Abstract
15-Deoxy-Δ12,14-prostaglandin J2 (15-d-PGJ2) is a reactive cyclopentenone eicosanoid generated from the dehydration of cyclooxygenase-derived prostaglandin D2 (PGD2). This compound possesses an α,β-unsaturated carbonyl moiety that can readily adduct thiol-containing biomolecules such as glutathione and cysteine residues of proteins via the Michael addition. Due to its reactivity, 15-d-PGJ2 is thought to modulate inflammatory and apoptotic processes and is believed to be an endogenous ligand for peroxisome proliferator-activated receptor-γ. However, the extent to which 15-d-PGJ2 is formed in vivo and the mechanisms that regulate its formation are unknown. Previously, we have reported the formation of PGD2 and PGJ2-like compounds, termed D2/J2-isoprostanes (D2/J2-IsoPs), produced in vivo by the free radical-catalyzed peroxidation of arachidonic acid (AA). Based on these findings, we investigated whether 15-d-PGJ2-like compounds are also formed via this nonenzymatic pathway. Here we report the generation of novel 15-d-PGJ2-like compounds, termed deoxy-J2-isoprostanes (deoxy-J2-IsoPs), in vivo, via the nonenzymatic peroxidation of AA. Levels of deoxy-J2-IsoPs increased 12-fold (6.4 ± 1.1 ng/g liver) in rats after oxidant insult by CCl4 treatment, compared with basal levels (0.55 ± 0.21 ng/g liver). These compounds may have important bioactivities in vivo under conditions associated with oxidant stress.
Keywords: 15-d-PGJ2, cyclopentenone eicosanoid, lipid peroxidation, oxidant stress, isoprostane
Cyclooxygenase 1 (COX-1) and COX-2 catalyze the committed step in the formation of prostaglandins (PG) from arachidonic acid (AA) by generating the unstable bicyclic endoperoxide intermediate, PGH2 (1–3). Tissue-specific synthases convert PGH2 to the parent PGs PGF2α, PGE2, PGD2, PGI2, and thromboxane (TXA2) (1). These molecules are formed in response to various stimuli, and they exert a plethora of biological effects through interaction with cell surface receptors (1).
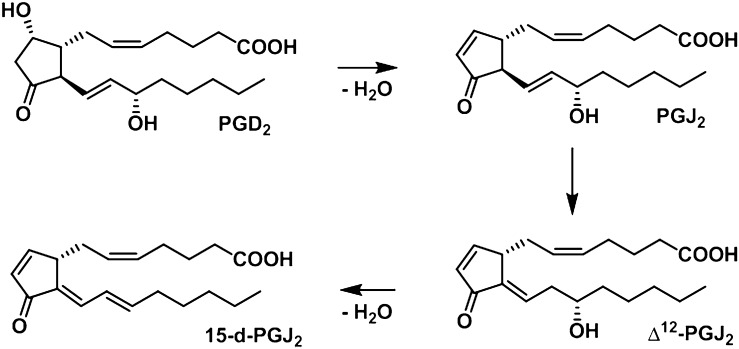
PGE2 and PGD2 are unstable and readily undergo dehydration in an aqueous solution to form cyclopentenone PGs PGA2 and PGJ2, respectively. Dehydration of PGE2 results in formation of PGA2 (4). Dehydration of PGD2 results in the formation of PGJ2 (5). PGJ2 can isomerize in vitro in the presence of albumin to Δ12-PGJ2 and then undergo dehydration to yield 15-deoxy-Δ12,14-PGJ2 (15-d-PGJ2) (Fig. 1) (5). Unlike other classes of PGs, cyclopentenone PGs are characterized by the presence of an electrophilic α,β-unsaturated carbonyl moiety in the prostane ring, rendering them susceptible to Michael addition with nucleophilic biomolecules, such as the free sulfhydryls of glutathione (GSH) and cysteine residues of proteins (6). These compounds have attracted considerable attention because they exhibit a unique spectrum of biological activities, presumably through reaction with cellular biomolecules (6). 15-d-PGJ2, one of the most well-defined cyclopentenone PGs, is particularly reactive because it possesses two electrophilic carbon centers that are susceptible to nucleophilic attack: one center is in the cyclopentenone ring (C-9), and the other is on the lower side chain (C-13). This compound is believed to mediate a number of cellular responses through covalent interaction with critical intracellular protein targets (7, 8). 15-d-PGJ2 is postulated to be an endogenous ligand of peroxisome proliferator-activated receptor-γ (PPAR-γ), which plays an important role in adipocyte differentiation and antiinflammatory processes (9). 15-d-PGJ2 has also been shown to exhibit antiinflammatory, antiproliferative, and proapoptotic activities independent of PPAR-γ in studies performed in vitro. However, the extent to which 15-d-PGJ2 is formed in vivo and the mechanisms regulating its formation remain unclear.
Fig. 1.
15-d-PGJ2 is formed from PGD2 through a sequence of dehydration and isomerization reactions.
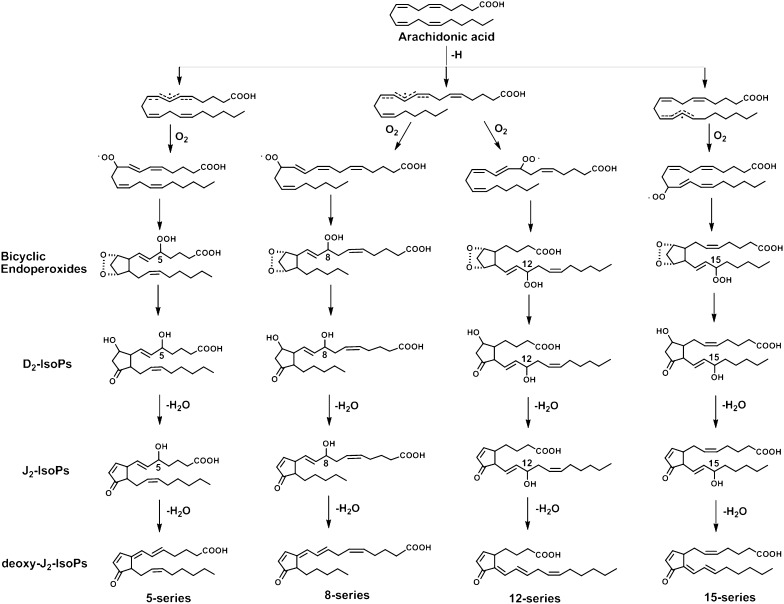
Oxidant stress has been increasingly implicated in the pathogenesis of a number of human diseases, including atherosclerosis, cancer, neurodegenerative disorders, and even the normal aging process (10–13). Lipid peroxidation is a central feature of oxidant stress. We have previously reported discovery of PG-like compounds, termed isoprostanes (IsoP), which are produced in vivo from free radical-induced peroxidation of AA, independent of COX (14). IsoPs are isomeric to PGs, differing in the stereochemical relationships of the two side chains on the prostane ring. PG side chains are in the trans configuration with respect to the prostane ring, whereas the majority of IsoP side chains are in the cis configuration. Additionally, unlike PGs, IsoPs are initially formed in situ in phospholipids and are then subsequently hydrolyzed to their free form by phospholipases (15, 16). The mechanism for the formation of IsoPs from AA has been well defined. Following abstraction of a bisallylic hydrogen from AA and the addition of a molecule of oxygen to form a peroxyl radical, the radical undergoes 5-exo cyclization, and a second molecule of oxygen is added to the backbone of the compound to form PGG2-like compounds (17, 18). These unstable bicycloendoperoxide intermediates can be reduced to PGF2α-like compounds, termed F2-IsoPs (14), or they undergo rearrangement to PGE2- and PGD2-like compounds (E2/D2-IsoPs) (19) or TXA2-like compounds (Tx-IsoPs). Based on this mechanism of formation, four regioisomers of each class of IsoPs derived from AA are generated, each of which can theoretically be composed of eight racemic diastereomers. Compounds are denoted 5-, 8- 12-, or 15-series regioisomers depending on the carbon atom to which the side chain hydroxyl is attached (20).
