Abstract
A growing body of evidence suggests that the multifunctional protein E4F1 is involved in signaling pathways that play essential roles during normal development and tumorigenesis. We generated E4F1 conditional knockout mice to address E4F1 functions in vivo in newborn and adult skin. E4F1 inactivation in the entire skin or in the basal compartment of the epidermis induces skin homeostasis defects, as evidenced by transient hyperplasia in the interfollicular epithelium and alteration of keratinocyte differentiation, followed by loss of cellularity in the epidermis and severe skin ulcerations. E4F1 depletion alters clonogenic activity of epidermal stem cells (ESCs) ex vivo and ends in exhaustion of the ESC pool in vivo, indicating that the lesions observed in the E4F1 mutant skin result, at least in part, from cell-autonomous alterations in ESC maintenance. The clonogenic potential of E4F1 KO ESCs is rescued by Bmi1 overexpression or by Ink4a/Arf or p53 depletion. Skin phenotype of E4F1 KO mice is also delayed in animals with Ink4a/Arf and E4F1 compound gene deficiencies. Our data identify a regulatory axis essential for ESC-dependent skin homeostasis implicating E4F1 and the Bmi1–Arf–p53 pathway.
Keywords: mouse model, bulge, long term label retaining cells, wound healing
E4F1 is an ubiquitously expressed transcription factor of the Gli–Kruppel family that was identified as a cellular target of the adenoviral oncoprotein E1A (1). Although several cellular targets of E1A (e.g., E2F/pRB, CBP/p300, PCAF, CtBP, ATF/Creb) have been studied extensively and are recognized as central regulators of cell proliferation and survival, the biological functions of E4F1 remain poorly investigated. E4F1 is a multifunctional protein with transcriptional and atypical ubiquitin E3 ligase activities. E4F1-mediated ubiquitylation modulates the p53 transcriptional activities involved in alternative cell fates, either growth arrest or apoptosis (2). The idea that E4F1 plays an important role in the p53 pathway is reinforced by other reports showing that E4F1 interacts directly not only with p53 itself (2, 3) but also with regulators/effectors of this pathway, including p14ARF (4), the polycomb member Bmi1 (5), and the p53 target gene FHL2 (6). However, the functions of E4F1 likely extend beyond the regulation of p53, however. Indeed, physical interactions between E4F1 and components of other oncogenic pathways, including RASSF1A, pRB, HMGA2, and Smad4, have been reported (7–10).
Using a gene targeting approach in mice, we previously showed that E4F1 constitutive inactivation results in embryonic lethality near the time of implantation. E4F1 KO blastocysts in culture exhibit mitotic defects, including lagging chromosomes, chronic activation of the mitotic checkpoint, and cell death (11). More recently, shRNA-mediated partial depletion of E4F1 was shown to rescue hematopoietic stem cell (HSC) exhaustion in mouse resulting from inactivation of the polycomb member Bmi1 (5), suggesting an important role for E4F1 in HSC homeostasis. To date, little information is available regarding in vivo functions of E4F1 in adult tissues. In the present work, we generated mouse strains to explore the roles of E4F1 in skin homeostasis.
Constant renewal of the interfollicular epithelium (IFE) and of hair follicles (HFs) relies on the recruitment of epidermal stem cells (ESCs) located in the basal layer of the IFE and in the bulge region of the HFs, respectively. ESCs fuel the highly proliferative transit amplifying compartments (TACs) in the basal layer of the IFE and in the bulbs of HFs. TAC cells then embark on differentiation programs to generate the spinous, granular, and cornified layers in the IFE or the different lineages of mature HFs (12–14). Several essential molecular circuitries that orchestrate ESC maintenance, including p63-, BMP-, TGF-β–, Wnt/β-catenin–, and Notch-initiated signaling cascades, have been described (12, 15, 16).
Here we report that inactivation of E4F1 in the entire skin or in the basal compartment of the epidermis results in severe epidermal defects in both neonatal and adult mice, revealing an as-yet unidentified regulatory axis essential for ESC-dependent skin homeostasis implicating E4F1 and the Bmi1–Ink4a/Arf–p53 pathway.
Results
E4F1 KO Induces Transient Hyperplasia in the Epidermis, Followed by Permanent Loss of Epidermal Cells and Severe Skin Ulcerations.
