Abstract
Meaningful body movements depend on the interplay between synaptic inputs to motoneurons and their intrinsic properties. Injury and disease often alter either or both of these factors and cause motoneuron and movement dysfunction. The ability of the motoneuronal membrane to generate persistent inward currents (PICs) is especially potent in setting the intrinsic excitability of motoneurons and can drastically change the motoneuron output to a given input. In this article, we review the role of PICs in modulating the excitability of spinal motoneurons during health, and their contribution to motoneuron excitability after spinal cord injury (SCI) and in amyotrophic lateral sclerosis (ALS) leading to exaggerated long-lasting reflexes and muscle spasms, and contributing to neuronal degeneration, respectively.
Keywords: Motor neuron, Motoneuron, Persistent inward current, Amyotrophic lateral sclerosis, Spinal cord injury
1. Introduction
Spinal motoneurons provide the final neural output of the central nervous system (CNS) through which motor commands to peripheral muscles are communicated. They were called the “final common pathway” by Sherrington (1906) because motor activity has to be executed through the activation of motoneurons. Although motoneurons are only a very small percentage of all neurons (<0.0005% of all neurons in humans, Kernell, 2006), their study provides unusually deep insights into the function of the motor system. First, they have large soma making their identification and penetration with microelectrodes relatively easy. Second, their direct connection to muscles gives their output clearly defined functional significance. Third, their action potentials are one to one with the muscle fibers that they innervate, so that motoneurons are the only neurons whose firing patterns can be readily measured in human subjects.
It has long been appreciated that motoneurons can generate steady repetitive firing to prolonged inputs (Granit et al., 1963; Kernell, 1965). The general model of the motoneuron was that its extensive dendritic tree passively transmitted synaptic current to the soma, where it was converted into spike trains (Powers and Binder, 2001). The realization that motoneurons are not simple integrate and fire cells emerged from two revolutionary results. In Seattle, Schwindt and Crill showed that motoneurons possessed large persistent inward currents (PICs) that could greatly increase firing rate and prolong firing after input ceased (Schwindt and Crill, 1977, 1980a,b, 1982). Then in Copenhagen, Hounsgaard, Kiehn, Hultborn and colleagues showed that large PICs were a natural consequence of endogenous neuromodulators released by axons originating in the brainstem (Hounsgaard and Kiehn, 1985, 1989; Hounsgaard et al., 1988a,b). The main neuromodulators were shown to be serotonin and norepinephrine. Several studies (Hounsgaard and Kiehn, 1993; Lee and Heckman, 1996, 1998a,b, 2000; Bennett et al., 1998) subsequently demonstrated that most of the PIC is generated in dendritic regions and hence that motoneuron dendrites are not passive but in fact highly active integrators of input. The dendritic PIC greatly amplifies synaptic input – as much as fivefold (Lee and Heckman, 