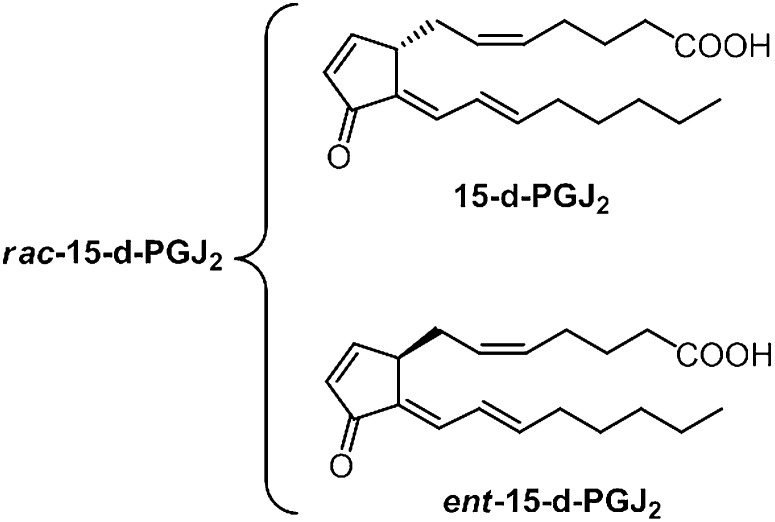
The extent to which cyclopentenone prostanoids are generated in vivo has been the subject of continuing controversy for the last 3 decades (21–24). We have previously reported that, analogous to the formation of PGA2 and PGJ2 from the dehydration of COX-generated PGE2 and PGD2, respectively, cyclopentenone IsoPs (A2/J2-IsoPs) are also formed in vivo from the dehydration of E2/D2-IsoPs (25). However, evidence for the formation of 15-d-PGJ2 in vivo is lacking. Hirata et al. (21) reported that the precursor of 15-d-PGJ2, Δ12-PGJ2, can be detected in human urine and that its formation is suppressed to some extent by COX inhibitors. Levels of free 15-d-PGJ2 excreted in human urine were found to be very low (22). Heretofore, it has been assumed that synthesis of 15-d-PGJ2 depends upon COX generation of PGD2. Given their structural and functional similarity, it is highly likely that similar to the dehydration of COX-derived PGD2, D2-IsoPs can undergo dehydration and isomerization to yield a series of 15-d-PGJ2-like compounds, termed deoxy-J2-IsoPs. A proposed mechanism for formation of the four regioisomers of deoxy-J2-IsoPs is shown in Fig. 2. It should be noted that dehydration of the 15- series D2-IsoPs followed by isomerization of the 13,14 double bond would result in loss of the chiral center at C-12, eliminating the stereochemical orientation of the lower side chain that distinguishes IsoPs from PGs. A single chiral center would be retained at C-8. Thus, a compound identical in all respects to 15-d-PGJ2 and its corresponding enantiomer would be expected to form from dehydration and isomerization of 15-D2-IsoP (Fig. 3). For purposes of discussion hereafter, the PG possessing a structure identical to that generated by COX is referred to as 15-d-PGJ2. The compound that is enantiomeric to COX-derived 15-d-PGJ2 is referred to as ent-15-d-PGJ2. The racemic mixture is termed rac-15-d-PGJ2. Dehydration of other regioisomers of D2-IsoPs yields various regioisomers of deoxy-J2-IsoPs (Fig. 2).
Fig. 2.
Proposed pathway for the formation of the deoxy-J2 -IsoPs via the nonenzymatic peroxidation of AA. For simplicity, the stereochemistry of both the lipid peroxidation intermediates and fi nal IsoPs are not shown.
Fig. 3.
Structures of rac-15-d-PGJ2, consisting of 15-d-PGJ2 and its enantiomer.
Herein, we report that 15-d-PGJ2 and 15-d-PGJ2-like compounds (deoxy-J2-IsoPs) are formed in significant abundance in vitro and in vivo via the nonenzymatic free radical-catalyzed peroxidation of AA, independent of COX enzymes. The finding that a series of deoxy-J2-IsoPs are generated in vivo through lipid peroxidation is of potential biological importance due to the predicted bioactivity of these compounds. Similar to 15-d-PGJ2, each of the deoxy-J2-IsoPs possesses reactive electrophilic carbon centers, which render them susceptible to nucleophilic addition reactions with thiol-containing biomolecules, such as GSH and cysteine residues of proteins. Because the biological effects of 15-d-PGJ2 are often likely to be mediated by the compound's reactivity with cellular thiols, all of the deoxy-J2-IsoPs would be expected to exert similar biological effects in vivo under settings of oxidant stress.
MATERIALS AND METHODS
Chemical reagents and supplies
Human serum albumin, CCl4, triphenyl-phosphine (TPP), and Apis mellifera venom phospholipase A2 (PLA2) were purchased from Sigma-Aldrich. PGD2, 15-d-PGJ2, and isotopically labeled 15-d-PGJ2 ([2H4]15-d-PGJ2) was purchased from Cayman Chemical Co. (Ann Arbor, MI). Arachidonic acid was purchased from NU-Check Prep, Inc. (Elysian, MN). The C18 Sep-Paks were purchased from Waters Corporation (Milford, MA). High-performance liquid chromatography (HPLC) columns were purchased from Phenomenex (Torrance, CA). All solvents were of HPLC quality and were purchased from EM Science (Gibbstown, NJ).
Oxidation of AA in vitro
Five mg of AA was dissolved in 50 μl of ethanol and added immediately to 4.95 ml of phosphate-buffered saline (PBS) (pH 7.4) containing 10 mM 2,2′-azobis(amidinopropane)dihydrochloride (AAPH), a free radical initiator. The AA oxidation reaction mixture was incubated at 37°C for 24 h. Following oxidation, excess triphenylphosphine (TPP) was added to the oxidized AA mixture (1 mg/ml) for 5 min at room temperature to reduce hydroperoxides to hydroxyls. Subsequently, the oxidized AA sample was extracted twice with 2 ml of ethyl acetate, and extracts were dried under nitrogen gas. Samples were stored in 200 μl of ethanol at −80°C until analyzed by LC/tandem mass spectrometry (MS/MS).
Isolation of 15-d-PGJ2-like compounds (deoxy-J2-IsoPs) from rat liver
CCl4 (1 mg/kg body weight in 1 ml of corn oil) was administered orogastrically to male Sprague-Dawley rats to induce lipid peroxidation (14). Control animals received corn oil alone. Four hours after treatment, the animals were anesthetized with pentobarbital (60 mg/kg, intraperitoneally) and euthanized. Livers were removed and immediately flash-frozen in liquid nitrogen and stored at −80°C. Tissues samples weighing 300–500 mg were homogenized in 5 ml of ice-cold chloroform-methanol (2:1, v/v) containing butylated hydroxytoluene (0.005%) to prevent ex vivo autooxidation. Esterified deoxy-J2-IsoPs in liver phospholipids were enzymatically hydrolyzed with A. mellifera venom PLA2 to liberate free deoxy-J2-IsoPs. Subsequently, free IsoPs were extracted over a C18 Sep-Pak cartridge that had been preconditioned by rinsing with methanol and deionized water, pH 3. The C18 Sep-Pak cartridge was rinsed with 10 ml each of deionized water (pH 3) and heptane, and the sample was eluted with 10 ml of 1:1 ethyl acetate-heptane. The solvent was removed by evaporation under a stream of N2, and samples were stored in 200 μl of ethanol at −80°C until LC/MS/MS analysis.
Analysis of 15-d-PGJ2-like compounds (deoxy-J2-IsoPs) by LC/MS/MS
After oxidized AA was extracted by using the C18 Sep-Pak method described above, the sample was suspended in 100 μl of 2:1 methanol-water, and deoxy-J2-IsoPs were analyzed by reverse-phase HPLC. Online HPLC was carried out using a Surveyor MS pump equipped with a Phenomenex Luna C18 column (50 × 2.00 mm, 3 μm) at a flow rate of 0.3 ml/min starting with 80% Phase 1 (water-phase 2-acetic acid, 95:5:0.1) to 30% from 1 to 27.0 min and holding at 100% Phase 2 (acetonitrile-methanol-acetic acid, 95:5:0.1) from 28.0 to 29.0 min and returning to 80% Phase 1 at 30.0 to 32.0 min. LC/electrospray ionization (ESI)-MS/MS was carried out with a ThermoFinnigan TSQ/Quantum Ultra mass spectrometer. The ESI source was fitted with a deactivated fused silica capillary (100-μm inner diameter), and the mass spectrometer was operated in the negative ion mode. Nitrogen was used as both the sheath gas and the auxiliary gas, at 31 and 17 psi, respectively. The capillary temperature was 300°C. The spray voltage was 5.0 kV, and the tube lens voltage was 80 V. In another set of experiments, deoxy-J2-IsoPs were analyzed by normal phase HPLC/atmospheric pressure chemical ionization (APCI)-MS for separation of deoxy-J2-IsoP regioisomers. For these experiments, deoxy-J2-IsoPs were extracted by C18 Sep-Pak chromatography as described previously, and samples were resuspended in mobile phase (93:7, hexane-isopropanol-acetic acid) for normal phase HPLC. Online HPLC was carried out using a Waters Alliance HPLC equipped with a Phenomenex Luna silica column (250 × 4.60 mm, 5 μm) at a flow rate of 1.0 ml/min with an isocratic gradient of 93:7:0.1, hexane-isopropanol-acetic acid for 20 min. LC/APCI-MS was carried out with a ThermoFinnigan TSQ/Quantum Ultra mass spectrometer operating in the negative ion mode with an APCI source. MS/MS analyses using collision-induced dissociation (CID) of the precursor ion m/z of 315 of putative 15-d-PGJ2 and deoxy-J2-IsoPs were performed from 18 to 27 eV under 1.5 mTorr of argon. Spectra shown were obtained at 24 eV. Spectra were displayed by averaging scans across chromatographic peaks. Selected reaction monitoring (SRM) was performed according to the characteristic fragmentation patterns of 15-d-PGJ2 determined by CID. The collision energy for SRM was 24 eV. Data acquisition and analysis were performed using Xcaliber version 2.0 software.