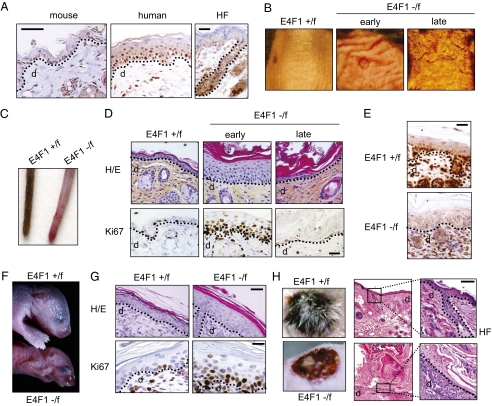
Immunohistochemistry (IHC) analyses of murine and human skin sections demonstrated nuclear expression of E4F1 in the basal and suprabasal layers of the IFE, as well as in the bulb and bulge regions of the HFs (Fig. 1A). To address the role of E4F1 in skin homeostasis, we generated mouse models with conditional homozygote deletion of the E4F1 gene in the entire skin or in the epidermis only. In short, we generated E4F1−/flox mice (Fig. S1) and intercrossed them with Cre-ERT2 KI/KI mice (RERT), which ubiquitously express tamoxifen-inducible Cre recombinase-ERT2 fusion protein (17). Topical applications of 4-hydroxytamoxifen (4OHT) on the tail skin or on a shaved area of the back skin of adult E4F1−/flox;RERT mice resulted in efficient recombination of the E4F1 locus in the skin, as monitored in genomic DNA, mRNA, and protein samples prepared from treated areas (Fig. S1). Between 1 and 2 wk after 4OHT treatment, E4F1-depleted back skin thickened and became wrinkled and ruffled. These early lesions evolved 1–2 wk later into severe skin ulcerative lesions (Fig. 1B). E4F1 KO in unshaved skin tails of E4F1−/flox;RERT mice resulted in complete alopecia by 6 wk after 4OHT application (Fig. 1C). Histological analyses revealed that skin thickening resulted from massive hyperplasia of the epidermis with increased cellularity in the IFE and the infundibulum (Fig. 1D). Consistent with hyperplasia, an abnormal proportion of epidermal cells proliferated in E4F1 KO skin, as indicated by increased Ki67 (Fig. 1D) and BrdU (Fig. S2) immunostainings of skin sections. This phenotype was skin-autonomous, as demonstrated by the recapitulation of similar hyperplasia on E4F1−/flox;RERT neonatal back skin engrafted onto nude mice (Fig. S3). At later time points (3–4 wk after 4OHT treatment), the initial hyperplasia of E4F1 KO;RERT skin was followed by broad disorganization of the IFE and massive hyperkeratosis, associated with partial or complete loss of cellularity in the IFE (Fig. 1D). Of note, sebaceous glands appeared to be unaltered in E4F1 KO skin, as suggested by Nile red staining on whole-mount experiments and H&E coloration (Fig. S4).
Fig. 1.
E4F1 KO triggers transient hyperplasia of the epidermis, followed by permanent loss of epidermal cells and severe skin ulcerations. (A) IHC analysis of E4F1 expression in human skin or murine back skin and HFs. (Scale bar: 40 μm.) The dotted line indicates the dermis (d)–epidermis junction. (B) Macroscopic alterations of back skin of adult E4F1−/flox;RERT mice 7 (early) or 15 d (late) after Cre-mediated E4F1 KO. (C) E4F1flox; RERT tails at late time points (6 wk) after 4OHT application. (D) H&E staining (Upper) and Ki67 IHC analyses (Lower) of dorsal skin sections prepared from 4OHT-treated E4F1−/flox;RERT or E4F1+/flox;RERT control adult mice at early and late time points. (Scale bar: 20 μm.) (E) IHC analysis of dorsal skin sections from E4F1+/flox;K5-Cre and E4F1−/flox;K5-Cre neonates using anti-E4F1 antibody. (Scale bar: 40 μm.) (F) E4F1+/flox and E4F1−/flox;K5-Cre neonates (P4) showing acute symptoms of dehydration. (G) H&E staining (Upper) and Ki67 IHC analysis (Lower) of dorsal skin sections prepared from E4F1flox;K5-Cre P4 neonates. (Scale bar: 20 μm.) (H) H&E-stained sections of back skin from E4F1+/flox;K5 or E4F1−/flox;K5-Cre P1 neonates at 2 wk after engraftment onto recipient nude mice. (Scale bar: 40 μm.)
To confirm that this phenotype resulted from epidermis-specific defects, we next crossed E4F1−/flox mice with keratin 5 (K5)-Cre transgenic mice expressing Cre in the basal cell layer of the epidermis from E15.5 onward (18). The E4F1−/flox;K5-Cre neonates exhibited efficient E4F1 KO in the epidermis (Fig. 1E and Fig. S1). At 3–4 d after birth, these mice developed epidermal hyperplasia and died exhibiting symptoms of acute dehydration (Fig. 1F). As in E4F1−/flox;RERT skin, hyperplasia was observed in E4F1−/flox; K5-Cre epidermis was associated with hyperproliferation and higher mitotic index of epidermal basal cells, as shown by increased Ki67 (Fig. 1G) and phospho-histone H3 (Ser10; PHH3) staining (Fig. S5D). Of note, we previously showed that E4F1 KO blastocysts exhibited abnormal mitotic figures associated with increased PHH3 staining (11). These abnormalities were not observed in E4F1 KO epidermal basal cells, indicating that the increased mitotic index likely reflected the overproliferation of basal cells, rather than mitotic progression defects.
To bypass neonatal lethality, we engrafted E4F1 KO;K5-Cre skin onto nude mice. At 2 wk after engraftment, the E4F1 KO grafts failed to regenerate a normal epidermis and exhibited marked epidermal hypocellularity, hyperkeratosis, severe ulcerative lesions, and lack of HFs (Fig. 1H), similar to the late phenotype of the E4F1−/flox;RERT mice. Taken together, these data show that E4F1 KO results in transient hyperplasia and ultimately leads to a permanent loss of epidermal cells in the IFE and HFs.
E4F1 KO Results in Expansion of Epidermal Basal Cells and Abnormal Differentiation in Vivo.