2000; Hultborn et al., 2003). It is now considered likely that PICs play a fundamental role in shaping motoneuron firing patterns during normal motor behavior (Heckman et al., 2008a,b, 2009). In this review, we briefly summarize the ionic mechanisms and functions of PICs in the normal state, and then concentrate on their potential involvement in two motor disorders: spasticity emerging after spinal cord injury (SCI) and the neurodegenerative disease amyotrophic lateral sclerosis (ALS).
2. Persistent inward currents in normal function
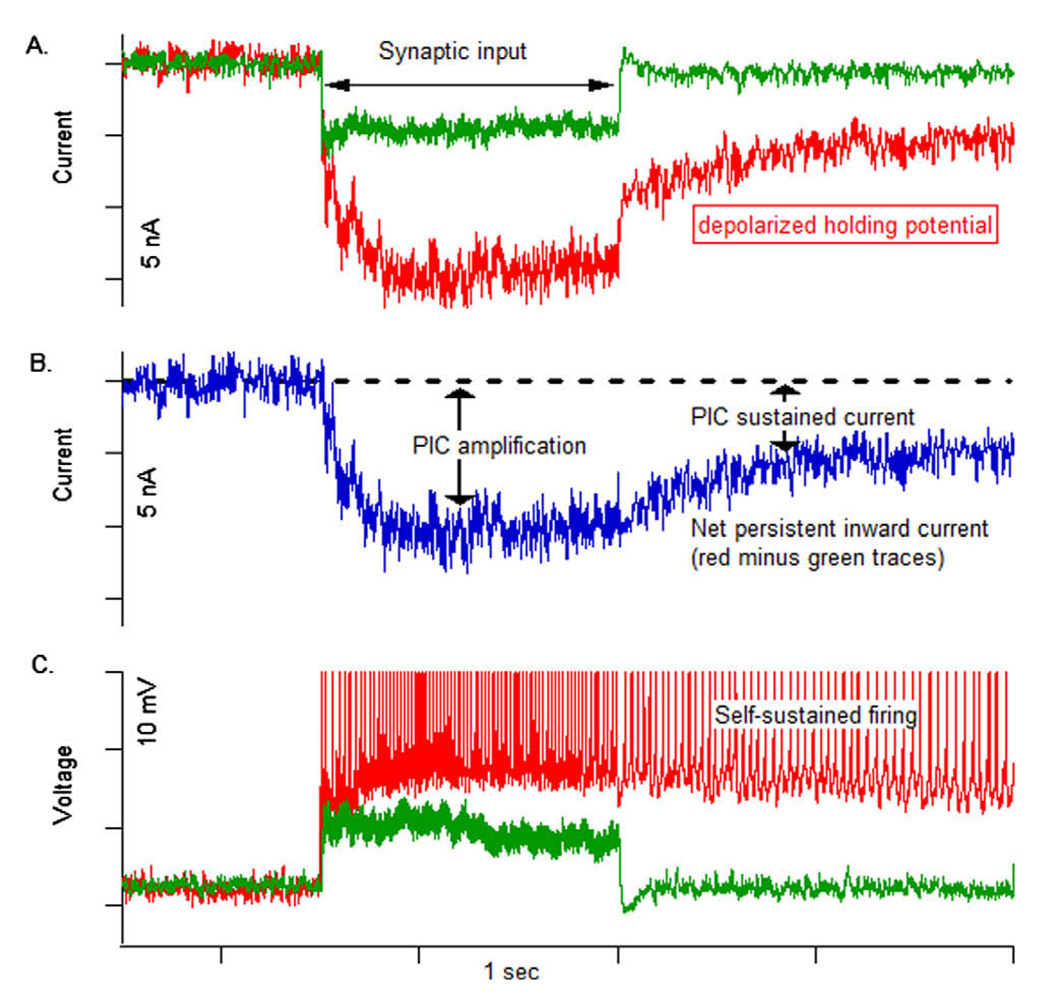
Schwindt and Crill (1977, 1980a,b) were the first to discover persistent inward currents (PICs) in spinal motoneurons. The PIC is a depolarizing inward current that activates as long as the membrane potential is depolarized (i.e., does not inactivate with prolonged depolarization). It is an intrinsic ionic mechanism that allows motoneurons to respond to brief synaptic input with prolonged firing activity, even after the cessation of the input (i.e., self-sustained firing, Fig. 1C) (Schwindt and Crill, 1980b; Hounsgaard et al., 1988b). This mechanism could be beneficial in situations when motoneurons need to be constantly activated (e.g., during postural control or isometric contractions) since steady synaptic input would not be required (Hounsgaard et al., 1988b; Lee and Heckman, 1998a,b). Motoneuron self-sustained firing can be terminated through inhibitory synaptic inputs, which hyperpolarize the membrane potential and deactivate the PIC (Hounsgaard et al., 1988b; Kuo et al., 2003; Bui et al., 2008).
Fig. 1.
The dendritic persistent inward current (PIC) amplified and prolonged synaptic input in a low-threshold, type S motoneuron. (A) At a hyperpolarized holding potential (−90 mV; green trace), synaptic input produced a steady current with a sharp onset and offset. At a depolarized holding potential (~−55 mV; red trace), the PIC is activated and amplifies and prolongs the same input. Baseline holding currents are removed to allow the traces to be superimposed. (B) The difference between the currents in A reflects the net PIC contribution (C) In current clamp, the same input produces a steady excitatory post-synaptic potential (EPSP) when the cell is hyperpolarized (~−90 mV; green trace). At a more depolarized level (−70 mV; red trace) [offset removed], the input evokes repetitive firing and then slower self-sustained firing when the input is removed. Data are from Lee and Heckman (1996). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
When the PIC is activated, it enhances intrinsic motoneuron excitability by increasing the gain of the frequency–current (F–I) relationship (which is the input–output function of the motoneuron), increasing the maximum firing rate of the motoneuron, and amplifying synaptic input (Fig. 1A and B, Lee and Heckman, 2000). Because the majority of the PIC-mediating channels are located on the dendrites (Hounsgaard and Kiehn, 1993; Lee and Heckman, 1996, 2000; Bennett et al., 1998), a small synaptic input that activates the dendritic PIC is amplified resulting in a large current reaching the soma (i.e., the synaptic current + the PIC) producing higher motoneuronal firing rates (Fig. 1B).