Adduction of deoxy-J2-IsoPs with glutathione in vitro
To isolate deoxy-J2-IsoPs in vitro, AA was oxidized as described above and extracted with ethyl acetate. The sample was dried under a stream of N2 and reconstituted in 100 μl of ethanol. This sample was dissolved in 0.5 ml of 1 N HCl and incubated for 5 h at 37°C to facilitate acid-catalyzed dehydration of D2/J2-IsoPs to deoxy-J2-IsoPs. Following incubation, the reaction mixture was diluted to 10 ml with 50 mM aqueous ammonium acetate (pH 3.4) and extracted three times with 1.5–2 ml of ethyl acetate. Extracts were pooled, dried under a stream of N2, and reconstituted in 100 μl of ethanol. This sample was dissolved in 1 ml of PBS (pH 6.5) and incubated in the presence of excess reduced GSH (∼1 mg) and ∼1 mg of equine liver glutathione S-transferase (GST) for 2 h at 37°C. A similar method was used for the incubation of chemically synthesized 15-d-PGJ2 in the presence of GSH and GST. After the incubation, GSH adducts of deoxy-J2-IsoPs and synthesized 15-d-PGJ2 were purified by extraction using a C18 Sep-Pak cartridge preconditioned with 10 ml of acetonitrile and 50 mM aqueous ammonium acetate (pH 3.4) and eluted with 10 ml of 95% ethanol, and dried under a stream of N2. Samples were suspended in a 3:1 mixture of Phase 1-Phase 2 (Phase 1, water-phase 2-acetic acid, 95:5:0.1; Phase 2, acetonitrile-methanol-acetic acid, 95:5:0.1), and deoxy-J2-IsoP-GSH adducts were definitively identified by LC/ESI-MS/MS. The reverse-phase HPLC conditions described above were used. For MS/MS experiments, CID analysis of the parent ion m/z 622 of 15-d-PGJ2-GSH adduct and putative deoxy-J2-IsoP-GSH adducts was performed at 18 to 22 eV under 1.5 mTorr of argon. Spectra shown were obtained at 22 eV.
RESULTS
Formation and characterization of 15-d-PGJ2 and 15-d-PGJ2-like compounds in vitro
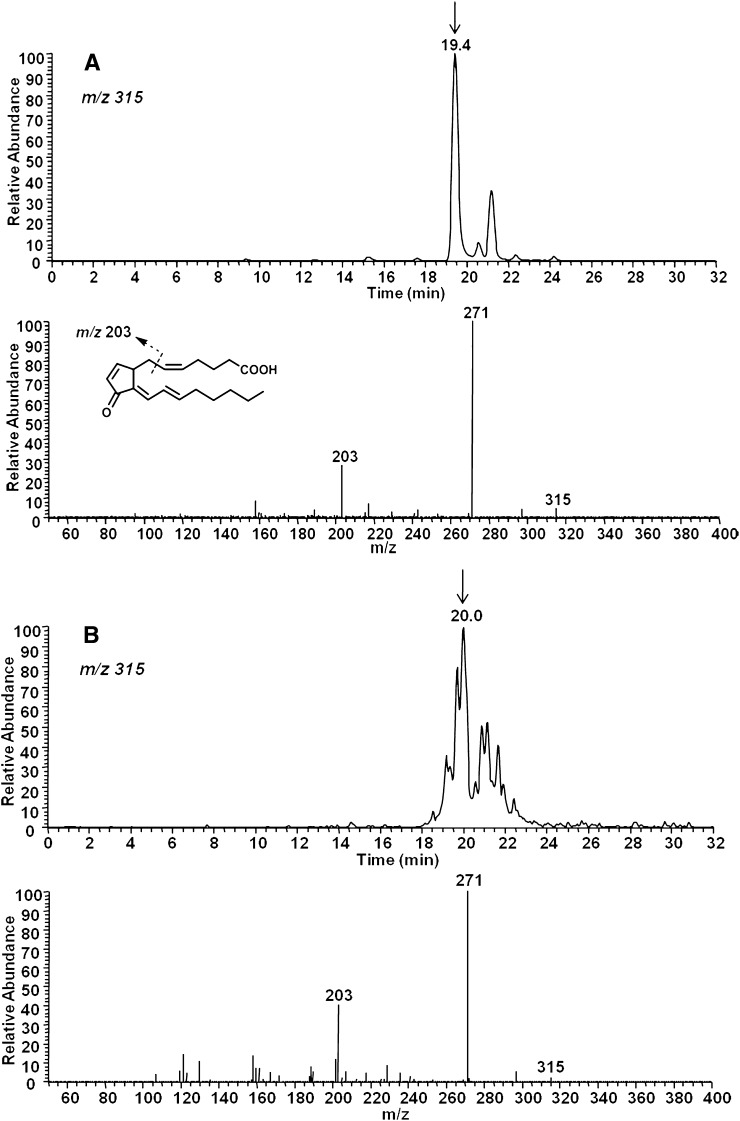
A representative selected ion monitoring (SIM) chromatogram obtained from LC/MS/MS analysis of chemically synthesized 15-d-PGJ2 is shown in Fig. 4A (upper chromatogram) . When analyzed by ESI-MS in the negative ion mode, the predicted precursor ion for 15-d-PGJ2 is m/z 315. The peak eluting at 19.4 min was analyzed by CID, and predicted fragments were obtained (Fig. 4A, lower spectrum). These included m/z 271 [M − CO2]− and m/z 203 (Fig. 4A, lower spectrum). The smaller peak in the upper chromatogram is likely a synthetic isomer of 15-d-PGJ2 as results from LC/ESI-MS/MS analysis of this peak were identical to that of the authentic 15-d-PGJ2.
Fig. 4.
LC/ESI-MS/MS analysis of 15-d-PGJ2 (A) and a mixture of 15-d-PGJ2-like compounds (deoxy-J2-IsoPs) obtained from the nonenzymatic oxidation of AA in vitro (B). A: LC/ESI-MS/MS analysis of a chemically synthesized 15-d-PGJ2 employing SIM (upper chromatogram) and CID (lower product ion spectrum). B: LC/ESI-MS/MS analysis of 15-d-PGJ2-like compounds obtained from the in vitro oxidation of AA employing SIM for the precursor ion m/z 315 (upper chromatogram) and CID (lower product ion spectrum) indicative of putative 15-d-PGJ2.
