were originally described as a peripheral ring of adhesion molecules surrounding a central accumulation of T-cell receptor (TCR)–peptide major histocompatibility complex (pMHC) interactions. Although the structure of these ‘classical’ ISs has been the subject of intense study, non-classical ISs have also been observed under a variety of conditions. Multifocal ISs, characterized by adhesion molecules dispersed among numerous small accumulations of TCR–pMHC, and motile ‘immunological kinapses’ have both been described. In this review, we discuss the conditions under which non-classical ISs are formed. Specifically, we explore the profound effect that the phenotypes of both T cells and APCs have on IS structure. We also comment on the role that IS structure may play in T-cell function.
Keywords: adhesion molecules, antigen presentation, immune synapse, T-cell receptor, T cells
Introduction
Recognition of peptide antigens by T cells and their subsequent activation, which are of central importance for adaptive immune responses, necessitate the physical interaction of T cells and antigen-presenting cells (APCs). Early studies of both cytotoxic T lymphocytes (CTLs) and CD4+ helper T (Th) cells interacting with APCs presenting cognate antigen demonstrated that the microtubule organizing centre (MTOC) and the cytoskeleton of the T cell are orientated towards the APC.1,2 These cytoskeletal changes, resulting in gross changes in T-cell morphology and a flattening of the T cell against the APC, occur almost immediately after antigen recognition.3,4 Experiments with supported planar lipid bilayers showed that peptide-loaded major histocompatility complexes (pMHCs) and the adhesion molecule intercellular adhesion molecule 1 (ICAM-1) are sufficient to stop migration and induce MTOC polarization in T cells.5 Seminal work from the Kupfer and Dustin laboratories demonstrated that, in addition to the morphological changes described above, receptor–ligand interactions and signalling molecules involved in adhesion and antigen recognition are organized into distinct domains or supramolecular activation clusters (SMACs) at the Th cell–APC interface.6,7 This organization is thought to function in the communication between the two cells and has therefore been termed the ‘immunological synapse’ (IS).8 In this review, we examine the kinetics of IS formation and the effect that T-cell differentiation state and APC phenotype have on IS structure. We also discuss the role that IS structure plays in T-cell activation and function.
The classical immunological synapse
Initial observations of the organization of molecules at the cell–cell interface during antigen-specific interactions between Th cells and a B-cell lymphoma line indicated that T-cell receptor (TCR)–pMHC interactions, along with the signalling molecules protein kinase C (PKC-θ) and lymphocyte-specific protein tyrosine kinase (Lck), are located in a central SMAC (cSMAC), while adhesion molecule interactions [lymphocyte function-associated antigen 1 (LFA-1)–ICAM-1] surround the cSMAC in a peripheral SMAC (pSMAC)7 (Fig. 1a). Similar structures are also seen when Th cells are introduced to supported planar bilayers containing fluorescently labelled pMHC and ICAM-1.6 Significantly, CD8+ CTLs also form ‘bull's-eye’ type ISs with a ring of adhesion molecules surrounding TCR–pMHC interactions both in vivo and in vitro, demonstrating that the phenomenon is not limited to CD4+ Th cells.9–11 Bull's-eye ISs have also been observed at the interface between natural killer (NK) cells and target cells, where a central accumulation of activating or inhibitory receptors is surrounded by adhesion molecules.12
Figure 1.