In spinal motoneurons, the PIC has two equal components mediated by two distinct types of ion channels, persistent Na+ (Na+ PIC, Hsiao et al., 1998; Lee and Heckman, 2001; Li and Bennett, 2003) and low voltage-activated L-type Ca2+ (Ca2+ PIC) (Cav1.3 type; Schwindt and Crill, 1980b; Hounsgaard and Kiehn, 1985, 1993) channels. The Na+ PIC is activated at subthreshold potentials, has fast activation/deactivation kinetics and partial time-dependent inactivation (Hsiao et al., 1998; Li and Bennett, 2003). The channels mediating the Na+ PIC appear to be located at the soma and/or proximal dendrites because the Na+ PIC is not hysteretic (i.e., the Na+ PIC activation and deactivation potentials are nearly the same on an ascending and descending voltage ramp) and has small tail currents (Li and Bennett, 2003). The Na+ PIC plays an essential role in the initiation of action potentials (APs) during rhythmic firing of motoneurons (Lee and Heckman, 2001). On the other hand, the Ca2+ PIC activation potential varies (could activate sub, at, or supra-threshold), has slow activation/deactivation kinetics and little or no time-dependent inactivation (Hounsgaard and Kiehn, 1989; Perrier et al., 2002; Li and Bennett, 2003). The Ca2+ PIC exhibits strong hysteresis (i.e., the Ca2+ PIC activation and deactivation potentials are different on an ascending and descending voltage ramp) and has long tail currents (Li and Bennett, 2003). These behaviors are an intrinsic component of the channel behavior (Moritz et al., 2007). The L-type Ca2+ channels mediating the Ca2+ PIC are located on the dendrites of motoneurons (Hounsgaard and Kiehn, 1993) and subsequently play a major role in the amplification of synaptic currents (Bennett et al., 1998; Lee and Heckman, 2000). Computer simulations of motoneurons indicate a mid dendritic location of the Ca2+ channels mediating the PIC (ElBasiouny et al., 2005; Bui et al., 2006). Furthermore, repeated depolarization of the membrane potential results in an increased activation of the Ca2+ PIC (Russo and Hounsgaard, 1994, 1996; Delgado-Lezama et al., 1999). This phenomenon is called “warm up” and is thought to result from depolarization-induced facilitation of the L-type Ca2+ channels in which membrane depolarization facilitates subsequent channel opening due to the increase in intracellular Ca2+ concentration (Dolphin, 1996; Perrier et al., 2000).
The activation of a dendritic PIC and its evoked behaviors were missed in the early electrophysiological studies because recordings were mostly performed in anaesthetized animals. Under anesthesia the activity in descending monoaminergic axons is greatly reduced and dendritic conductances are suppressed (Hultborn and Kiehn, 1992; Guertin and Hounsgaard, 1999); thus, motoneuron dendrites were largely dominated by their passive properties (Powers and Binder, 2001). However, the PICs and their evoked behaviors became evident when K+ channel blockers were used to unmask the PIC, as in the initial studies by Schwindt and Crill (1980a), or when the decerebrate preparation was used, in which the monoaminergic drive to motoneurons is not reduced, as in the following studies by Hounsgaard et al. (1984).
The discovery by Hounsgaard et al. (1988b) that endogenous neurotransmitters strongly facilitate the PIC revolutionized our understanding of motoneuron behavior in normal function and set the stage for many subsequent studies (reviewed in: Alaburda et al., 2002; Hultborn et al., 2004; Heckman et al., 2008a,b). The level of the monoaminergic drive from the brainstem to spinal cord varies between motor behaviors and is proportional to the level of required motor activity (low during sleep, medium during moderate motor activity such as standing or walking, and high during ultimate motor activities such as during the “fight or flight” states) (Jacobs et al., 2002; Aston-Jones and Cohen, 2005; Heckman et al., 2005).
The primary neurotransmitters that control the PIC are the monoamines serotonin (5-HT) and norepinephrine (NE). Axons that release 5-HT and NE from the brainstem originate from the raphe nucleus and the locus coeruleus nucleus (Maxwell et al., 1996; Patel et al., 1997), respectively, whereas there are little endogenous sources (~10%) of 5-HT or NE in the spinal cord (Newton and Hamill, 1988; Shapiro, 1997). Interestingly, monoamines have differential effects on the ventral versus dorsal horn of the spinal cord. In the ventral horn, monoamines have excitatory effects on motoneurons and interneurons (Hammar and Jankowska, 2003). For instance, 5-HT and NE have been shown to facilitate the activation of PICs (Lee and Heckman, 1999), reduce the resting leak conductance (Elliott and Wallis, 1992), induce membrane depolarization (Hsiao et al., 1997), enhance the hyperpolarization-activated inward current (Hsiao et al., 1997), and decrease the amplitude of the medium duration afterhyperpolarization (Berger et al., 1992). Collectively, these effects increase motoneuron excitability, increase the gain of the F-I relationship, and reduce the amount of synaptic current required to recruit the motoneuron or to generate self-sustained firing (Berger et al., 1992; Lindsay and Feldman, 1993; Hultborn, 1999; Heckman et al., 2005).
Conversely, monoamines have inhibitory effects in the dorsal horn. They inhibit sensory inputs to motoneurons (Jacobs and Fornal, 1997) such as cutaneous inputs (Clarke et al., 2002) and high-threshold muscle afferents (groups III and IV) through presynaptic inhibition (Cleland and Rymer, 1990; Jankowska, 1992; Miller et al., 1995). They also suppress inputs to deep dorsal horn interneurons from high-threshold afferents (groups III and IV) (Garraway and Hochman, 2001). The differential control of the monoaminergic drive on the spinal cord is attained through the effect of monoamines on the different metabotropic G-protein coupled receptors which influence the voltage-gated ion channels. For example, PIC facilitation in motoneurons is achieved through 5-HT2 (Perrier and Hounsgaard, 2003) and NE α1 receptors (Lee and Heckman, 1999), whereas inhibition of cutaneous inputs and high-threshold muscle afferent inputs is achieved through 5-HT1b/d and NE α2 receptors (Bras et al., 1990; Miller et al., 1995).