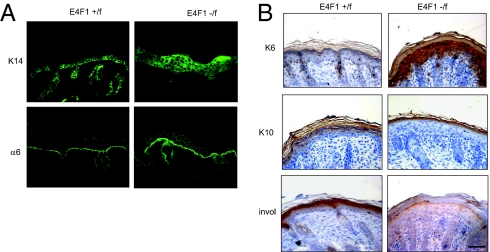
In both 4OHT-treated E4F1−/flox;RERT and E4F1−/flox;K5-Cre epidermis, cells of the hyperplasic IFE stained positive for basal cell–specific K14 and integrin-α6 markers (Fig. 2A and Fig. S6A). Expression of these markers extended to upper cellular layers, in contrast to the normal IFE of control animals, in which marker expression was restricted to the basal cell layer. E4F1-floxed IFE also massively expressed K6 (Fig. 2B and Fig. S6B), which is normally restricted to sweat glands and HFs in normal skin but can be expressed in IFE undergoing abnormal proliferation or wound healing (19). We also noticed an absence of suprabasal and granular layers in KO epidermis, as illustrated by the lack of expression of early (K1 and K10) and late (involucrin, loricrin, filaggrin) differentiation markers (Fig. 2B and Fig. S6B). Of note, no significant cell death was observed in hyperplasic lesions, suggesting that hyperproliferative cells had finally undergone an abnormal differentiation program of stratification, rather than apoptosis, leading to the observed hyperkeratosis. These data indicate that E4F1 KO in the IFE results in massive expansion of keratinocytes with basal/TAC properties and also alters the differentiation of epidermal lineages.
Fig. 2.
E4F1 KO results in expansion of the basal cell compartment of the epidermis and in abnormal keratinocyte differentiation in vivo. (A) Immunostainings of dorsal skin sections from E4F1+/flox and E4F1−/flox;K5-Cre neonates with anti-K14 and anti–α6 integrin antibodies. (B) IHC analysis of dorsal skin sections from E4F1+/flox and E4F1−/flox;K5-Cre neonates with anti-K6, anti-K10, or anti-involucrin (invol) antibodies. (Scale bar: 40 μm.)
E4F1 KO Induces Epidermal Stem Cell Defects.
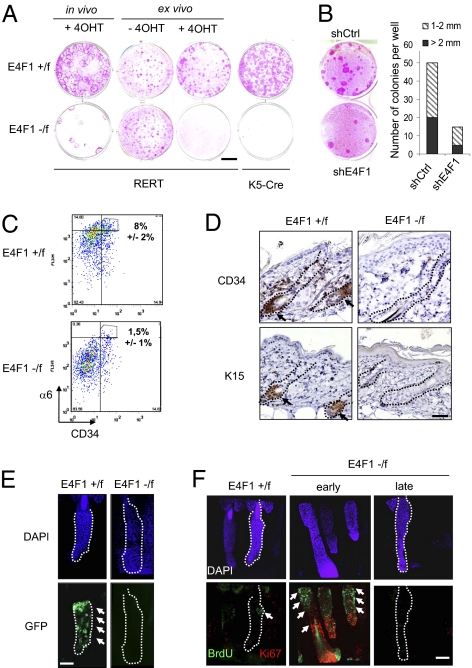
Several scenarios could explain how E4F1 KO leads to a transient expansion of the basal/TAC compartment of the epidermis. First, the overproliferation of basal cells could reflect an increase in the intrinsic proliferation capability of basal/TAC keratinocytes. This scenario is unlikely, however, given that E4F1 KO primary keratinocytes isolated from E4F1−/flox;K5-Cre p1 neonates did not recapitulate in culture the overproliferation observed in skin sections, as demonstrated by BrdU/propidium iodide (PI) or PHH3/PI labeling (Fig. S5 A and C). Similar findings were seen in primary human keratinocytes upon shRNA-mediated depletion of endogenous E4F1 (Fig. S5B). These data, together with the withering and transient aspect of the hypertrophy phenotypes, led us to investigate an alternative hypothesis involving perturbations of resident ESCs. Thus, inactivation in the epidermis of key signaling molecules, including Rac1, Myc, Smad4, and NFATC1, causes ESCs to exit their normal microenvironment to enter into the TACs, where they become transiently proliferating keratinocytes on exposure to natural promitogenic signals present in this amplification compartment (20–24). This results in transient hyperplasia, followed by a permanent exhaustion of the ESC pool. To examine this scenario, we first performed clonogenic assays with primary keratinocytes isolated from in vivo recombined hyperplasic regions of E4F1−/flox;RERT adult mice, E4F1−/flox;K5-Cre p1 neonates, and control littermates. After 10–15 d in culture, typical holoclones, corresponding to the long-term clonal outgrowth of epidermal cells with stem cell properties (25, 26), were detected in controls, but not under E4F1 KO conditions (Fig. 3A). Importantly, similar reduction was recapitulated when E4F1 KO was induced ex vivo by adding 4OHT in the culture medium of E4F1−/flox;RERT cells (Fig. 3A), demonstrating that these defects are stem cell autonomous. Similarly, shRNA-mediated partial depletion of E4F1 in primary human keratinocytes resulted in a 3-fold reduction in the number of holoclones (Fig. 3B).
Fig. 3.