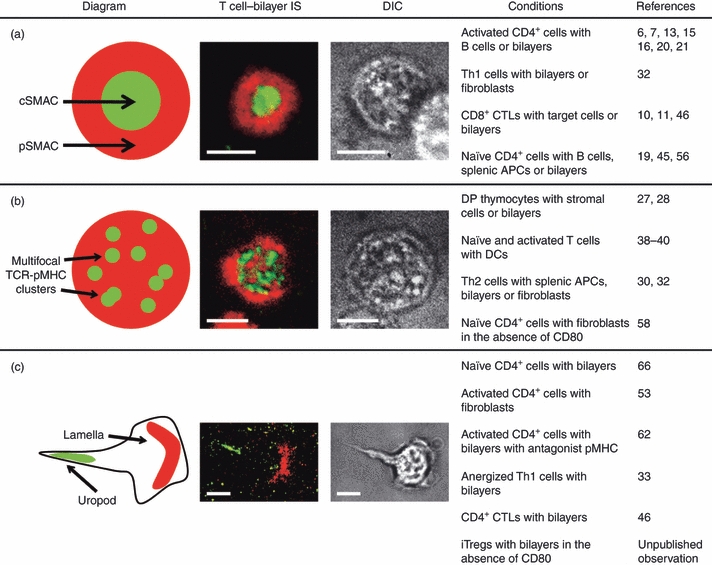
Immunological synapse and kinapse structures. Examples of classical (a) and multifocal (b) immunological synapse (ISs) and immunological kinapses (c) are shown. The fluorescent images are from experiments where CD4+ T cells were introduced to supported planar bilayers containing fluorescently labelled peptide major histocompatibility complex (pMHC) and intercellular adhesion molecule 1 (ICAM-1). T-cell receptor (TCR)–pMHC and lymphocyte function-associated antigen 1 (LFA-1)–ICAM-1 interactions are green and red, respectively, in both the representative diagrams and fluorescent images. Differential interference contrast (DIC) images are also shown. Note that the cell forming an immunological kinapse (c) has a polarized shape with a well-defined lamella and uropod. The conditions under which these phenotypes are seen and the corresponding references are also given. The scale bars represent 5 μm. APC, antigen-presenting cell; cSMAC, central supramolecular activation cluster; CTL, cytotoxic T lymphocyte; DC, dendritic cell; DP, double-positive; iTreg, induced T regulatory cell; pSMAC, peripheral supramolecular activation cluster.
Immunological synapse structure varies with time
The observations described above were made at relatively late time-points (more than 10 min after conjugate formation). Experiments with conjugates fixed at various time-points and studies utilizing time-lapse microscopy have demonstrated that IS formation is a dynamic process involving a number of intermediate structures. Early images of CD4+ T cells forming ISs with supported planar bilayers demonstrated that the majority of TCR–pMHC interactions exist at the periphery of the interface at early time-points, but consolidate in the cSMAC within 5 min, while the opposite pattern occurs for LFA-1–ICAM-1.6 The large phosphatase CD45 is present in the centre of the contact zone at early time-points,13 but is cleared from the cSMAC by 7 min post-conjugation.13,14 However, experiments using supported planar bilayers as APCs demonstrated that under some experimental conditions CD45 does accumulate at the cSMAC in addition to the strong peripheral accumulation.15,16 The spatiotemporal location of CD45 relative to the TCR during IS formation is of interest because, while dephosphorylation of the Src-family kinase Lck is critical for the initiation of a signal through the TCR, CD45 is also capable of dephosphorylating activated components of the proximal TCR signalling complex.17 The conflicting data obtained for CD45 localization in experiments using different types of APC (planar bilayers and B lymphoma cells in this case) demonstrate the important effect that experimental parameters can have on results and conclusions, and the hazard in generalizing results derived from one experimental system. The influence of the type of APC on IS structure is discussed in detail below.
In an experimental tour de force, Wülfing et al. used live cell imaging to examine the spatiotemporal patterns of 30 green fluorescent protein (GFP)-conjugated signalling sensors and found that the TCR and a group of proximal signalling molecules, including zeta-chain associated protein kinase of 70 kDa (ZAP-70), linker for activation of T cells (LAT), phospholipase C-γ and PKC-θ, are rapidly recruited to the cSMAC,18 in agreement with previous results.7,13 This large accumulation of TCR-proximal signalling molecules in the cSMAC could be interpreted as evidence that IS formation is required for the initiation of signalling; however, this is not the case because phosphorylated signalling proteins, including Lck and ZAP-70, are found at the T cell–APC interface prior to the formation of mature, bull's-eye ISs.13,19 In fact, the majority of phosphorylated signalling molecules are found in the periphery of the T cell–APC contact.19 This result was explained by the discovery that TCR microclusters, containing 40–150 TCR molecules, form immediately upon contact with planar bilayers containing pMHC and ICAM-1.20 The microclusters exclude CD45 and contain activated signalling molecules, including pLck, pZAP-70 and pLAT, as well as CD28 and PKC-θ,16,20–22 and are reminiscent of the small signalling clusters seen when Jurkat cells are introduced to anti-TCR coated coverslips.23 These microclusters move centripetally in an F-actin and myosin motor-dependent fashion, resulting in the formation of a TCR-rich cSMAC.16,20,24,25 However, as the TCR microclusters move towards the centre of the interface, they become dissociated from the TCR-proximal signalling molecules, as well as CD28 and PKC-θ.20,22 New microclusters are continuously generated in the periphery and move centripetally, even after the formation of a mature, bull's-eye IS,20,21 while the presence of a marker for multivesicular bodies and the endosomal sorting complex required for transport I ubiquitin-recognition complex at the cSMAC indicates that this is a site of active TCR down-modulation.16,26 Thus, IS formation is best viewed as a two-stage process. Stage I occurs immediately upon T cell–APC conjugation and involves the formation and coalescence of TCR microclusters, resulting in a large-scale, actin-dependent rearrangement of receptors, downstream signalling molecules and adhesion molecules into SMACs. Stage II is characterized by relative stability in the macro-structure of the IS and the centripetal movement of newly generated microclusters from the periphery to the cSMAC where signalling is extinguished and the TCR is down-modulated.