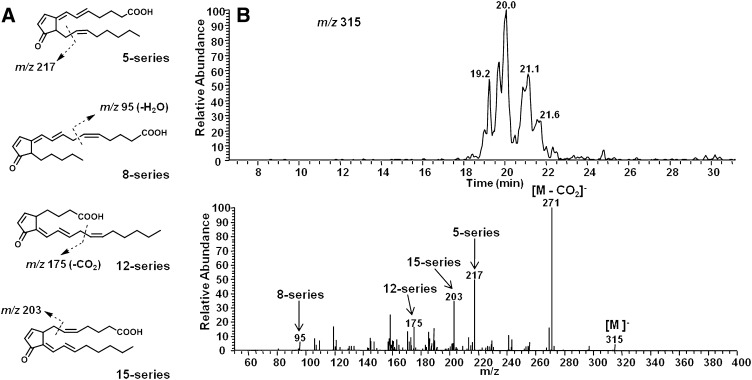
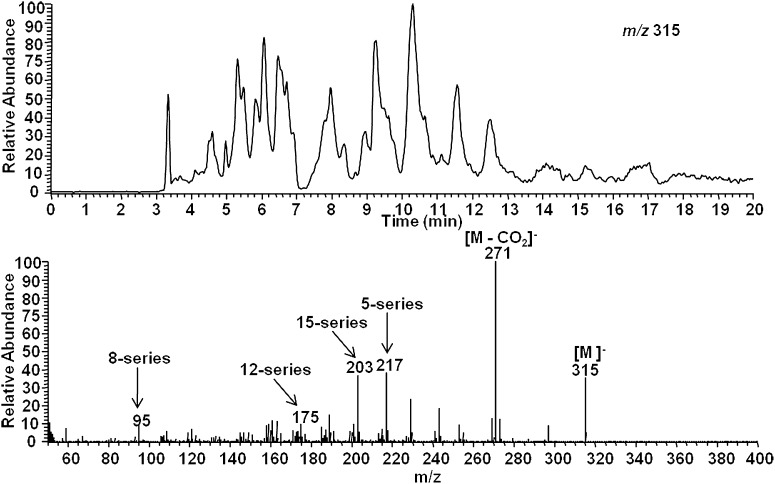
AA was then oxidized in vitro using the free radical initiator AAPH, and extracts were analyzed for 15-d-PGJ2 and other 15-d-PGJ2-like compounds (deoxy-J2-IsoPs). When analyzed by LC/ESI-MS/MS in negative ion mode, the predicted precursor ion for deoxy-J2-IsoPs is m/z315. The SIM chromatogram of this ion is shown in Fig. 4B. As is evident, multiple chromatographic peaks are present that presumably represent different deoxy-J2-IsoP regioisomers obtained from the nonenzymatic peroxidation of AA. The chromatographic peak eluting at 20.0 min has nearly the same retention time as authentic 15-d-PGJ2. An authentic sample of isotopically labeled 15-d-PGJ2 ([2H4]15-d-PGJ2) showed the identical retention time upon co-injection (see below). (Analyses described in legends to Fig. 4A and B were performed on separate days, accounting for differences in LC retention time.) This peak was analyzed by CID to obtain structural information, and the CID spectrum is shown in Fig. 4B. The observed product ions, m/z 271 and m/z 203, are consistent with fragmentation of 15-d-PGJ2. The series of compounds representing deoxy-J2-IsoPs elute in multiple chromatographic peaks at approximately 19–22 min. CID analysis of this series of chromatographic peaks supports the contention that these are 15-d-PGJ2-like compounds derived from different IsoP regioisomers, including compounds of the 5-, 8-, 12-, and 15-series (Fig. 5). The composite CID spectrum obtained by summing scans over the entire series of chromatographic peaks reveals the product ion m/z 271 [M − Co2]− as well as product ions resulting from specific fragmentation of each of the four deoxy-J2-IsoPs regioisomers, including m/z 217 (5-series), m/z 95 (8-series), m/z 175 (12-series), and m/z 203 (15-series). Additionally, we used normal phase HPLC to obtain chromatographic separation of each of the deoxy-J2-IsoPs regioisomers. Shown in Fig. 6 is a representative chromatogram of the different regioisomers of deoxy-J2-IsoPs analyzed by normal phase LC/APCI-MS/MS. Again, CID analysis of the series of chromatographic peaks is consistent with fragmentation of each of the deoxy-J2-IsoP regioisomers (Fig. 6).
Fig. 5.
LC/ESI-MS/MS analysis of multiple regioisomers of deoxy-J2-IsoPs generated from the nonenzymatic oxidation of AA in vitro. A: Structurally specific fragmentation of the different deoxy-J2-IsoP regioisomers. B: SIM chromatogram of the precursor ion at m/z 315 (upper) and CID spectrum (lower) obtained by summing scans over the chromatographic peaks, revealing structurally specific fragmentation of the different deoxy-J2-IsoP regioisomers.
Fig. 6.
Normal phase LC/APCI-MS/MS analysis of multiple regioisomers of deoxy-J2 -IsoPs generated from the nonenzymatic peroxidation of AA in vitro. SIM chromatogram of the parent ion at m/z 315 (upper) and CID spectrum (lower) obtained by summing scans over the series of chromatographic peaks reveals structurally specific fragmentation of the different deoxy-J2 -IsoP regioisomers.
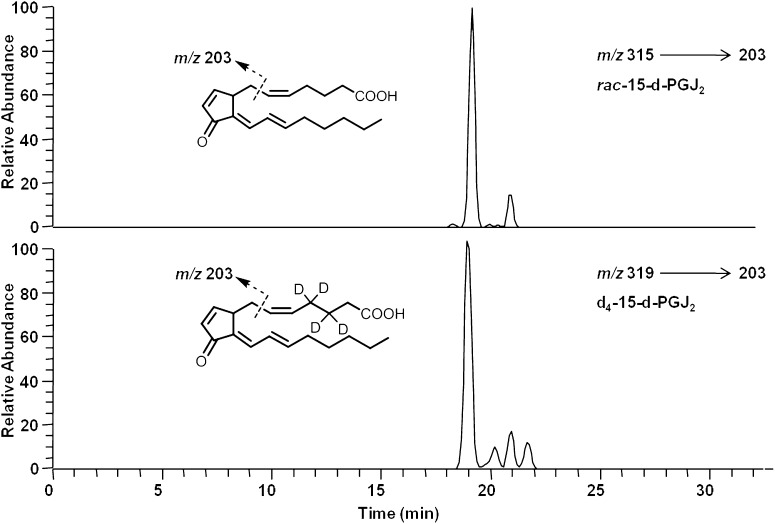
Due to their nonenzymatic formation, compounds generated from the free radical-catalyzed peroxidation of AA would be predicted to be racemic. Thus, 15-series deoxy-J2-IsoPs are predicted to comprise a racemic mixture of a compound identical in all respects to COX-derived 15-d-PGJ2 and its enantiomer, ent-15-d-PGJ2. The racemic mixture is referred to as rac-15-d-PGJ2 (Fig. 3). To confirm the formation of rac-15-d-PGJ2 from the nonenzymatic oxidation of AA, we used LC/MS/MS analysis with SRM. The major product ion unique to rac-15-d-PGJ2 is m/z 203; thus, we monitored the precursor-to-product transition of m/z 315 to m/z 203 to selectively detect rac-15-d-PGJ2. [2H4]15-d-PGJ2 (d4-15-d-PGJ2) was used as an internal standard for these analyses. The precursor ion of d4-15-d-PGJ2 is m/z 319 due to the presence of four deuterium atoms on the upper side chain of the molecule, and CID analysis of d4-15-d-PGJ2 yields the major product ions m/z 275 [M − Co2]− and m/z 203 (data not shown). Shown in Fig. 7 is a representative chromatogram for the SRM analysis of rac-15-d-PGJ2 generated from the nonenzymatic oxidation of AA, and these compounds coelute with d4-15-d-PGJ2. Additional support for the identification of these compounds as 15-d-PGJ2 and ent-15-d-PGJ2 was obtained through LC/ultraviolet (UV) analysis, which showed that these compounds possess a UV absorbance maximum (λmax) of 306 nm, identical to that of authentic 15-d-PGJ2 (data not shown). To assess the total amount of deoxy-J2-IsoPs formed from the nonenzymatic oxidation of AA, LC/MS quantification was performed by measuring the abundance of compounds representing deoxy-J2-IsoPs relative to that of the d4-15-d-PGJ2 internal standard. The mass spectrometer was operated in SRM mode in the negative ion mode, monitoring the precursor-to-product transition from m/z 315 to m/z 271 for the deoxy-J2-IsoPs and monitoring the parent-to-product transition from m/z 319 to m/z 275 for d4-15-d-PGJ2. Deoxy-J2-IsoPs are generated in significant abundance from the nonenzymatic oxidation of AA in vitro, and levels are 16.0 ± 3.4 ng/mg AA (n = 4). In additional studies, when we oxidized a phospholipid containing sn-2-AA (palmitoyl-arachidonyl-phosphatidylcholine [PAPC]) in vitro, we found that 15-d-PGJ2 was formed on the intact phospholipid (data not shown).
Fig. 7.
Analysis of putative rac-15-d-PGJ2 in vitro by LC/MS/MS using SRM. LC/MS/MS analysis of rac-15-d-PGJ2 (A) from the nonenzymatic oxidation of AA in vitro and chemically synthesized [2H4]15-d-PGJ2 (d4-15-d-PGJ2) (B). The mass spectrometer was operated in negative ion mode using SRM to monitor the precursor-to-product transition of m/z 315–203 for rac-15-d-PGJ2 (chromatogram A) and the precursor-to-product transition of m/z 319–203 for d4-15-d-PGJ2 (chromatogram B).