E4F1 KO results in ESC exhaustion in vivo and ex vivo. (A) Clonogenic assays performed with primary murine keratinocytes prepared from back skin of E4F1flox;RERT adult mice 6 d after 4OHT applications (in vivo), from E4F1flox;RERT neonatal skins and treated with 4OHT in culture medium (ex vivo), or from E4F1flox;K5-Cre neonates (P1). Rhodamine B staining was performed after 15 d of culture. Data shown are representative of experiments performed in duplicate and repeated at least three times. (Scale bar: 1 cm.) (B) (Left) Clonogenic assays performed with human primary keratinocytes transduced with retroviral vectors expressing shRNAs targeting either human E4F1 (shE4F1) or a nonrelevant sequence (shCtrl). (Right) Quantification of the total number and size (diameter, in mm) of clones from three independent experiments. (C) FACScan analyses of primary keratinocytes isolated from E4F1flox;K5-Cre P3 neonates after α6-integrin and CD34 immunostainings. The percentage of α6high/CD34+ epidermal stem cells is indicated (average of three independent experiments ± SD). (D) IHC analyses of back skin sections from E4F1flox;RERT adult mice at 3 wk after 4OHT application with antibodies directed against CD34 and K15. Arrows indicate K15- or CD34-positive stem cells located in the bulge region of HFs. (Scale bar: 80 μm.) (E) Whole mounts of tail epidermis prepared from E4F1flox;RERT;K15-GFP mice at 4 wk after 4OHT application. White arrows indicate K15-GFP+ ESCs located in the bulge region of HFs in control animals (E4F1+/flox) treated with 4OHT (Left). (Scale bar: 80 μm.) (F) Analyses of BrdU LRCs in whole mounts of tail epidermis prepared from 16-wk-old mice. Anti-BrdU (green) and anti-Ki67 (red) immunostainings of E4F1+/flox;RERT and E4F1−/flox;RERT HFs at 3 wk (early) or 6 wk (late) after E4F1 KO showing transient hyperproliferation followed by a loss of LRCs (white arrows). LRCs are located in the bulge region of HFs from control skin (E4F1+/flox) treated with 4OHT for 6 wk. (Scale bar: 80 μm.)
We next investigated whether E4F1 KO also resulted in exhaustion of the stem cell pool in vivo by analyzing the expression of various ESC markers. FACscan flow cytometry analyses revealed that E4F1−/flox;K5-Cre neonatal epidermis contained fewer ESCs coexpressing CD34 and higher levels of α6-integrin (CD34+/α6high) compared with control epidermis (8% ± 2% vs. 1.5% ± 1%; n = 3) (Fig. 3C). Similarly, immunostainings of back skin sections and whole mounts of tail epidermis showed significant reductions in HF bulge markers CD34 and K15 in adult E4F1−/flox;RERT animals at 4–6 wk after E4F1 KO (Fig. 3D and Fig. S7A).
We also crossed E4F1−/flox;RERT animals with K15-EGFP transgenic mice expressing the GFP reporter under the control of the K15 promoter (27). In 7- to 12-wk-old K15-GFP;E4F1+/flox;RERT control animals, GFP expression was restricted to the bulge region of unsynchronized HFs. In contrast, a complete loss of GFP-positive cells was observed at 4–6 wk after E4F1 KO, confirming the exhaustion of HF ESCs (Fig. 3E). Finally, we tracked HF bulge/ESCs as BrdU long-term retaining cells (LRCs) using an in vivo labeling protocol that marks self-renewing and multipotent epidermal cells (26, 28). E4F1−/flox;RERT and E4F1+/flox;RERT neonates were injected with BrdU, and after a chase period of 3 months, the poorly proliferative adult ESCs were identified on whole mounts of tail epidermis as BrdU-positive LRCs. At 2–3 wk after E4F1 KO, the LRC zone was extended, and increased numbers of LRCs were colabeled with Ki67 in E4F1 KO HFs compared with control HFs (Fig. 3F, early and Fig. S7B), suggesting that E4F1 KO induced transient proliferation of HF ESCs. At later time points (6 wk), although LRCs were still restricted to the HF bulges in controls, BrdU staining finally disappeared in the E4F1 KO HFs (Fig. 3F, late), further suggesting that E4F1 KO results in exhaustion of the ESC pool. ESCs also play a major role in regenerating hairy skin after injury (26). Accordingly, wound healing was strongly altered in E4F1 KO skin (Fig. S8). Taken together, these findings indicate that E4F1 is essential for ESC maintenance in vivo and ex vivo, and strongly suggest that the hyperplasia and subsequent loss of cellularity of E4F1 KO epidermis are due primarily to cell-autonomous perturbations of the ESC pool that supplies TAC compartments.
Down-Regulation of the Bmi1-Ink4a/Arf-p53 Axis Partly Rescues ESC Clonogenic Potential and Skin Lesions of E4F1 KO.