T-cell differentiation state influences IS structure
Although most studies have focused on the formation and structure of classical ISs with well-defined SMACs, the literature contains many examples of T cells forming non-classical ISs under a variety of conditions. The differentiation state of T cells has a particularly profound effect on IS structure. Experiments examining the ISs formed between double-positive (DP) thymocytes and thymic stromal cells in vitro showed an inability of DP thymocytes to form a cSMAC with a centrally located accumulation of TCRs during negative selection.27 Furthermore, DP thymocytes interacting with bilayers containing pMHC and ICAM-1 fail to form classical ISs.28 Instead, these cells form ‘multifocal’ ISs characterized by a T cell–APC interface with ICAM-1 interspersed among multiple small accumulations of TCR–pMHC and phosphorylated signalling molecules.28 These multifocal ISs are hypothesized to be the result of relatively low TCR expression by DP thymocytes compared with peripheral T cells.29
The observation that DP thymocytes fail to form classical ISs leads to the question of whether there are T-cell subsets in the periphery that also form alternative IS structures. Several groups, including our own, have conducted experiments examining the ISs formed by differentiated Th1 and Th2 cells and found that Th2 cells do not form classical ISs under a variety of conditions.18,30–32 Bottomly et al. discovered that Th2 cells fail to cluster TCR–pMHC interactions at the interface when forming conjugates with resting B cells,30 and found that this defect was attributable to relatively high levels of cytotoxic T-lymphocyte antigen (CTLA)-4 in Th2 cells compared with Th1 cells.31 Th2 cells are also less efficient than Th1 cells at clustering TCR–pMHC in a cSMAC when B lymphoma cells are used as APCs.18 In a recent report, we showed that ISs formed by Th1 and Th2 cells have a strikingly different morphology when transfected fibroblasts or planar bilayers containing pMHC and ICAM-1 are used as APCs.32 While Th1 cells form classical, bull's-eye ISs, Th2 ISs are multifocal, with small accumulations of TCR–pMHC that exclude ICAM-1 spread throughout the interface (Fig. 1b). Unlike Th1 ISs, CD45 is excluded from the TCR–pMHC foci but not the interface as a whole. The foci did co-localize with phosphotyrosine staining, suggesting that these small TCR–pMHC accumulations are sites of active signalling.32 The multifocal pattern of TCR–pMHC, together with the continuous association of these foci with active signalling molecules, that we observed in Th2 ISs is similar to the IS structure reported for DP thymocytes.28
Several studies have described non-classical ISs formed by T cells that have been anergized and are hyporesponsive to subsequent activation. Th1 cells anergized via treatment with ionomycin initially form classical ISs with planar bilayers, but these ISs are highly unstable compared with those formed by control Th1 cells.33 Also, the recruitment of the TCR and some signalling molecules to the T cell–APC interface is abnormal for anergic cells under some experimental conditions.34–36 However, we have shown that, when T cells are anergized by activation in the absence of costimulation, the macro-structure of the ISs formed with transfected fibroblast APCs is similar to that of controls.37 The disparate results obtained in these studies probably indicate the importance of specific anergizing conditions in determining the structure of ISs formed by hyporesponsive cells.