Adduction of deoxy-J2-IsoPs with GSH in vitro
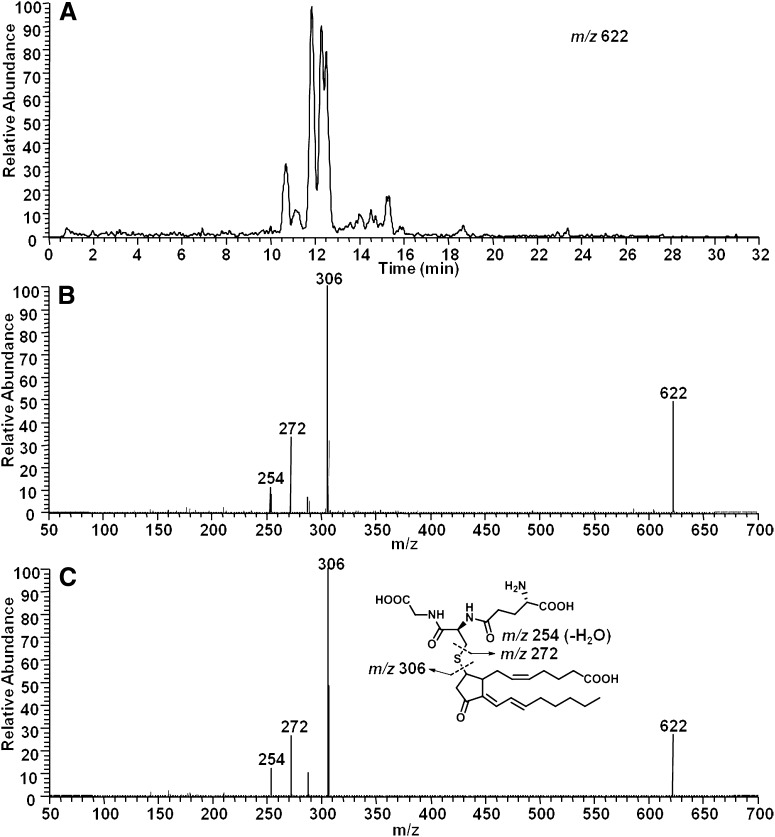
One of our primary interests in the formation of 15-d-PGJ2 and cyclopentenone deoxy-J2-IsoPs in vivo is related to the fact that these molecules contain electrophilic α,β-unsaturated carbonyl moieties which render them susceptible to Michael addition with cellular nucleophiles. Because the biological effects of 15-d-PGJ2 are thought to be mediated largely through its interaction with cellular thiols, all of the deoxy-J2-IsoPs would be expected to exert similar biological effects through adduction to cellular thiols. Thus, as a further characterization of these compounds, we sought to determine whether deoxy-J2-IsoPs would adduct GSH, an abundant cellular thiol, in the presence of GST. We have previously shown that 15-d-PGJ2 and other cyclopentenone eicosanoids readily adduct GSH in the presence of GST in vitro (26–30). In the present study, we incubated deoxy-J2-IsoPs generated from the nonenzymatic oxidation of AA in vitro with GSH in the presence of GST. Adducts were partially purified from an incubation mixture by C18 Sep-Pak extraction and subjected to LC/MS as described above. Putative deoxy-J2-IsoP-GSH adducts were definitively identified by LC/ESI-MS/MS. The predicted precursor ion for deoxy-J2-IsoP-GSH adducts in negative ion mode is m/z 622. SIM analysis showed multiple peaks, with this m/z eluting from 10.7 to 15.3 min, presumably representing the GSH adducts of different deoxy-J2-IsoP regioisomers (Fig. 8A).These adducts elute at a retention time similar to that of the GSH adduct of 15-d-PGJ2. CID experiments confirmed the molecules at m/z 622 to be deoxy-J2-IsoP-GSH adducts. A composite CID spectrum was obtained by summing scans over these peaks (Fig. 8B). The precursor ion, M−, is present at m/z 622. Relevant product ions include m/z 306 [M − deoxy-J2-IsoP]− (loss of the prostanoid portion of the molecule); m/z 272 [M − 350]− (retro-Michael fragmentation); and m/z 254 [272 − H2O]−. A similar CID spectrum was observed when chemically synthesized 15-d-PGJ2 was incubated with GST and GSH (Fig. 8C). It should be noted that the major product ions generated from CID analysis of deoxy-J2-IsoP-GSH adducts were indistinguishable from those generated from the CID analysis of the synthetic 15-d-PGJ2-GSH adduct. Together, these data confirm that the molecules putatively identified as cyclopentenone deoxy-J2-IsoPs are reactive and capable of undergoing nucleophilic addition reactions with GSH.
Fig. 8.
Analysis of GSH adducts of deoxy-J2 -IsoPs and 15-d-PGJ2 by LC/ESI-MS/MS. A: SIM chromatogram of putative deoxy-J2 -IsoP-GSH adducts with the precursor ion m/z 622. B: Composite CID spectrum obtained by summing scans over the series of chromatographic peaks representing deoxy-J2 -IsoP-GSH adducts. C: CID spectrum of the synthesized 15-d-PGJ2 -GSH adduct.
Isolation and quantification of deoxy-J2-IsoPs from rat liver
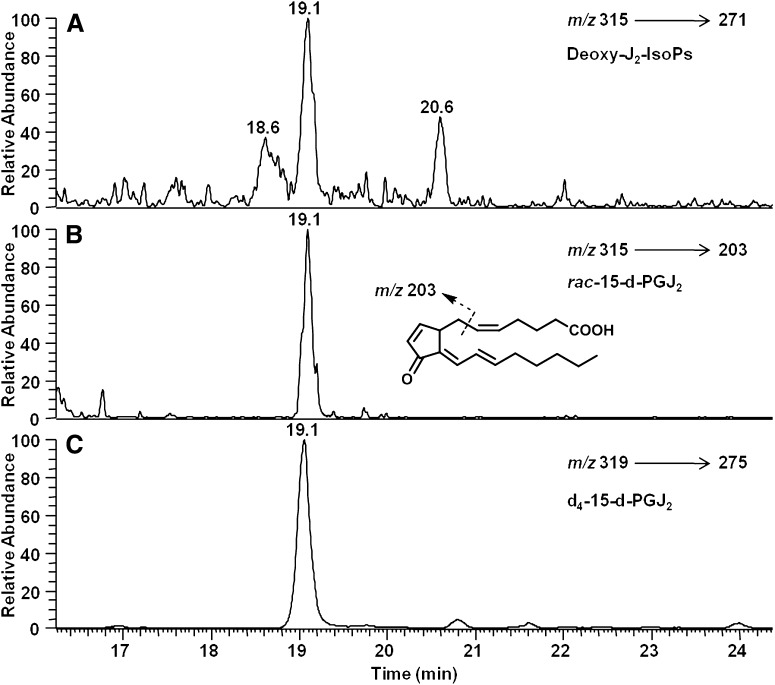
After confirming the formation of rac-15-d-PGJ2 and deoxy-J2-IsoPs via the nonenzymatic oxidation of AA in vitro, we explored whether these compounds are formed in vivo. IsoPs are initially formed in situ, esterified in phospholipids, and hydrolyzed to their free form by platelet-activating factor acetylhydrolase and possibly other phospholipases (15). To quantify F2-IsoPs esterified in tissues, the tissue is initially treated with base to release F2-IsoPs, which are then quantified. However, base treatment will induce dehydration of D2-IsoPs to form J2-IsoPs. Therefore, to quantify esterified deoxy-J2-IsoPs formed in tissue, the tissue was subjected to enzymatic hydrolysis using bee venom PLA2. We found that this method of hydrolysis results in <0.3% dehydration of PGD2 to 15-d-PGJ2. We have previously shown that treatment of rats with CCl4 induces a dramatic increase in esterified IsoPs in the liver (19, 25). Therefore, we examined whether deoxy-J2-IsoPs are formed in vivo esterified in liver phospholipids in rats that had been treated with CCl4. Shown in Fig. 9 is a representative chromatogram from SRM analysis of deoxy-J2-IsoPs (Fig. 9A), including rac-15-d-PGJ2 (Fig. 9B), generated in vivo in liver phospholipids in the rat after CCl4 treatment. Again, the entire series of deoxy-J2-IsoPs was quantified by LC/ESI-MS using SRM as described previously. Levels of deoxy-J2-IsoPs in vivo esterified in liver phospholipids in control rats were near the limit of detection (0.55 ± 0.21 ng/g tissue; n = 5) for this method. However, in animals treated with CCl4, levels increased by ∼12-fold to 6.4 ± 1.1 ng/g tissue (n = 5; P < 0.0001 compared with control). Additionally, we have identified phosphatidylcholine (PC) containing esterified 15-d-PGJ2 (15-d-PGJ2-PC) generated in vivo in rats after oxidant insult (data not shown). These findings provide evidence that deoxy-J2-IsoPs are produced in significant abundance in vivo as products of free radical-induced lipid peroxidation.
Fig. 9.
Analysis of putative deoxy-J2-IsoPs in vivo by LC/MS/MS using SRM. LC/MS/MS analysis of (A) deoxy-J2-IsoPs regioisomers and (B) the 15-series deoxy-J2-IsoPs (rac-15-d-PGJ2) generated in vivo esterified in liver phospholipids in rats after treatment with CCl4 and (C) chemically synthesized [2H4]15-d-PGJ2 (d4-15-d-PGJ2). The mass spectrometer was operated in the negative ion mode with SRM for the precursor-to-product transitions of m/z 315–271 for all deoxy-J2-IsoPs regioisomers (chromatogram A), m/z 315–203 for rac-15-d-PGJ2 (chromatogram B), and m/z 319–275 for d4-15-d-PGJ2 (chromatogram C).