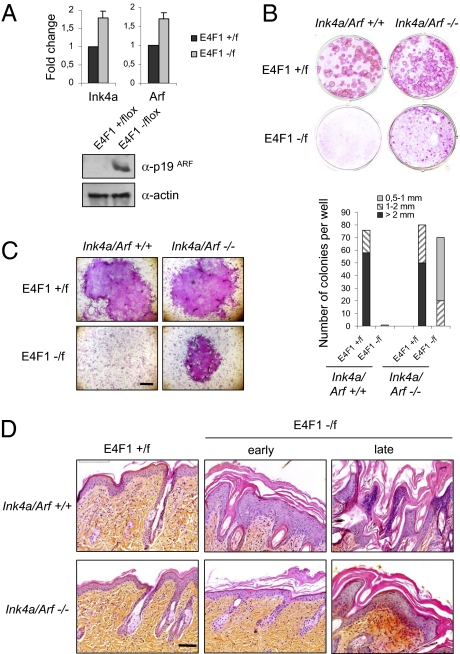
We next addressed the molecular pathway by which E4F1 could regulate ESC maintenance. E4F1 associates with the polycomb member Bmi1 and the tumor suppressors Arf, p53, and pRb (2–5, 8). Although some evidence suggests that these factors have independent functions, they define a functional cascade with Bmi1 triggering the repression of the Ink4a/Arf locus, whose products, p19Arf and p16Ink4a, act as potent inhibitors of p53- and pRb-dependent activities, respectively (29). Repression of Ink4a/Arf expression is considered a central event in stem cell renewal in several tissues, including the epidermis (30–34). Strikingly, E4F1 KO keratinocytes exhibited increased levels of Ink4a and Arf mRNA and p19Arf protein compared with control cells (Fig. 4A). To assess the impact of this deregulation in the E4F1 KO phenotype, we generated E4F1flox;RERT;Ink4a/Arf−/− compound mice. Clonogenic assays performed with keratinocytes isolated from these animals showed that Ink4a/Arf KO partly restored long-term outgrowth of E4F1 KO ESCs ex vivo (Fig. 4 B and C). Accordingly, the in vivo skin phenotype induced by E4F1 KO was delayed in these animals. The epidermal hyperplasia was almost undetectable at early time points after 4OHT treatment (Fig. 4D, early), but moderate hyperplasia and hyperkeratosis were evident at later time points (Fig. 4D, late).
Fig. 4.
Deletion of the Ink4a/Arf locus partially rescues E4F1 KO skin defects in vivo and ex vivo. (A) Quantitative RT-PCR analyses of Ink4a and Arf mRNA levels (Upper) and p19ARF immunoblot analyses (Lower) performed on E4F1+/flox or E4F1−/flox;K5-Cre freshly isolated primary keratinocytes. (B) Clonogenic assays performed with E4F1flox;RERT;Ink4a/Arf−/− or E4F1flox;RERT;Ink4a/Arf+/+ primary keratinocytes in the presence of 4OHT. Shown is one representative experiment of three independent experiments performed in duplicate. Quantitative analyses of the number and size (diameter, in mm) of clones was performed after 15 d in culture. (C) High-magnification photograph of representative clones. (Scale bar: 0.5 mm.) (D) H&E staining of dorsal skin sections prepared from 4OHT-treated E4F1flox;RERT;Ink4a/Arf+/+ 12-wk-old mice and E4F1flox;RERT;Ink4a/Arf−/− mice.
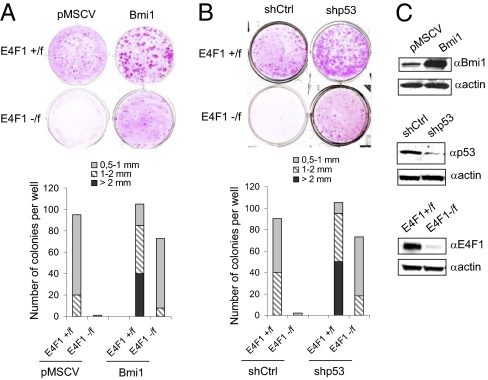
A partial rescue of the clonogenic potential of E4F1 KO ESCs was also obtained on overexpression of Bmi1, a main repressor of the Ink4a/Arf locus (Fig. 5 A and C), providing additional evidence for a role of the Ink4a/Arf–pRb/p53 cascades in the E4F1 KO phenotype. To assess which Ink4a/Arf downstream targets were involved, we repeated clonogenic assays on shRNA-mediated depletion of either murine RB1 or p53 (Fig. 5 B and C). p53 depletion, but not pRb depletion, restored clonal outgrowth of E4F1 KO keratinocytes, highlighting the role of the Bmi1-Arf-p53 axis rather than the Bmi1-p16-pRb axis in E4F1 KO skin phenotypes. Of note, E4F1 KO rescued clones that developed on inactivation of Ink4a/Arf, depletion of p53, or overexpression of Bmi1 were significantly smaller than those growing from control cells (Figs. 4 B and C and 5 A and B), suggesting that E4F1 also might also impinge on other molecular circuitries that orchestrate ESC maintenance.
Fig. 5.
Down-regulation of the Bmi1-Arf-p53 axis rescues E4F1 KO ESC defects. (A) Primary keratinocytes isolated from E4F1flox;RERT neonates were transduced with control (pMSCV) or Bmi1-encoding retroviruses (Bmi1) and used for clonogenic assays in the presence of 4OHT. (B) Clonogenic assays performed with E4F1flox;RERT primary keratinocytes transduced with control (shCtrl) or p53 (shp53) shRNAs encoding lentiviruses in presence of 4OHT. A and B present photographs (Upper) and quantifications (Lower) of a representative experiment. Three independent experiments were performed in duplicate. Quantitative analyses of the number and size (diameter, in mm) of clones were performed after 10 d. (C) Western blot analyses showing ectopic overexpression of Bmi1 in rescued E4F1 KO clones or shRNA-mediated depletion of p53 in rescued E4F1 KO clones.