Antigen-presenting cells influence IS structure
In addition to T-cell differentiation state, the phenotype of APCs can profoundly affect IS structure. Dendritic cells (DCs) have been shown to form multifocal ISs with naïve CD4+ and CD8+ cells38 and activated CD4+ cells.39,40 There is evidence suggesting that this multifocal structure is the result of T cells interacting with microvilli on the DC surface.39 The activation state of DCs may also play a role in determining IS structure, as LPS treatment increased the frequency of classical ISs in one study;41 however, others have found that activated DCs promote the formation of multifocal ISs.40 It is interesting to note that, unlike B cells,42 DCs polarize their actin cytoskeleton towards the T cell in an antigen-specific manner, and poisoning the DC cytoskeleton results in decreased T-cell activation.43,44 It is possible that the involvement of the DC cytoskeleton encourages the formation of multifocal ISs. Unlike DCs, B cells, tumour cell targets and supported planar bilayers seem to promote the formation of classical, bull's-eye ISs by both naïve and activated T cells.6,7,10,11,45 One explanation for these observations is that a classical IS with clearly defined SMACs is not required for T-cell activation by DCs during the priming phase of an immune response, but is necessary for the targeted delivery of certain effector functions, including cytolytic granules, inflammatory cytokines such as IFN-γ and preformed CD40L.11,46–52
Given the importance of costimulation during T-cell activation, the influence of CD28–CD80/86 interactions on IS formation and structure is of interest. We have shown that blocking of CD28–CD80 interactions decreases the accumulation of TCR–pMHC and alters the morphology of the ISs formed between activated T cells and transfected fibroblast APCs,53 in agreement with other studies demonstrating that a lack of CD28 signalling results in diffuse ISs that lack a clearly defined cSMAC.54,55 Additionally, activated but not resting B cells are capable of promoting TCR clustering in ISs formed by Th2 cells, and treating activated B cells with anti-CD80/86 decreases TCR accumulation in both Th1 and Th2 ISs.31 However, the differentiation state of the T cell may be critical in determining the effect of CD80 on IS formation, as experiments examining the ISs formed by naïve CD4+ T cells with planar bilayers containing pMHC and ICAM-1 with or without CD80 did not detect a CD80-dependent difference in IS frequency or structure.56 Also, CD28 ligation does not alter the ISs formed by naïve CD8+ cells, which have been reported to form diffuse ISs without clearly defined SMACs.57 However, experiments with naïve CD4+ cells and chinese hamster ovary cell APCs showed that multifocal ISs are formed in the absence of CD28 ligation, while cSMACs are formed when CD80 is present.58
Signalling through the TCR is critical for IS formation and maintenance,21,59 and thus the role that the strength of TCR signalling plays in IS structure is worth exploring. In elegant experiments using fluorescently labelled peptides, Davis et al. showed that as few as 10 agonist pMHC molecules are sufficient to induce cSMAC and pSMAC formation.60 Reducing the amount of available antigen by over 100-fold does not significantly alter the structure of ISs formed between T-cell blasts or Th1 cells and planar bilayers.6,32 However, Th2 cells are more likely to have a single, centrally located cluster of TCR–pMHC than a multifocal phenotype at low levels of antigen, but fail to form ISs with a peripheral LFA-1-–ICAM-1 ring at any antigen dose.32 Thus, decreasing the availability of antigen can alter IS structure, but these effects depend on the differentiation state of the T cell and are not seen universally. Non-classical ISs are also formed by T cells interacting with APCs presenting altered peptide ligands with a lower affinity for the TCR. In the planar bilayer system, weak agonist and antagonist peptides induce decreased TCR–pMHC accumulation compared with strong agonists, and T-cell blasts interacting with antagonist-loaded bilayers fail to form classical ISs.6 Similarly, ISs with well-defined cSMACs are not seen when T-cell blasts are conjugated with a B-cell lymphoma line presenting a weak agonist peptide.61 Interestingly, when T-cell blasts are introduced to planar bilayers or B cells containing agonist mixed with an excess of antagonist peptides, the T cells flux calcium, but accumulate ICAM-1 in a crescent shape and fail to stop, suggesting that weak signalling through the TCR encourages T cells to adopt a migratory phenotype.62 Additional examples of T cells assuming a motile phenotype upon antigen recognition, instead of forming stable ISs, are discussed below.
Immunological kinapses
While most studies of T cell–APC interactions have focused on stable conjugates, it is known from in vitro experiments that dynamic T cell–DC interactions, characterized by brief, migratory interactions, are sufficient for the induction of calcium flux, activation and proliferation.63 Furthermore, in vivo imaging of naïve T cell–DC interactions indicates that T cells go through three stages during priming: several hours of high T-cell motility and short-lived T cell–DC contacts, followed by a phase of relatively long-lived contacts and decreased motility, and finally rapid motility and short contacts concomitant with proliferation.64,65 Of note, even cells forming relatively long-lived contacts with APCs retain some motility.64,65 Thus, T cells undergo periods of varying motility while integrating signals through the TCR.