DISCUSSION
We report finding that a series of 15-d-PGJ2-like compounds, termed deoxy-J2-IsoPs, are formed in vitro and in vivo via free radical-catalyzed peroxidation of AA. Among the deoxy-J2 products formed during this process, the 15-series deoxy-J2-IsoPs (rac-15-d-PGJ2) comprises a racemic mixture of a molecule identical in all respects to 15-d-PGJ2 and its corresponding enantiomer. The formation of 15-d-PGJ2 and 15-d-PGJ2-like compounds in vivo is biologically relevant because 15-d-PGJ2 is thought to be an important lipid mediator. Despite significant interest in the bioactivity of 15-d-PGJ2, the extent to which this compound is formed in vivo and the mechanisms that regulate its formation have been unclear. In the present study, we have shown for the first time the formation of 15-d-PGJ2 and 15-d-PGJ2-like compounds in vivo via free radical-catalyzed lipid peroxidation, independent of the COX pathway. Our results suggest that a second pathway exists for the biosynthesis of 15-d-PGJ2 in vivo, and these findings provide further insight into the biochemical mechanisms that can contribute to the endogenous generation of reactive lipid mediators.
15-d-PGJ2 was originally identified as a dehydration product of COX-derived PGD2 (5). Our initial interest in determining whether 15-d-PGJ2 and 15-d-PGJ2-like compounds are formed via the free radical-catalyzed pathway emerged from the observation that cyclopentenone PGA2 and PGJ2-like compounds (A2/J2-IsoPs) are generated in vivo in significant quantities esterified in liver phospholipids in rats following oxidant insult (25). In addition, recent studies by our laboratory support the contention that PGs can be generated through the IsoP pathway, independent of COX enzymes (31, 32). Gao et al. (31) reported the formation of PGE2 and PGD2 and their respective enantiomers in vitro and in vivo through the nonenzymatic peroxidation of AA by epimerization of E2/D2-IsoPs, specifically 15-E2t-IsoP and 15-D2c-IsoP, respectively. More recently, Yin et al. (32) reported the formation of PGF2α in vivo via the IsoP pathway and demonstrated that the majority of putative PGF2α in human urine is derived from free radical-initiated peroxidation of AA, independent of COX enzymes. In the present study, we have found that J2-IsoPs derived from D2-IsoPs can also undergo further dehydration and isomerization in vivo to yield the cyclopentenone deoxy-J2-IsoPs. Dehydration of the 15-series J2-IsoPs yields a compound identical in all respects to COX-derived 15-d-PGJ2 and its enantiomer.
Cyclopentenone eicosanoids as a class have received considerable attention over the past 3 decades due their reactivity (6, 33). These compounds are characterized by the presence of an electrophilic α,β-unsaturated carbonyl moiety in the prostane ring. This functional group renders them susceptible to nucleophilic addition reactions with thiol-containing biomolecules, which has been attributed to their biological effects. 15-d-PGJ2, the most extensively studied compound of the cyclopentenone eicosanoids, is particularly reactive due to the presence of two electrophilic carbons. The presence of two electrophilic centers in 15-d-PGJ2 is thought to contribute to its reactivity and biological potency which are increased compared with those of other cyclopentenone eicosanoids (34). The finding that in addition to 15-d-PGJ2, a series of deoxy-J2-IsoPs are formed in significant abundance in vivo is notable due to the fact that these compounds also possess the reactive electrophilic carbon centers that render them susceptible to reaction with cellular nucleophiles. Like other IsoPs generated via the nonenzymatic peroxidation of AA, deoxy-J2-IsoPs are formed as a series of four regioisomers, each consisting of a mixture of stereoisomers. Deoxy-J2-IsoPs are derived from the dehydration and isomerization of the different D2-IsoP and J2-IsoP regioisomers, including the 5-, 8-, 12-, and 15-series. Of note, the 15-series deoxy-J2-IsoPs comprise a racemic mixture of 15-d-PGJ2 and its enantiomer and are termed rac-15-d-PGJ2. Consistent with the presence of the polyunsaturated carbonyl moiety in each of the deoxy-J2-IsoPs, we have shown that these compounds are reactive and readily undergo nucleophilic addition with GSH. Because this electrophilic reactivity is often likely to be responsible for the bioactivity of 15-d-PGJ2, all of the deoxy-J2-IsoPs are expected to have similar bioactivities related to covalent interaction with intracellular proteins. In this regard, the deoxy-J2-IsoPs represent a new class of lipid peroxidation products that are predicted to have more potent bioactivity than the previously identified cyclopentenone (A2/J2) IsoPs, due to the presence of two reactive electrophilic carbons. These compounds may be important lipid mediators involved in the pathophysiology of oxidant stress.
The extent to which both 15-d-PGJ2 and other deoxy-J2-IsoPs are produced in vivo is biologically relevant because 15-d-PGJ2 has been shown to mediate a wide range of biological activities, including modulating inflammatory and apoptotic processes (35–41). Unlike other PGs, which elicit their biological responses by binding specific G-protein-coupled receptors (GPCRs), 15-d-PGJ2 exerts its biological effects through interaction with key intracellular targets. Some of its activity may relate to the observation that 15-d-PGJ2 activates the nuclear receptor PPAR-γ (9). Activation of PPAR-γ by 15-d-PGJ2 has been recently shown to occur through covalent binding of 15-d-PGJ2 to a critical cysteine residue in the PPAR-γ ligand binding pocket (42). Additionally, 15-d-PGJ2 has been shown to inhibit the inflammatory response in a number of cell types, including monocytes and macrophages, by blocking nuclear factor kappa B (NF-κB)-dependent gene expression via covalent modification of a critical cysteine residue in IκB kinase and the DNA-binding domains of NF-κB subunits (38–41). 15-d-PGJ2 is also thought to be involved in the resolution of inflammation by enhancing apoptosis in activated macrophages through a p38 mitogen-activated protein kinase (MAPK)-dependent increase in reactive oxygen species formation (38). Furthermore, 15-d-PGJ2 activates the antioxidant response pathway via covalent modification of reactive cysteine residues in Keap1, leading to Nrf2 activation and induction of antioxidant proteins, including HO-1, peroxiredoxin 1, γ-GCL, and heat shock protein 70 (43–47). Finally, 15-d-PGJ2 has been reported to inhibit proliferation of vascular smooth muscle cells by inducing cell cycle arrest (48, 49).
The finding that 15-d-PGJ2 and other deoxy-J2-IsoPs are generated in vivo via free radical-induced lipid peroxidation has important implications for the pathophysiological role of these compounds. Generation of 15-d-PGJ2 via the COX pathway has been associated with settings of inflammation (50–52). In the present study, we provide evidence that 15-d-PGJ2 and 15-d-PGJ2-like compounds, the deoxy-J2-IsoPs, are generated in vivo under conditions of oxidant stress. As a result of being generated through the COX and free radical-catalyzed pathways, these compounds may have additive effects in the pathophysiology of diseases, such as atherosclerosis, in which inflammation and oxidant stress are considered to play a critical role (53–56). Previous studies have shown that 15-d-PGJ2 is synthesized during mammalian inflammatory responses, and its production may represent a feedback mechanism for the resolution of inflammation (50–52). 15-d-PGJ2 has, in fact, been reported to be present in significant amounts in human atherosclerotic lesions and to reside in foam cells (50). 15-d-PGJ2 has also been detected in the inflammatory exudates from carrageenan-induced pleurisy in rats, using enzyme immunoassay (51), and it was detected by LC/MS in the cell-free inflammatory exudates of mice during resolving peritonitis (52). Additionally, stimulation of selenium-supplemented murine macrophages with lipopolysaccharide was shown to result in a time-dependent increase in 15-d-PGJ2 formation, quantified in cell lysates by enzyme immunoassay (57). This suggests that 15-d-PGJ2 and deoxy-J2-IsoPs may be produced in vivo in settings of inflammation in which COX activity is increased as well as under conditions of oxidant stress.
Further support for the free radical-catalyzed generation of 15-d-PGJ2 and other deoxy-J2-IsoPs in vivo, independent of COX enzymes, was provided by the finding that these compounds are formed in situ esterified in membrane phospholipids in rat liver after induction of oxidant injury by CCl4. Hydrolysis of deoxy-J2-IsoPs by bee venom PLA2 permitted detection and quantification of these compounds by LC/MS/MS. Free 15-d-PGJ2, which would be generated through the COX pathway, was not detectable in the liver of control rats and rats treated with CCl4. This may be due to the rapid reactivity of 15-d-PGJ2. Previous studies have indicated that cyclopentenone eicosanoids rapidly undergo conjugation with GSH in vitro and in vivo (26–30). Brunoldi et al. (26) reported that HepG2 cells convert 15-d-PGJ2 to a GSH conjugate in which the carbonyl on the prostane ring is reduced to a hydroxyl, and the GSH portion of the molecule is subsequently hydrolyzed to a cysteine conjugate. Additionally, Milne et al. (58) showed that the cyclopentone eicosanoid 15-A2t-IsoP is metabolized primarily in vivo by conjugation with GSH and that the compound is excreted into the urine as the N-acetyl cysteine sulfoxide conjugate of 15-A2t-IsoP in which the carbonyl on the prostane ring of the IsoP is reduced to a hydroxyl. Preliminary studies suggest that, like other cyclopentenone eicosanoids, 15-d-PGJ2 is rapidly metabolized in vivo via conjugation with GSH and excreted into the urine as a metabolized GSH adduct (data not shown). Further studies are required to identify the major urinary metabolite of 15-d-PGJ2 to develop a biomarker of its systemic production in humans. These studies are critical to determine to what extent 15-d-PGJ2 is generated in vivo in humans and elucidate the mechanisms that regulate its production.