3. PICs and spinal cord injury
3.1. Spasticity after spinal cord injury (SCI)
Spasticity, or hyper-reflexia, is a common complication after a number of neurological disorders (e.g., stroke, cerebral palsy, multiple sclerosis, brain injury or SCI). It is commonly defined as “a velocity-dependent increase in the tonic stretch reflex (muscle tone) with exaggerated tendon jerks, resulting from the hyperexcitability of the stretch reflex” (Lance, 1980). Despite the extensive citation of this definition of spasticity, it has not been fully validated (c.f. Pandyan et al., 2005). The causative relationship between the hyperexcitable stretch and exaggerated tendon reflexes implied by the definition has not been confirmed experimentally. Instead, the magnitude of the tendon reflex was found to have no significant correlation with that of the stretch reflex (Fellows et al., 1993). Moreover, the classical definition of spasticity is considered to be narrow and restrictive (Young, 1994; Barnes, 2001; Pandyan et al., 2005). More specifically, because spasticity results from lesions in the pyramidal and extrapyramidal pathways (Burke, 1988), its pathophysiology varies depending on the site of neurological lesion. For instance, following stroke excessive muscle tone in the antigravity muscles is the prominent feature of spasticity with lesser involvement of muscle spasms, whereas following SCI excessive muscle spasms in flexor and extensor muscles are the prominent features of spasticity with lesser involvement of muscle tone.
The mechanisms underlying spasticity in stroke and SCI appear to be different as well, neural mechanisms are thought to be more involved in spasticity following SCI, whereas biomechanical mechanisms (i.e., changes in intrinsic properties of muscles) are thought to be more involved in spasticity following stroke (Pierrot-Deseilligny and Burke, 2005). Accordingly, the term “spasticity” has been increasingly used to refer to several features emerging after lesions to the descending corticospinal tract (i.e., upper motor neuron syndrome) (Edwards, 2002; Pandyan et al., 2005). In addition to muscle hypertonus, spasticity following SCI could also involve hyperreflexia (increased reflex gain), clonus (oscillating reflex activation of the muscle), clasp-knife responses (abrupt relaxation of the stretched muscle after initial resistance), long-lasting cutaneous reflexes, and large involuntary muscle contractions (i.e., muscle spasms) evoked by brief non-noxious cutaneous stimuli (Lance and Burke, 1974; Young, 1994). Our focus in this review is on spasticity emerging after SCI.
Spasticity usually develops several months after SCI, mainly in antigravity muscles. However, immediately following SCI, the spinal cord becomes areflexic for a period of time that lasts for weeks (Nacimiento and Noth, 1999). This period is called spinal shock and is characterized by loss of tendon reflexes below the level of the lesion, muscle paralysis, and flaccid muscle tone (Bastian, 1890). Days-to-weeks after injury, various muscle reflexes such as the tendon reflex, the flexor withdrawal reflex, and the Babinski sign recover gradually (Hiersemenzel et al., 2000). The threshold of the flexor reflexes, which are usually evoked by cutaneous stimulation, decreases over time until a brief stimulation of the foot plantar surface becomes capable of generating long-lasting strong contraction of the flexor muscles (Kuhn and Macht, 1948; Ashby and McCrea, 1987).
Extensor reflexes, which are usually evoked by proprioceptive stimuli, recover later than the flexor reflexes, and sometimes flexor/extensor muscle co-contractions can occur and can last for several seconds (Kuhn and Macht, 1948; Ashby and McCrea, 1987). Furthermore, intense muscle spasms lasting for several seconds can be triggered by various other stimuli such as heat/cold sensation and bladder distention (Little et al., 1989). Interestingly, extensor spasms in the leg muscles which make the leg rigid can at times provide assistance in dressing or walking (Barnes, 2001). Nonetheless, these spasms are usually painful and can reduce the functional outcome of the residual voluntary drive in individuals with incomplete SCI spasticity; thus, compromising rehabilitation efforts. This makes spasticity one of the most debilitating side effects of spinal cord injury (Little et al., 1989; Delwaide and Pennisi, 1994).