Time-lapse studies of naïve CD4+ T cells interacting with planar bilayers containing pMHC, ICAM-1 and CD80 showed that these cells alternate between forming stable, symmetrical bull's-eye ISs and forming migratory ‘immunological kinapses’.66 The actin-regulatory protein Wiskott-Aldrich syndrome protein was found to promote the reformation of ISs, while PKC-θ destabilizes symmetrical ISs, causing a transition to immunological kinapses.66 CD4+ CTLs, Th cells, anergized Th1 cells and T cells recognizing antagonist pMHC have all been observed forming migratory, immunological kinapse-like structures.33,46,53,62 These structures are characterized by a crescent-shaped accumulation of LFA-1–ICAM-1 in the middle of the cell (lamella) pointing towards the direction of migration and clusters of TCR–pMHC in the trailing uropod (Fig. 1c). We have found that induced T regulatory cells form immunological kinapses in the absence of costimulation, but transition to stable, symmetrical ISs in the presence of high, but not low, levels of CD80 (T.J. Thauland and D.C. Parker, manuscript in preparation). It is likely that the level of TCR stimulation and costimulation, the differentiation state of the T cell and the phenotype of the APC combine to determine whether cells predominantly form symmetrical ISs or kinapses or transition between the two modes.
Common themes
Given the diversity of IS structures described in this review, it is interesting to consider what characteristics all ISs have in common. Large-scale molecular rearrangements at the T cell–APC interface are the hallmark of IS formation, and this repositioning is dependent on the cytoskeleton, because poisoning the T-cell actin cytoskeleton, but not microtubule function, results in the failure of large clusters of TCR–pMHC to accumulate at the T cell–APC interface.53,67,68 In addition to large-scale molecular rearrangements, the actin cytoskeleton is also critical for the formation of new TCR microclusters and for the centripetal movement of microclusters of signalling proteins and adhesion molecules.16,25 The segregation of signalling and adhesion molecules into separate domains also appears to be a common phenotype of all ISs and kinapses, and occurs even at the level of microclusters early after conjugation, before IS formation is complete.25 Microclusters containing small numbers of receptors and associated signalling molecules are found not only in T cell–APC ISs, but also in the ISs formed by B cells and NK cells.69–71 It is likely that actin-associated microclusters of signalling and adhesion molecules are a basic unit of all ISs and kinapses. The formation of the classical ISs has been proposed to occur via centripetal movement of TCR and LFA-1 microclusters, with highly actin-dependent clusters of adhesion molecules failing to enter the nascent cSMAC because of the relative paucity of actin at the centre of the T cell–APC contact zone.25
What is the role of microclusters in forming multifocal ISs? We propose that microclusters are present in the ISs formed by DP thymocytes and Th2 cells and in T cell–DC ISs, but that F-actin is not completely excluded from the centre of the cell–cell interface in these ISs, perhaps because of differences in the initial signalling events after TCR stimulation. Microclusters have been observed fusing together to form larger clusters prior to consolidation in a cSMAC.16 A similar coalescence may take place in nascent multifocal ISs, but with the presence of actin and actin-associated adhesion molecules in the centre of the contact zone preventing cSMAC formation. Instead, these larger clusters of TCR–pMHC might create a barrier to the diffusion of adhesion molecules, as has been observed in the cSMAC,25 resulting in multifocal ISs with adhesion molecules interspersed among, but excluded from, TCR–pMHC accumulations. Experiments designed to observe F-actin dynamics and microcluster formation in nascent multifocal ISs will be necessary to test this hypothesis.
Why is there such diversity in IS structure?