Taken together, our findings provide evidence that E4F1 is essential for ESC-dependent skin homeostasis and identify a regulatory axis involved in this process, implicating Bmi1 and the Arf–p53 pathway.
Discussion
We have shown that E4F1 KO in the epidermis leads to neonatal lethality resulting from defects in skin homeostasis. E4F1 depletion in E4F1 KO;K5-Cre neonates and E4F1−/flox;RERT adult skin led to rapid thickening of the IFE with increased numbers of proliferative basal keratinocytes. This hyperplasia was transient, and was followed by severe disorganization and an almost complete loss of viable epithelial cell layers in the IFE and HFs. In addition, perturbations of epidermal differentiation were observed in both E4F1 KO models. Strikingly, E4F1 depletion in murine or human primary keratinocytes in culture did not recapitulate the overproliferation observed in skin sections, ruling out the possibility that hyperplasia originates from an intrinsic increase in the proliferative capacity of E4F1 KO TAC keratinocytes. As has been described for other gene deficiencies in epidermis (20, 22), our data suggest that the E4F1 KO phenotypes resulted from cell-autonomous perturbations in resident stem cells. E4F1 depletion in vivo or ex vivo dramatically impaired the clonogenic potential of both murine and human epidermal cell populations and resulted in a strong reduction in the expression of various ESC markers in E4F1 KO skin. BrdU labeling of LRCs suggested that E4F1 KO HF stem cells first exited their normal location in the bulge region of HFs to enter into a transient phase of proliferation before disappearing. Accordingly, exhaustion of the resident stem cell pool in E4F1 KO skin ultimately led to a complete loss of cellularity in the IFE and altered wound healing.
The clonogenic potential of E4F1 KO ESCs was restored upon targeting of the Bmi1-Ink4a/Arf-p53 axis, but not by pRb depletion, highlighting the role of the Bmi-Arf-p53 axis rather than the Bmi-p16-pRb axis in E4F1 KO skin phenotypes. Consistently, E4F1 KO was correlated with increased Ink4a/ARF expression, and hyperplasia was delayed and reduced in mice with Ink4a/Arf and E4F1 compound gene deficiencies. This calls into question the poorly documented physiological functions of the Bmi1-Ink4a/Arf-p53 axis in ESC homeostasis. This axis might play its usual “gatekeeper” function, notably in response to genotoxic or developmental stress, as has been reported in mice with dysfunctional telomeres (35, 36) and in p63 KO mice (34, 37). As has been described in other tissues, this axis also could play a role in ESC self-renewal, symmetric division, or maintenance programs, as has been reported in hematopoietic multipotent progenitors (38), neural stem cells (39), and mammary gland progenitor cells (40). This raises questions about the role of p53 (and, indirectly, of E4F1) in the epidermis, where proper columnar stratification and tissue organization are driven, at least in part, by oriented, asymmetric cell divisions (41, 42).
How E4F1 inactivation affects Ink4a/Arf levels also remains unclear. As a bona fide transcription factor, E4F1 might directly transactivate or might cooperate/interfere with other transcriptional regulators of the Ink4a/Arf locus. Consistent with this scenario, E4F1 physically interacts with Bmi1, a main repressor of this locus (5). We found that, similar to Ink4a/Arf inactivation, Bmi1 ectopic overexpression partly rescued the clonogenic potential of E4F1 KO ESCs. Nevertheless, the genetic links between E4F1 and Bmi1 in stem cell maintenance are likely complex and might differ among tissues. Indeed, other investigators have shown that shRNA-mediated depletion of E4F1 rescues premature senescence of Bmi1 KO hematopoietic stem cells in an Ink4a/Arf- and p53-independent manner (5). E4F1 might also exert other, as-yet unidentified cellular functions independent from Bmi1 and the Arf-p53 axis, as suggested by our finding that targeting the Bmi1-Arf-p53 axis did not fully restore ESC clonal outgrowth and normal skin phenotype. Thus, it could be proposed that E4F1 also impinges on other molecular circuitries that orchestrate ESC maintenance, including TGFβ/BMP-, Wnt/β-catenin–, Notch-, and p63-initiated signaling cascades (10, 12–14). Indeed, E4F1 might act as a transcriptional regulator of the p63 gene or as a posttranslational modifier of p63 activity, as has been described for p53. This is unlikely, however, given that mRNA levels of ΔNp63 and of several hiherto described transcriptional targets of p63 (Perp, Claudin1, Fibronectin1, Eva1, Runx1, and Redd1) (43) remained unchanged in E4F1 KO samples (Fig. S9). Concerning the Notch pathway, we did not detect changes in Notch1 transcripts levels in E4F1 KO keratinocytes (Fig. S9), suggesting that this pathway is not directly involved in the E4F1 KO phenotype.
In conclusion, we have provided evidence that E4F1, through its connection with the Bmi1 and the Ink4a/Arf-p53 axis, plays a role in stem cell–dependent skin homeostasis. This finding, together with reports showing that E4F1 is targeted by several oncoproteins and tumor suppressors, raise questions about E4F1’s role in the development and maintenance of cancer stem cells whose presence is suspected in skin carcinoma and malignant melanoma.
Materials and Methods
E4F1−/flox Mice and Experimental Treatment of Mice.