3.2. Role of PICs in spasticity after SCI
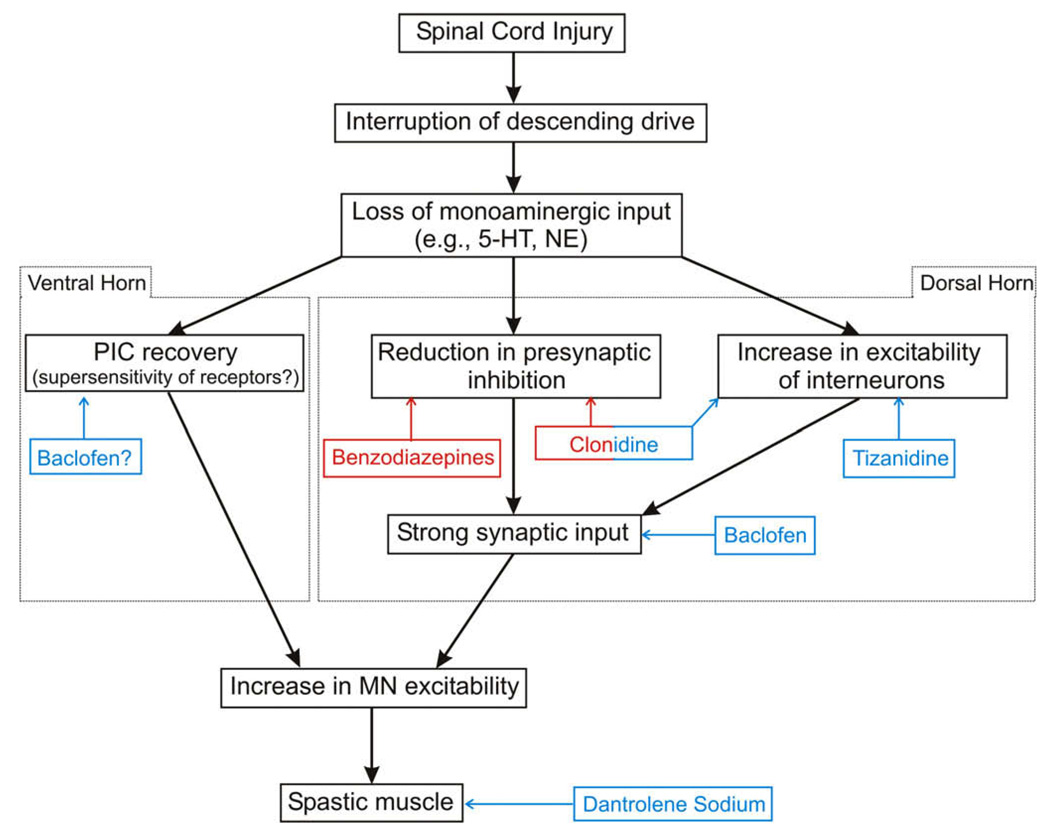
The role of PICs in the pathogenesis of spasticity after SCI has been studied over the last decade mainly in the chronic spinal rat model of spasticity (Bennett et al., 1999), which allowed for establishing the ionic basis of the long-lasting exaggerated reflexes and muscle spasms associated with spasticity. Immediately following SCI (acute injury), the loss of the monoaminergic drive from the brainstem to spinal cord causes disfacilitation (i.e., removal of excitation) of the ventral horn and disinhibition (i.e., removal of inhibition) of the dorsal horn (Fig. 2). The former effect reduces the excitability of motoneurons, whereas the latter effect increases the size and duration of the polysynaptic EPSPs of sensory inputs mediated through the dorsal horn (Bennett et al., 2004). At this acute stage, long-lasting reflexes are not activated due to the reduced excitability of motoneurons.
Fig. 2.
Block diagram illustrating the flow of events following SCI. The differential effects of monoamines on the dorsal and ventral horns are indicated. The site of action and nature of effect of the various drugs used for the management of spasticity after SCI are illustrated. Red color indicates a positive action, i.e., increase, whereas a blue color indicates a negative action, i.e., reduction. Figure adapted from Elbasiouny et al. (2009). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Conversely, in chronic injury, the motoneuronal PIC recovers, via as yet unclear mechanisms, and restores the motoneuronal excitability. It has been shown that motoneurons become highly sensitive to monoamines after long-term injury and this could play a role in the reactivation of the PIC. The supersensitivity of receptors could allow the residual monoamines available below the level of the lesion (from the autonomic system and blood vessels) to facilitate the reactivation of PICs after injury (Harvey et al., 2006a,b; Li et al., 2007). The lack of descending control over PIC activation coupled with the aberrant enhancement in excitability of other spinal mechanisms (see next section) allow synaptic inputs to evoke prolonged high motoneuronal firing activity (Fig. 2). At this stage, prolonged EPSPs generated by sensory stimuli can easily reactivate the PICs and trigger long-lasting reflexes and muscle spasms (Bennett et al., 2004).
In human subjects, where intracellular recordings are not feasible, the PIC is measured indirectly via monitoring the firing rates of motor units (Paired motor unit analysis technique, Kiehn and Eken, 1997; Gorassini et al., 2002). In this technique, the firing rates of two motor units are recorded simultaneously and compared (the lower-threshold unit is the control, whereas the higher-threshold unit is the test unit). The difference in firing rate of the control unit at the recruitment and de-recruitment of the test unit (Δf) is measured and used to estimate the PIC magnitude in the test unit (Gorassini et al., 2002). Careful examination of this technique in decerebrate cats, in which independent measurements of Δf values and PIC amplitudes are feasible, showed that this technique is dependent on a number of factors other than the PIC amplitude (Powers et al., 2008). These factors include: (1) amplitude and mode of PIC activation (graded versus all-or-none) in the two units, (2) amount of rate modulation in the control unit, (3) choice of the control unit, and (4) the relationship, whether linear or non-linear, between the net synaptic excitation received by the two units (Powers et al., 2008). Despite these constraints, motor unit recordings in spastic SCI patients using this technique suggest that spasticity in humans involves similar Na+ and Ca2+ ionic mechanisms to those found in animal models (Gorassini et al., 2004; Norton et al., 2008). More specifically, long-lasting reflexes and self-sustained firing seen during muscle spasms were ascribed to the activation of the Ca2+ PIC, whereas the slow and regular firing of motor units of chronic SCI subjects seen after muscle spasms or voluntary contraction were ascribed to the activation of the Na+ PIC. In agreement with that, computer models of motoneurons incorporating Na+ and Ca2+ PICs were able to exhibit self-sustained firing, plateau potentials, and sustained depolarization of the membrane potential similar to those seen during spasticity (ElBasiouny et al., 2005, 2006).