There is evidence that the cSMAC plays a role in modulating signals through the TCR,61,72,73 and serves as a site of TCR down-regulation.16,26 We propose that these functions happen at sites of TCR–pMHC accumulation, but do not necessarily require the formation of a cSMAC. Thus, TCR down-modulation could occur in the TCR–pMHC clusters in multifocal ISs or in the uropod of T cells forming kinapses. The propensity of DP thymocytes, naïve T cells (when forming ISs with DCs) and Th2 cells to form multifocal ISs suggests that bull's-eye ISs with well-defined SMACs are not required for thymocyte selection, T-cell priming or Th2 function, at least under some conditions.28,32,38
Well-defined SMACs are a hallmark of the ISs formed by NK cells, CD8+ CTLs and Th1 cells.12,32,50 In fact, CD8+ CTLs are capable of forming antigen-independent LFA-1–ICAM-1 rings, suggesting that these cells may be ‘primed’ to form classical ISs.74 Cytolytic granules are delivered to target cells at the cSMAC,11,46,47 and the pSMAC is critical for efficient target-cell lysis46,75. Additionally, Th cells are capable of secreting some cytokines, including IFN-γ and IL-10, directly at the APC, while others, such as IL-4, are secreted multidirectionally.48 Thus, while multifocal ISs and immunological kinapses are sufficient for signal integration and T-cell priming, the delivery of certain effector molecules, such as the inflammatory cytokine IFN-γ, and cytolytic granules necessitates the formation of a classical, bull's-eye IS. We suggest that IS macro-structure is linked to function. Cells being selected in the thymus, primed in peripheral lymphoid organs or secreting Th2 cytokines form immunological kinapses and non-classical ISs, while cells that function by delivering inflammatory or cytotoxic effector functions specifically to antigen-presenting targets are poised to form classical ISs with well-defined SMACs.
Acknowledgments
We wish to thank Susan Murray and Yoshinobu Koguchi for their critical reading of the manuscript. This work was supported by NIH grant AI50823 to D.C.P. T.J.T. was supported by training grant T32-AI078903.
Disclosures
The authors have no financial disclosures.
References
- 1.Geiger B, Rosen D, Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol. 1982;95:137–43. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupfer A, Swain SL, Singer SJ. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987;165:1565–80. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delon J, Bercovici N, Liblau R, Trautmann A. Imaging antigen recognition by naive CD4+ T cells: compulsory cytoskeletal alterations for the triggering of an intracellular calcium response. Eur J Immunol. 1998;28:716–29. doi: 10.1002/(SICI)1521-4141(199802)28:02<716::AID-IMMU716>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Donnadieu E, Bismuth G, Trautmann A. Antigen recognition by helper T cells elicits a sequence of distinct changes of their shape and intracellular calcium. Curr Biol. 1994;4:584–95. doi: 10.1016/s0960-9822(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 5.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997;94:3909–13. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 7.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 8.Bromley SK, Burack WR, Johnson KG, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–96. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 9.Barcia C, Thomas CE, Curtin JF, et al. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med. 2006;203:2095–107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potter TA, Grebe K, Freiberg B, Kupfer A. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc Natl Acad Sci USA. 2001;98:12624–29. doi: 10.1073/pnas.221458898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–61. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 12.Krzewski K, Strominger JL. The killer's kiss: the many functions of NK cell immunological synapses. Curr Opin Cell Biol. 2008;20:597–605. doi: 10.1016/j.ceb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–7. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 14.Leupin O, Zaru R, Laroche T, Muller S, Valitutti S. Exclusion of CD45 from the T-cell receptor signaling area in antigen-stimulated T lymphocytes. Curr Biol. 2000;10:277–80. doi: 10.1016/s0960-9822(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KG, Bromley SK, Dustin ML, Thomas ML. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc Natl Acad Sci USA. 2000;97:10138–43. doi: 10.1073/pnas.97.18.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–27. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–37. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 18.Singleton KL, Roybal KT, Sun Y, Fu G, Gascoigne NR, van Oers NS, Wulfing C. Spatiotemporal patterning during T cell activation is highly diverse. Sci Signal. 2009;2:ra15. doi: 10.1126/scisignal.2000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–42. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 20.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–62. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 21.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–6. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C q translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–75. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10:531–9. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci USA. 2007;104:20296–301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vardhana S, Choudhuri K, Varma R, Dustin ML. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity. 2010;32:531–40. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richie LI, Ebert PJ, Wu LC, Krummel MF, Owen JJ, Davis MM. Imaging synapse formation during thymocyte selection: inability of CD3z to form a stable central accumulation during negative selection. Immunity. 2002;16:595–606. doi: 10.1016/s1074-7613(02)00299-6. [DOI] [PubMed] [Google Scholar]