Generation of the E4F1flox allele and of E4F1−/flox mice is detailed in SI Materials and Methods. Experimental groups were composed of E4F1+/flox or E4F1−/flox;RERTKI/KI, E4F1+/flox or E4F1−/flox;RERTKI/KI; Ink4a/Arf+/+ or Ink4a/Arf −/−, and E4F1+/flox or E4F1−/flox;RERTKI/KI;K15-GFP Tg mice (17, 18, 27, 44). Compound mice were maintained on a mixed 129Sv/J/DBA/C57BL/6 background, and phenotypic characterization was performed in parallel on 12- to 16-wk-old E4F1+/flox and E4F1−/flox littermates. E4F1 recombination in adult E4F1flox;RERT skin was induced by four topical applications of 4OHT (in ethanol, 2 mg/day; Sigma-Aldrich) on shaved back or tail skin. For skin grafting experiments, dorsal skin from E4F1flox;RERT or E4F1flox;K5-Cre neonate donors were transplanted onto athymic nude recipient mice (Charles River) as described previously (45). For visualization of BrdU LRCs, 10-d-old mice were injected with BrdU (50 mg/kg; Sigma-Aldrich) every 12 h for a total of four injections. To achieve short-term labeling of cells undergoing DNA synthesis in vivo (for IHC experiments), BrdU (50 mg/kg) was injected i.p. 4 h before euthanasia. All experiments were approved by the University of Montpellier's Ethics Committee for Animal Welfare.
Histochemistry, Immunolabeling of Skin Sections and Whole Mounts, and Immunoblot Analysis.
Immunolabeling of skin sections were as described in SI Materials and Methods using the following antibodies: anti-E4F1 (B-21 rabbit polyclonal, from our laboratory), anti-Ki67 (SP6; Neomarkers), anti-K6 (SPM269; Abcam), anti-K10 (PRB-159P; Covance), anti-involucrin (Sc15230; Santa Cruz Biotechnology), anti-CD34 (RAM34; BD Pharmingen), anti-K15 (LHK15; Vector Laboratories), anti–α6-integrin (GoH3; BD Biosciences), anti-K14 (AF64; Covance), and anti-BrdU antibody (BD Biosciences). Immunoblots were probed with anti-E4F1 (8), anti-p53 (1C12; Cell Signaling), Bmi1 (F6; Millipore), anti-p19ARF (Ab80; Abcam), and anti–β-actin (Sigma-Aldrich). Whole mounts of tail epidermis and detection of LRCs were performed as described previously (28) and as detailed in SI Materials and Methods. For FACScan analyses, cells were probed with FITC-conjugated anti-CD34 (RAM34; BD Biosciences) and PE-Cy5–conjugated anti–α6-integrin antibodies.
Culture of Primary Keratinocytes and Clonogenic Assays.
Murine primary keratinocytes were isolated from newborn or adult back skin, and clonogenic assays were perfomed as described in SI Materials and Methods. Cre-mediated recombination of E4F1 flox alleles was achieved by adding 4OHT (1 μM) to the culture medium. Human primary keratinocytes were isolated from skin biopsy specimens in accordance with the Declaration of Helsinki and cultured as described in SI Materials and Methods (46).
Retroviral and Lentiviral Particle Production and Infections.
Viral particles were produced as described in SI Materials and Methods from pMSCV-Bmi1 (29), pMKO vector encoding either control or anti human E4F1 shRNAs, pLKO1 encoding shRNAs directed against murine RB1 or p53 (MISSION NM_011640.1–625s1c1; Sigma-Aldrich), or control irrelevant sequences.
Quantitative RT-PCR.
The primers and conditions used for quantitative RT-PCR analyses are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank members of L.L.C.’s and C.S.’s laboratories, C. Jacquet, E. Jouffre, and P. Cavelier, for providing technical help and critical readings; Institut de la clinique de la souris (Strasbourg) for performing injections of E4F1 flox ES cells into blastocysts; and C. Blanpain (University of Brussels, Brussels), M. Van Lohuizen (National Cancer Institute, Amsterdam), J. Jorcano and A. Ramirez (Centro de investigaciones energéticas medioambientales y tecnalogicas, Madrid), A. Gandarillas (Instituto de Formación e Investigación Marqués de Valdecilla, Santander, Spain), M. Barbacid, and M. Serrano (Centro nacional de investiganioces oncologicas, Madrid) for providing reagents and mice. Imaging and histological analyses were performed at the Montpellier Rio Imaging and Réseau d'Histologie Expérimentale de Montpellier core facilities (Montpellier), respectively. C.S. and J.C. are supported by the Agence Nationale pour la Recherche, the American Institute for Cancer Research, la Fondation pour la Recherche Médicale, and institutional supports from Centre National de la Recherche Scientifique. L.L.C. is supported by the INSERM Avenir Program, the Association pour la Recherche contre le Cancer (ARC), and the Ligue Contre le Cancer. M.L. and E.H. are supported by fellowships from ARC.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010167107/-/DCSupplemental.