In summary, we report the formation of cyclopentenone eicosanoids 15-d-PGJ2 and deoxy-J2-IsoPs in vivo via free radical-catalyzed lipid peroxidation, independent of COX. Deoxy-J2-IsoPs represent a new class of reactive lipid peroxidation products that may exert unique biological actions relevant to the pathobiology of oxidant stress. This study provides the rational basis with which to examine the extent to which deoxy-J2-IsoPs are generated in vivo in humans. Additionally, future studies aimed at exploring the biological activities of deoxy-J2-IsoPs will likely yield insights into the pathophysiological consequences of their formation in settings of oxidant stress.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- AAPH
- 2,2′-azobis(amidinopropane)dihydrochloride
- APCI
- atmospheric pressure chemical ionization
- CID
- collision-induced dissociation
- COX
- cyclooxygenase
- GSH
- glutathione
- GST
- glutathione S-transferase
- HPLC
- high-performance liquid chromatography
- IsoPs
- isoprostanes
- NF-κB
- nuclear factor kappa B
- PC
- phosphatidylcholine
- PG
- prostaglandin
- PLA2
- phospholipase A2
- PPAR-γ
- peroxisome proliferator-activated receptor-γ
- RT
- retention time
- SIM
- selected ion monitoring
- SRM
- selected reaction monitoring
- TXA2
- thromboxane
This research was supported by National Institutes of Health Grants GM15431 and ES13125. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. K.D.H. is supported by National Institutes of Health Ruth L. Kirschstein National Research Service Award 1F31AG035483-01 from the National Institute of Aging.
REFERENCES
- 1.Hardman J. G., Limbird L. E., 2001. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th edition McGraw-Hill Book Co, NY: 669–679. [Google Scholar]
- 2.Marnett L. J. 2000. Cyclooxygenase mechanisms. Curr. Opin. Chem. Biol. 4: 545–552. [DOI] [PubMed] [Google Scholar]
- 3.Marnett L. J., Rowlinson S. W., Goodwin D. C., Kalgutkar A. S., Lanzo C. A. 1999. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J. Biol. Chem. 274: 22903–22906. [DOI] [PubMed] [Google Scholar]
- 4.Hamberg M., Samuelsson B. 1966. Prostaglandins in human seminal plasma. Prostaglandins and related factors 46. J. Biol. Chem. 241: 257–263. [PubMed] [Google Scholar]
- 5.Fitzpatrick F. A., Wynalda M. A. 1983. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J. Biol. Chem. 258: 11713–11718. [PubMed] [Google Scholar]
- 6.Straus D. S., Glass C. K. 2001. Cyclopentenone prostaglandins: new Insights on biological activities and cellular targets. Med. Res. Rev. 21: 185–210. [DOI] [PubMed] [Google Scholar]
- 7.Kim E. H., Surh Y. 2006. 15-deoxy-Δ12,14-Prostaglandin J2 as a potential endogenous regulator of redox-sentitive transcriptor factors. Biochem. Pharmacol. 72: 1516–1528. [DOI] [PubMed] [Google Scholar]
- 8.Uchida K., Shibata T. 2008. 15-deoxy-Δ12,14-prostaglandin J2: an electrophilic trigger of cellular responses. Chem. Res. Toxicol. 21: 138–144. [DOI] [PubMed] [Google Scholar]
- 9.Forman B. M., Tontonoz P., Jasmine C., Brun R. P., Spiegelman B. M., Evans R. M. 1995. 15-deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 83: 803–812. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B., Gutteridge J. M. 1990. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 186: 1–85. [DOI] [PubMed] [Google Scholar]
- 11.Southorn P. A., Powis G. 1988. Free radicals in medicine. II. Involvement in human disease. Mayo Clin. Proc. 63: 390–408. [DOI] [PubMed] [Google Scholar]
- 12.Ames B. N. 1983. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 221: 1256–1264. [DOI] [PubMed] [Google Scholar]
- 13.Harman D. 1981. The aging process. Proc. Natl. Acad. Sci. U S A. 78: 7124–7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow J. D., Hill K. E., Burk R. F., Nammour T. M., Badr K. F., Roberts L. J., II 1990. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. U S A. 87: 9383–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stafforini D. M., Sheller J. R., Blackwell T. S., Sapirstein A., Yull F. E., McIntyre T. M., Bonventre J. V., Prescott S. M., Roberts L. J., II 2006. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J. Biol. Chem. 281: 4616–4623. [DOI] [PubMed] [Google Scholar]
- 16.Morrow J. D., Awad J. A., Boss H. J., Blair I. A., Roberts L. J., II 1992. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA. 89: 10721–10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pryor W. A., Stanley J. P., Blair E. 1976. Autoxidation of polyunsaturated fatty acids: II. A suggested mechanism for the formation of TBA-reactive materials from prostaglandin-like endoperoxides. Lipids. 11: 370–379. [DOI] [PubMed] [Google Scholar]
- 18.Morrow J. D., Harris T. M., Roberts L. J., II 1990. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal. Biochem. 184: 1–10. [DOI] [PubMed] [Google Scholar]
- 19.Morrow J. D., Minton T. A., Mukundan C. R., Campbell M. D., Zachert W. E., Daniel V. C., Badr K. F., Blair I. K., Roberts L. J., II 1994. Free radical-induced generation of isoprostanes in vivo: evidence for the formation of D-ring and E-ring isoprostanes. J. Biol. Chem. 269: 4317–4326. [PubMed] [Google Scholar]
- 20.Taber D. F., Morrow J. D., Roberts L. J., II 1997. A nomenclature system for the isoprostanes. Prostaglandins. 53: 63–67. [DOI] [PubMed] [Google Scholar]
- 21.Hirata Y., Hayashi H., Ito S., Kikawa Y., Ishibashin M., Sudo M., Miyazaki H., Fukushima M., Narumiya S., Hayaishi O. 1988. Occurrence of 9-deoxy-Δ9,Δ12-13,14-dihydroprostaglandin D2 in human urine. J. Biol. Chem. 263: 16619–16625. [PubMed] [Google Scholar]
- 22.Bell-Parikh L. C., Ide T., Lawson J. A., McNamara P., Reilly M., FitzGerald G. A. 2003. Biosynthesis of 15-deoxy-Δ12,14-PGJ2 and the ligation of PPARγ. J. Clin. Invest. 112: 945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attallah A., Payakkapan W., Lee J., Carr A., Brazelton E. 1974. PGA: fact, not artifact. Prostaglandins. 5: 69–71. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson H. T., Middledtich B. S., Schexnayder M. A., Desiderio D. M. 1976. 11,15,19-trihydroxy-9-ketoprost-13-enoic acid and 11,15,19-trihydroxy-9-ketoprosta-5, 13-dienoic acid in human seminal fluid. J. Lipid Res. 17: 1–6. [PubMed] [Google Scholar]
- 25.Chen Y., Morrow J. D., Roberts L. J., II 1999. Formation of reactive cyclopentenone compounds in vivo as products of the isoprostane pathway. J. Biol. Chem. 274: 10863–10868. [DOI] [PubMed] [Google Scholar]
- 26.Brunoldi E. M., Zanoni G., Vidari G., Sasi S., Freeman M. L., Milne G. L., Morrow J. D. 2007. Cyclopentenone prostaglandin, 15-deoxy-Δ12,14-PGJ2 is metabolized by Hep G2 cells via conjugation with glutathione. Chem. Res. Toxicol. 20: 1528–1535. [DOI] [PubMed] [Google Scholar]
- 27.Hubatsch I., Mannervik B., Gao L., Roberts L. J., II, Chen Y., Morrow J. D. 2002. The cyclopentenone product of lipid peroxidation, 15-A2t-isoprotane (8-isprostaglandin A2), is efficiently conjugated with glutathione by human and rat glutathione transferase A4–4. Chem. Res. Toxicol. 15: 1113–1118. [DOI] [PubMed] [Google Scholar]
- 28.Milne G. L., Zanoni G., Porta A., Sasi S., Vidari G., Musiek E. S., Freeman M. L., Morrow J. D. 2004. The cyclopentenone product of lipid peroxidation, 15-A2t-isoprostane, is efficiently metabolized by HepG2 cells via conjugation with glutathione. Chem. Res. Toxicol. 17: 17–25. [DOI] [PubMed] [Google Scholar]