- 28.Hailman E, Burack WR, Shaw AS, Dustin ML, Allen PM. Immature CD4+ CD8+ thymocytes form a multifocal immunological synapse with sustained tyrosine phosphorylation. Immunity. 2002;16:839–48. doi: 10.1016/s1074-7613(02)00326-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Hori Y, Chakraborty AK. Low T cell receptor expression and thermal fluctuations contribute to formation of dynamic multifocal synapses in thymocytes. Proc Natl Acad Sci USA. 2003;100:4383–8. doi: 10.1073/pnas.0630563100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balamuth F, Leitenberg D, Unternaehrer J, Mellman I, Bottomly K. Distinct patterns of membrane microdomain partitioning in Th1 and Th2 cells. Immunity. 2001;15:729–38. doi: 10.1016/s1074-7613(01)00223-0. [DOI] [PubMed] [Google Scholar]
- 31.Jackman RP, Balamuth F, Bottomly K. CTLA-4 differentially regulates the immunological synapse in CD4 T cell subsets. J Immunol. 2007;178:5543–51. doi: 10.4049/jimmunol.178.9.5543. [DOI] [PubMed] [Google Scholar]
- 32.Thauland TJ, Koguchi Y, Wetzel SA, Dustin ML, Parker DC. Th1 and Th2 cells from morphologically distinct immunological synapses. J Immunol. 2008;181:393–9. doi: 10.4049/jimmunol.181.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heissmeyer V, Macian F, Im SH, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–65. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 34.Carlin LM, Yanagi K, Verhoef A, et al. Secretion of IFN-g and not IL-2 by anergic human T cells correlates with assembly of an immature immune synapse. Blood. 2005;106:3874–9. doi: 10.1182/blood-2005-03-0996. [DOI] [PubMed] [Google Scholar]
- 35.Ise W, Nakamura K, Shimizu N, Goto H, Fujimoto K, Kaminogawa S, Hachimura S. Orally tolerized T cells can form conjugates with APCs but are defective in immunological synapse formation. J Immunol. 2005;175:829–38. doi: 10.4049/jimmunol.175.2.829. [DOI] [PubMed] [Google Scholar]
- 36.Zambricki E, Zal T, Yachi P, Shigeoka A, Sprent J, Gascoigne N, McKay D. In vivo anergized T cells form altered immunological synapses in vitro. Am J Transplant. 2006;6:2572–9. doi: 10.1111/j.1600-6143.2006.01517.x. [DOI] [PubMed] [Google Scholar]
- 37.Doherty M, Osborne DG, Browning DL, Parker DC, Wetzel SA. Anergic CD4+ T Cells Form Mature Immunological Synapses with Enhanced Accumulation of c-Cbl and Cbl-b. J Immunol. 2010;184:3598–608. doi: 10.4049/jimmunol.0902285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brossard C, Feuillet V, Schmitt A, Randriamampita C, Romao M, Raposo G, Trautmann A. Multifocal structure of the T cell - dendritic cell synapse. Eur J Immunol. 2005;35:1741–53. doi: 10.1002/eji.200425857. [DOI] [PubMed] [Google Scholar]
- 39.Fisher PJ, Bulur PA, Vuk-Pavlovic S, Prendergast FG, Dietz AB. Dendritic cell microvilli-a novel membrane structure associated with multifocal synapse and T cell clustering. Blood. 2008;112:5037–45. doi: 10.1182/blood-2008-04-149526. [DOI] [PubMed] [Google Scholar]
- 40.Tseng SY, Waite JC, Liu M, Vardhana S, Dustin ML. T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase C-q. J Immunol. 2008;181:4852–63. doi: 10.4049/jimmunol.181.7.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benvenuti F, Lagaudriere-Gesbert C, Grandjean I, Jancic C, Hivroz C, Trautmann A, Lantz O, Amigorena S. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J Immunol. 2004;172:292–301. doi: 10.4049/jimmunol.172.1.292. [DOI] [PubMed] [Google Scholar]
- 42.Kupfer A, Swain SL, Janeway CA, Singer SJ. The specific direct interaction of helper T cells and antigen-presenting B cells. Proc Natl Acad Sci USA. 1986;83:6080–3. doi: 10.1073/pnas.83.16.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al-Alwan MM, Liwski RS, Haeryfar SM, Baldridge WH, Hoskin DW, Rowden G, West KA. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J Immunol. 2003;171:4479–83. doi: 10.4049/jimmunol.171.9.4479. [DOI] [PubMed] [Google Scholar]
- 44.Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–6. doi: 10.4049/jimmunol.166.3.1452. [DOI] [PubMed] [Google Scholar]