Additional evidence for PIC activation in normal and spastic individuals after SCI can be inferred from the large muscle contractions elicited in response to high-frequency electrical stimulation of muscles (Collins et al., 2001; Nickolls et al., 2004). Electrical stimulation of constant frequency and intensity of human muscles resulted in progressive generation of higher forces (up to 40% of maximal voluntary contraction) that exceeded those obtained from direct stimulation of motor axons. With triangular patterns of stimulation, abrupt muscle forces were also generated that persisted even after the decline of stimulation frequency. The activation of these additional forces appear to be an intrinsic central mechanism, which does not depend on volitional drive to motoneurons, consistent with PIC activation because the additional forces: (1) disappeared when the nerve was blocked proximal to the stimulation site, (2) could be triggered in sleeping subjects, (3) could be triggered at mild stimulation intensities that activated large sensory, but not motor axons, and (4) could be turned off by inhibitory synaptic input from the contraction of antagonist muscles or cutaneous nerve stimulation (Collins et al., 2001, 2002; Nickolls et al., 2004).
3.3. Potential for control of spasms via PICs
Long-lasting spasms are often triggered via hyperexcitable dorsal horn circuitry and then amplified and prolonged by motoneuron PICs. Thus drugs that target 5-HT2 and NE α1 receptors that facilitate motoneuron PICs used in combination with agonists of 5-HT1b/d and NE α2 receptors that inhibit sensory processing in the dorsal horn may prove to be the optimal approach for controlling spasms. Application of direct current (DC) and alternating current (AC) electrical fields across the spinal cord was also proposed through computer simulations as a means for reducing the motoneuronal hyperexcitability via the modulation of the dendritic PIC (ElBasiouny and Mushahwar, 2007). Non-invasive application of these electrical fields in spastic rats produced moderate reduction in spasticity (14%, ElBasiouny et al., 2010), but more studies are need to establish the effectiveness of this technique.
3.4. Other spinal mechanisms contributing to spasticity after SCI
The complete pathophysiology of spasticity is still unclear, and the reemergence of PICs after SCI appears to be one of the contributing factors to the manifestation of spasticity. However, the hallmark of spasticity is the abnormally increased excitability of the stretch reflex (Lance, 1980). Many spinal pathways, both excitatory and inhibitory, converge onto the motoneuron and are involved in the modulation of the excitability of the stretch reflex (e.g., Ia-reciprocal inhibition, recurrent inhibition, presynaptic inhibition, and interneurons). Thus, enhancement in the excitability of the stretch reflex could equally result from malfunctions that cause enhancements in the excitability of an excitatory pathway or suppression of the excitability of an inhibitory pathway.
Experimental evidence demonstrated that a reduction in the excitability of various inhibitory pathways, in addition to an enhancement in the excitability of excitatory pathways does indeed occur after SCI in humans and in animal models of spasticity. For instance, reductions in post-activation depression (Hultborn and Nielsen, 1998; Thompson et al., 1998), presynaptic inhibition (Delwaide, 1973; Faist et al., 1994), and Ia-reciprocal inhibition (Boorman et al., 1996), in addition to increases in the excitability of motoneurons (Hiersemenzel et al., 2000; Li et al., 2004a,b) and excitatory interneurons (Kitzman, 2006, 2007), were reported following SCI.
It is of great importance for the development of effective rehabilitation strategies to assess the likelihood of involvement and the relative significance these mechanisms might have in the pathophysiology of spasticity (for detailed reviews on this topic see: Pierrot-Deseilligny and Burke, 2005; ElBasiouny et al., 2010). Therapeutic interventions targeting one or more of the likely and/or significant mechanisms contributing to spasticity may be especially effective in reducing the severity of spasticity and related motor disabilities in people with SCI. Interestingly, when the intensity of spasticity, as assessed on the Ashworth scale, was examined versus the level of aberration in the various proposed spinal pathways (e.g., motoneuron hyperexcitability, reduction in presynaptic inhibition, and Ia-reciprocal inhibition), no statistically significant correlation was found (Faist et al., 1994; Hiersemenzel et al., 2000; Marque et al., 2001; Pierrot-Deseilligny and Burke, 2005). This led to the assumption that the intensity of spasticity is not a function of one specific abnormality.