References
- 1.Raychaudhuri P, Rooney R, Nevins JR. Identification of an E1A-inducible cellular factor that interacts with regulatory sequences within the adenovirus E4 promoter. EMBO J. 1987;6:4073–4081. doi: 10.1002/j.1460-2075.1987.tb02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Cam L, et al. E4F1 is an atypical ubiquitin ligase that modulates p53 effector functions independently of degradation. Cell. 2006;127:775–788. doi: 10.1016/j.cell.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 3.Sandy P, et al. p53 is involved in the p120E4F-mediated growth arrest. Oncogene. 2000;19:188–199. doi: 10.1038/sj.onc.1203250. [DOI] [PubMed] [Google Scholar]
- 4.Rizos H, et al. Association of p14ARF with the p120E4F transcriptional repressor enhances cell cycle inhibition. J Biol Chem. 2003;278:4981–4989. doi: 10.1074/jbc.M210978200. [DOI] [PubMed] [Google Scholar]
- 5.Chagraoui J, et al. E4F1: A novel candidate factor for mediating BMI1 function in primitive hematopoietic cells. Genes Dev. 2006;20:2110–2120. doi: 10.1101/gad.1453406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul C, et al. The LIM-only protein FHL2 is a negative regulator of E4F1. Oncogene. 2006;25:5475–5484. doi: 10.1038/sj.onc.1209567. [DOI] [PubMed] [Google Scholar]
- 7.Fenton SL, et al. Identification of the E1A-regulated transcription factor p120 E4F as an interacting partner of the RASSF1A candidate tumor-suppressor gene. Cancer Res. 2004;64:102–107. doi: 10.1158/0008-5472.can-03-2622. [DOI] [PubMed] [Google Scholar]
- 8.Fajas L, et al. pRB binds to and modulates the transrepressing activity of the E1A-regulated transcription factor p120E4F. Proc Natl Acad Sci USA. 2000;97:7738–7743. doi: 10.1073/pnas.130198397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tessari MA, et al. Transcriptional activation of the cyclin A gene by the architectural transcription factor HMGA2. Mol Cell Biol. 2003;23:9104–9116. doi: 10.1128/MCB.23.24.9104-9116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nojima J, et al. Dual roles of smad proteins in the conversion from myoblasts to osteoblastic cells by bone morphogenetic proteins. J Biol Chem. 2010;285:15577–15586. doi: 10.1074/jbc.M109.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Cam L, Lacroix M, Ciemerych MA, Sardet C, Sicinski P. The E4F protein is required for mitotic progression during embryonic cell cycles. Mol Cell Biol. 2004;24:6467–6475. doi: 10.1128/MCB.24.14.6467-6475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanpain C, Fuchs E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotsarelis G. Epithelial stem cells: A folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 14.Brouard M, Barrandon Y. Controlling skin morphogenesis: Hope and despair. Curr Opin Biotechnol. 2003;14:520–525. doi: 10.1016/j.copbio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27:5115–5123. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Candi E, et al. p63 in epithelial development. Cell Mol Life Sci. 2008;65:3126–3133. doi: 10.1007/s00018-008-8119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerra C, et al. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4:111–120. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez A, et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- 19.Weiss RA, Eichner R, Sun TT. Monoclonal antibody analysis of keratin expression in epidermal diseases: A 48- and 56-kd keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984;98:1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal deletion of Rac1. Science. 2005;309:933–935. doi: 10.1126/science.1113579. [DOI] [PubMed] [Google Scholar]
- 21.Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell. 2008;132:299–310. doi: 10.1016/j.cell.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Wang L, Yang X. Disruption of Smad4 in mouse epidermis leads to depletion of follicle stem cells. Mol Biol Cell. 2009;20:882–890. doi: 10.1091/mbc.E08-07-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- 24.Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–568. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 25.Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Morris RJ, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 28.Braun KM, et al. Manipulation of stem cell proliferation and lineage commitment: Visualisation of label-retaining cells in whole mounts of mouse epidermis. Development. 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 30.Janzen V, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamurthy J, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 32.Molofsky AV, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Signer RA, Montecino-Rodriguez E, Witte ON, Dorshkind K. Aging and cancer resistance in lymphoid progenitors are linked processes conferred by p16Ink4a and Arf. Genes Dev. 2008;22:3115–3120. doi: 10.1101/gad.1715808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su X, et al. Rescue of key features of the p63-null epithelial phenotype by inactivation of Ink4a and Arf. EMBO J. 2009;28:1904–1915. doi: 10.1038/emboj.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martínez P, et al. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev. 2009;23:2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flores I, Blasco MA. A p53-dependent response limits epidermal stem cell functionality and organismal size in mice with short telomeres. PLoS ONE. 2009;4:e4934. doi: 10.1371/journal.pone.0004934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su X, et al. TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell. 2009;5:64–75. doi: 10.1016/j.stem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akala OO, et al. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453:228–232. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
- 39.Armesilla-Diaz A, et al. p53 regulates the self-renewal and differentiation of neural precursors. Neuroscience. 2009;158:1378–1389. doi: 10.1016/j.neuroscience.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 40.Cicalese A, et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 41.Clayton E, et al. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 42.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carroll DK, et al. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol. 2006;8:551–561. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- 44.Serrano M, et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 45.Barrandon Y, Li V, Green H. New techniques for the grafting of cultured human epidermal cells onto athymic animals. J Invest Dermatol. 1988;91:315–318. doi: 10.1111/1523-1747.ep12475646. [DOI] [PubMed] [Google Scholar]
- 46.Bitoun E, et al. LEKTI proteolytic processing in human primary keratinocytes, tissue distribution and defective expression in Netherton syndrome. Hum Mol Genet. 2003;12:2417–2430. doi: 10.1093/hmg/ddg247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.