- 45.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, Gunzer M. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–29. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 46.Beal AM, Anikeeva N, Varma R, Cameron TO, Norris PJ, Dustin ML, Sykulev Y. Protein kinase C-q regulates stability of the peripheral adhesion ring junction and contributes to the sensitivity of target cell lysis by CTL. J Immunol. 2008;181:4815–24. doi: 10.4049/jimmunol.181.7.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beal AM, Anikeeva N, Varma R, Cameron TO, Vasiliver-Shamis G, Norris PJ, Dustin ML, Sykulev Y. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 2009;31:632–42. doi: 10.1016/j.immuni.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–55. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 49.Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat Immunol. 2008;9:1105–11. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stinchcombe JC, Griffiths GM. The role of the secretory immunological synapse in killing by CD8+ CTL. Semin Immunol. 2003;15:301–5. doi: 10.1016/j.smim.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 51.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–5. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 52.Koguchi Y, Thauland TJ, Slifka MK, Parker DC. Preformed CD40 ligand exists in secretory lysosomes in effector and memory CD4+ T cells and is quickly expressed on the cell surface in an antigen-specific manner. Blood. 2007;110:2520–7. doi: 10.1182/blood-2007-03-081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wetzel SA, McKeithan TW, Parker DC. Live cell dynamics and the role of costimulation in immunological synapse formation. J Immunol. 2002;169:6092–101. doi: 10.4049/jimmunol.169.11.6092. [DOI] [PubMed] [Google Scholar]
- 54.Wülfing C, Sumen C, Sjaastad MD, Wu LC, Dustin ML, Davis MM. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol. 2002;3:42–7. doi: 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- 55.Huang J, Lo PF, Zal T, Gascoigne NR, Smith BA, Levin SD, Grey HM. CD28 plays a critical role in the segregation of PKC q within the immunologic synapse. Proc Natl Acad Sci USA. 2002;99:9369–73. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bromley SK, Iaboni A, Davis SJ, Whitty A, Green JM, Shaw AS, Weiss A, Dustin ML. The immunological synapse and CD28-CD80 interactions. Nat Immunol. 2001;2:1159–66. doi: 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- 57.O'Keefe JP, Blaine K, Alegre ML, Gajewski TF. Formation of a central supramolecular activation cluster is not required for activation of naive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:9351–6. doi: 10.1073/pnas.0305965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tseng SY, Liu M, Dustin ML. CD80 cytoplasmic domain controls localization of CD28, CTLA-4, and protein kinase C-θ in the immunological synapse. J Immunol. 2005;175:7829–36. doi: 10.4049/jimmunol.175.12.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–55. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 60.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–9. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 61.Cemerski S, Das J, Locasale J, et al. The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse. Immunity. 2007;26:345–55. doi: 10.1016/j.immuni.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sumen C, Dustin ML, Davis MM. T cell receptor antagonism interferes with MHC clustering and integrin patterning during immunological synapse formation. J Cell Biol. 2004;166:579–90. doi: 10.1083/jcb.200404059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, Kampgen E, Friedl P. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–32. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 64.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 65.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–56. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sims TN, Soos TJ, Xenias HS, et al. Opposing effects of PKCq and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–85. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 67.Krummel MF, Sjaastad MD, Wülfing C, Davis MM. Differential clustering of CD4 and CD3z during T cell recognition. Science. 2000;289:1349–52. doi: 10.1126/science.289.5483.1349. [DOI] [PubMed] [Google Scholar]
- 68.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–59. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 69.Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, Marchbank KL, Tybulewicz VL, Batista FD. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol. 2008;9:63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- 70.Sohn HW, Tolar P, Jin T, Pierce SK. Fluorescence resonance energy transfer in living cells reveals dynamic membrane changes in the initiation of B cell signaling. Proc Natl Acad Sci USA. 2006;103:8143–8. doi: 10.1073/pnas.0509858103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Treanor B, Lanigan PM, Kumar S, et al. Microclusters of inhibitory killer immunoglobulin-like receptor signaling at natural killer cell immunological synapses. J Cell Biol. 2006;174:153–61. doi: 10.1083/jcb.200601108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–22. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee KH, Dinner AR, Tu C, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–22. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 74.Somersalo K, Anikeeva N, Sims TN, et al. Cytotoxic T lymphocytes form an antigen-independent ring junction. J Clin Invest. 2004;113:49–57. doi: 10.1172/JCI200419337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anikeeva N, Somersalo K, Sims TN, Thomas VK, Dustin ML, Sykulev Y. Distinct role of lymphocyte function-associated antigen-1 in mediating effective cytolytic activity by cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 2005;102:6437–42. doi: 10.1073/pnas.0502467102. [DOI] [PMC free article] [PubMed] [Google Scholar]