However, there are numerous factors that could have contributed to the low correlation between the level of spasticity and the degree of abnormalities in the various spinal pathways. First, experimental volunteers with spasticity resulting from different pathological conditions (e.g., stroke, SCI, cerebral palsy, and multiple sclerosis) were sometimes pooled together in clinical and research studies. Even in studies involving SCI volunteers only, small sample sizes are encountered as well as the pooling of results from people with different levels and severity of lesions. Second, the methods used for the assessment of excitability of spinal pathways were not selective (e.g., tendon vibration reduces the magnitude of the H-reflex due to the concurrent effects of post-activation depression and presynaptic inhibition). Third, the excitability of spinal pathways was assessed under variable conditions (i.e., rest versus contraction), thus leading to conflicting results (e.g., post-activation depression disappears as the level of muscle contraction increases). Finally, the alteration in excitability of spinal pathways that may contribute to the pathophysiology of spasticity do not covary (Pierrot-Deseilligny and Burke, 2005). This could explain the discrepancy in reports regarding the nature of alteration in some mechanisms during spasticity. For instance, contradictory results were reported on Ia-reciprocal inhibition after SCI in which enhancement (Boorman et al., 1991), reduction (Boorman et al., 1996), and reciprocal facilitation (i.e., reciprocal inhibition was replaced by excitation, Crone et al., 2003) were reported. In general, recent assessment of the likelihood of involvement and relative significance of the various spinal mechanisms might have in spasticity indicated that hyperexcitability of motoneurons and interneurons are most likely involved and play significant roles in the pathophysiology of spasticity (ElBasiouny et al., 2010a). Axonal sprouting, reductions in presynaptic inhibition, post-activation depression, and Ia-reciprocal inhibition were determined to be likely involved in the pathophysiology of spasticity, but had various levels of significance (high, moderate, uncertain, and unclear, respectively) (ElBasiouny et al., 2010a).
4. PICs and amyotrophic lateral sclerosis
4.1. Clinical features of amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a predominantly adult onset disease in which progressive motoneuron degeneration occurs. Since the first clinico-pathological description of ALS by Charcot and Joffroy (1869), the cause of motoneuron death has been a point of active research. Both sporadic and familial forms of the disease exist with the majority (90%) being the sporadic form. Of the familial cases, seven genes have been identified through linkage and positional cloning studies (Gros-Louis et al., 2006; Pasinelli and Brown, 2006; Dunckley et al., 2007) with mutations in the superoxide dismutase 1 (SOD1) gene accounting for 20% of familial cases (Rosen et al., 1993). The rate of mortality is staggering with most patients dying from respiratory failure within 5 years of the symptom onset (Beghi et al., 2006; Nelson and McGuire, 2006). Today the primary cause of motoneuron degeneration is still not clear and it is likely a combination of pathologies that trigger degeneration.
The clinical diagnosis of ALS is often not confirmed until nearly a year after symptoms are detected (Brooks, 2000; Zoccolella et al., 2006). Generally, ALS presents itself with both upper and lower motoneuron symptoms and is generally classified as arm, leg, or bulbar onset. Upper motoneuron signs include hyper-reflexia, increased tone, and clonus while lower motoneuron signs include weakness, fatigue, muscle atrophy, non-specific muscle cramping, and fasciculations (Brooks et al., 2000). At the onset of clinical symptoms, significant motoneuron degeneration has already occurred (Wohlfart, 1959; Swash and Ingram, 1988), which is mirrored in a mouse model of ALS (Hegedus et al., 2007, 2008). In this model, a 22% and 60% reduction in motor units of the fast twitch muscles, medial gastrocnemius and tibialis anterior, were seen presymptomatically at 40 and 60 days of age, respectively (Hegedus et al., 2007, 2008). Motor units from the slow twitch muscle, soleus, decreased only after symptom onset indicting a distinction between the degeneration of fast (type F, large) motoneurons and slow fatigue resistant (type S, small) motoneurons (Hegedus et al., 2007). The delay between large motoneuron degeneration, overt symptoms, and clinical diagnosis may be one reason for the failure of so many clinical drug trials. The disease may simply have progressed too far by the time interventions are implemented.
The identification of mutations in the SOD1 gene in some familial ALS patients, paved the way for transgenic mouse models of ALS to be developed. Expression of mutated human SOD1 protein (mSOD1) in mice mirrors many of the pathological features and time course of ALS in familial and sporadic cases but differences do exist between the mouse models and patients and also between mouse models expressing different SOD1 mutations and protein expression levels (Durand et al., 2006). However, mouse models are the only way to assess possible presymptomatic changes in ALS patients and may provide crucial data on very early abnormalities which lead to neuronal degeneration and possibly to the development of an early diagnostic tool. For instance, the time course of neuronal degeneration investigated in various mouse models of ALS with different SOD1 mutations (G85R, G93A, and G37R) showed that significant loss in neurons was correlated with the appearance of phosphorylated neurofilament inclusions and the onset of reactive astrocytosis (Chiu et al., 1995; Dal Canto and Gurney, 1995; Wong et al., 1995; Bruijn et al., 1997; Brett et al., 1998).
To this end, recent behavioral studies of neonatal mSOD1 mice have provided evidence of developmental changes. Although not documented to date in human patients, transient, subtle sensory-motor deficits are discernable during the first postnatal week in mSOD1 mice. The exact behaviors affected appear to depend on specific mutations, however, in two mouse models (G85R and G93A), a transient delay in the righting response was present (Amendola et al., 2004; Van Zundert et al., 2008). These early behavioral deficits suggest that pathological changes are present long before motoneuron death and stress the need to discover early markers of the disease.