- 29.Cox B., Murphey L. J., Zackert W. E., Chinery R., Graves-Deal R., Boutaud O., Oates J. A., Coffey R. J., Morrow J. D. 2002. Human colorectal cancer cells efficiently conjugate the cyclopentenone prostaglandin, prostaglandin J2, to glutathione. Biochim. Biophys. Acta. 1584: 37–45. [DOI] [PubMed] [Google Scholar]
- 30.Atsmon J., Sweetman B. J., Baertschi S. W., Harris T. M., Roberts L. J., II 1990. Formation of thiol conjugates of 9-deoxy-Δ9,Δ12(E)-prostaglanding D2 and Δ12(E)-prostaglandin D2. Biochemistry. 29: 3760–3765. [DOI] [PubMed] [Google Scholar]
- 31.Gao L., Zackert W. E., Hasford J. J., Danekis M. E., Milne G. L., Remmert C., Reese J., Yin H., Tai H., Dey S. K., et al. 2003. Formation of prostaglandins E2 and D2 via the isoprostane pathway: a mechanism for the generation of bioactive prostaglandins independent of cyclooxygenase. J. Biol. Chem. 278: 28479–28489. [DOI] [PubMed] [Google Scholar]
- 32.Yin H., Gao L., Tai H., Murphey L. J., Porter N. A., Morrow J. D. 2007. Urinary prostaglandin F2α is generated from the isoprostane pathway and not the cyclooxygenase in humans. J. Biol. Chem. 282: 329–336. [DOI] [PubMed] [Google Scholar]
- 33.Milne G. L., Musiek E. S., Morrow J. D. 2005. The cyclopentenone (A2/J2) isoprostanes—unique, highly reactive products of arachidonic acid peroxidation. Antioxid. Redox Signal. 7: 210–220. [DOI] [PubMed] [Google Scholar]
- 34.Brechbuhl H. M., Min E., Kariya C., Frederick B., Raben D., Day B. J. 2009. Selective cyclopentenone prostaglandins trigger glutathione efflux and the role of ABCG2 transport. Free Radic. Biol. Med. 47: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop-Bailey D., Hla T. 1999. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Δ12,14-prostaglandin J2. J. Biol. Chem. 274: 17042–17048. [DOI] [PubMed] [Google Scholar]
- 36.Levonen A. L., Dickinson D. A., Moellering D. R., Mulcahy R. T., Forman H. J., Darley-Usmar V. M. 2001. Biphasic effects of 15-deoxy-Δ12,14-prostaglandin J2 on glutathione induction and apoptosis in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 21: 1846–1851. [DOI] [PubMed] [Google Scholar]
- 37.Clay C. E., Monjazeb A., Thorburn J., Chilton F. H., High K. P. 2002. 15-Deoxy-delta12,14-prostaglandin J2-induced apoptosis does not require PPARgamma in breast cancer cells. J. Lipid Res. 43: 1818–1828. [DOI] [PubMed] [Google Scholar]
- 38.Hortelano S., Castillo A., Alvarez A. M., Bosca L. 2000. Contribution of cyclopentenone prostaglandins to the resolution of inflammation through potentiation of apoptosis in activated macrophages. J. Immunol. 165: 6525–6531. [DOI] [PubMed] [Google Scholar]
- 39.Rossi A., Kapahi P., Natoli G., Takahashi T., Chen Y., Karin M., Santoro M. G. 2000. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 403: 103–108. [DOI] [PubMed] [Google Scholar]
- 40.Straus D. S., Pascual G., Li M., Welch J. S., Ricote M., Hsiang C., Sengchangthalangs L. L., Ghosh G., Glass C. K. 2000. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc. Natl. Acad. Sci. U S A. 97: 4844–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Sala D., Cernuda-Morollon E., Pineda-Molina E., Canada F. J. 2002. Contribution of covalent protein modification to the anti-inflammatory effects of cyclopentenone prostaglandins. Ann. N. Y. Acad. Sci. 973: 533–536. [DOI] [PubMed] [Google Scholar]
- 42.Shiraki T., Kamiya N., Shiki S., Kodama T. S., Kakizuka A., Jingami H. 2005. α,β-Unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor γ. J. Biol. Chem. 280: 14145–14153. [DOI] [PubMed] [Google Scholar]
- 43.Dinkova-Kostova A. T., Holtzclaw W. D., Cole R. N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. 2002. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U S A. 99: 11908–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itoh K., Mochizuki M., Ishii Y., Ishii T., Shibata T., Kawamoto Y., Kelly V., Sekizawa K., Uchida K., Yamamoto M. 2004. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Δ12,14-prostaglandin J2. Mol. Cell. Biol. 24: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim E. H., Kim D. H., Na H. K., Surh Y. J. 2004. Effects of cyclopentenone prostaglandins on the expression of heme oxygenase-1 in MCF-7 cells. Ann. N. Y. Acad. Sci. 1030: 493–500. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z. H., Yoshida Y., Saito Y., Sekine A., Noguchi N., Niki E. 2006. Induction of adaptive response and enhancement of PC12 cell tolerance by 7-hydroxycholesterol and 15-deoxy-Δ12,14 prostaglandin J2 through up-regulation of cellular glutathione via different mechanisms. J. Biol. Chem. 281: 14440–14445. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X., Lu L., Dixon C., Wilmer W., Song H., Chen X., Rovin B. H. 2004. Stress protein activation by the cyclopentenone prostaglandin 15-deoxy-Δ12,14-prostaglandin J2 in human mesangial cells. Kidney Int. 65: 798–810. [DOI] [PubMed] [Google Scholar]
- 48.Miwa Y., Sasaguri T., Inoue H., Taba Y., Ishida A., Abumiya T. 2000. 15-Deoxy-delta(12,14)-prostaglandin J(2) induces G(1) arrest and differentiation marker expression in vascular smooth muscle cells. Mol. Pharmacol. 58: 837–844. [DOI] [PubMed] [Google Scholar]
- 49.Wakino S., Kintscher U., Kim S., Yin F., Hsueh W. A., Law R. E. 2000. Peroxisome proliferator-activated receptor gamma ligands inhibit retinoblastoma phosphorylation and G1→S transition in vascular smooth muscle cells. J. Biol. Chem. 275: 22435–22441. [DOI] [PubMed] [Google Scholar]
- 50.Shibata T., Kondo M., Osawa T., Shibata N., Kobayashi M., Uchida K. 2002. 15-Deoxy-Δ12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes. J. Biol. Chem. 277: 10459–10466. [DOI] [PubMed] [Google Scholar]
- 51.Gilroy D. W., Colville-Nash P. R., Willis D., Chivers J., Paul-Clark M. J., Willoughby D. A. 1999. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 5: 698–701. [DOI] [PubMed] [Google Scholar]
- 52.Rajakariar R., Hilliard M., Lawrence T., Trivedi S., Colville-Nash P., Bellingan G., Fitzgerald D., Yaqoob M. M., Gilroy D. W. 2007. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxy-Δ12–14PGJ2. Proc. Natl. Acad. Sci. USA. 104: 20979–20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berliner J. A., Navab M., Fogelman A. M., Frank J. S., Demer L. L., Edwards P. A., Watson A. D., Luis A. J. 1995. Atherosclerosis: basic mechanisms – oxidation, inflammation, and genetics. Circulation. 91: 2488–2496. [DOI] [PubMed] [Google Scholar]
- 54.Berliner J., Leitinger N., Watson A., Huber J., Fogelman A., Navab M. 1997. Oxidized lipids in atherogenesis: formation, destruction and action. Thromb. Haemost. 78: 195–199. [PubMed] [Google Scholar]
- 55.Heinecke J. W. 1998. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis. 141: 1–5. [DOI] [PubMed] [Google Scholar]
- 56.Chisolm G. M., Steinberg D. 2000. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic. Biol. Med. 18: 1815–1826. [DOI] [PubMed] [Google Scholar]
- 57.Vunta H., Davis F., Palempalli U. D., Bhat D., Arner R. J., Thompso J. T., Peterson D. G., Reddy C. C., Prabhu K. S. 2007. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Δ12,14-prostaglandin J2 in macrophages. J. Biol. Chem. 282: 17964–17973. [DOI] [PubMed] [Google Scholar]
- 58.Milne G. L., Gao L., Porta A., Zanoni G., Vidari G., Morrow J. D. 2005. Identification of the major urinary metabolite of the highly reactive cyclopentenone isoprostane 15-A2t-Isoprostane in Vivo. J. Biol. Chem. 280: 25178–25184. [DOI] [PubMed] [Google Scholar]