4.2. Proposed mechanisms for motoneuron degeneration in ALS
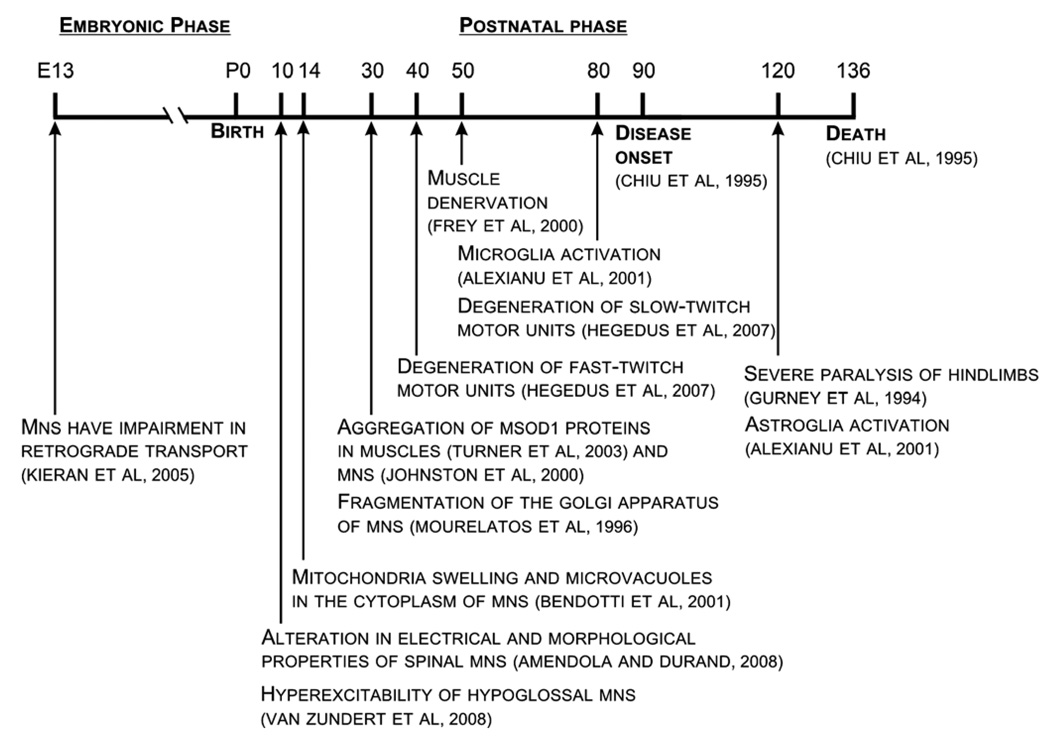
Identified after clinical diagnosis in ALS patients and throughout the lifespan of mSOD1 mice, a wide range of cellular pathologies are present which likely contribute to motoneuron degeneration including mitochondrial and axon transport dysfunction (reviewed in: Rao and Nixon, 2003; De Vos et al., 2008; Magrane and Manfredi, 2009; Shi and Gal, 2009), the accumulation of misfolded proteins (reviewed in: Chattopadhyay and Valentine, 2009), alteration in mechanisms which could lead to glutamate-induced excitotoxicity (reviewed in: Van Den Bosch et al., 2006; Foran and Trotti, 2009), and glial activation (reviewed in: Sargsyan et al., 2005) [see Fig. 3 for a summary of symptom timeline in ALS mouse models]. The process of identifying the primary mechanism(s) leading to motoneuron degeneration in ALS is compounded by the fact that these stressors are interrelated (primary versus secondary pathologies have not been distinguished yet) and any of them could trigger/enhance the other mechanisms resulting in progressive motoneuron loss. For instance, increased Ca2+ entry through excessive glutamate receptor activation can lead to mitochondria Ca2+ overloading resulting in production of reactive oxygen species (ROS) and additional mitochondria damage (Carriedo et al., 2000; Chang and Reynolds, 2006; Grosskreutz et al., 2007). Mitochondrial damage can enhance glutamate-induced excitotoxicity by depolarizing the resting membrane potential and releasing the Mg2+ block of NMDA receptors (Heath and Shaw, 2002). Furthermore, mitochondrial damage may result in energy generation dysfunctions which hamper axon transport, metabolic processes, and protein synthesis (Chang and Reynolds, 2006). Identifying abnormalities occurring before symptom onset and at early stages of the disease in transgenic mice could be one way to dissociate these mechanisms. For the purpose of this review, we focus on the excitotoxicity hypothesis in ALS because of its relevance to PICs and motoneuronal excitability.
Fig. 3.
A timeline summary showing the progression of events in the various animal models of ALS. (See above-mentioned references for further information.)
4.3. Motoneuron vulnerability in ALS
Despite the extensive cellular abnormalities seen in mSOD1 mice, these abnormalities do not explain the prominent, early degeneration of motoneurons in contrast to most other cells types, and it may be intrinsic motoneuron properties which cause the initial vulnerability. Motoneurons have high energy demands due to both their size (large soma, extensive dendrites, and very long axons) and function (often producing sustained repetitive firing) making them particularly susceptible to the axon transport, mitochondria, and metabolic dysfunctions prominent in ALS (Magrane and Manfredi, 2009). Interestingly, the dendritic morphology and branching complexity of mSOD1 motoneurons have been shown to increase significantly, long before symptom onset (at P8 in the G85R model; Amendola and Durand, 2008). A similar increase in the dendritic morphology of mSOD1 motoneurons was also observed in the G93A model (low expressor line, personal communication with the Durand group). It is unclear at the moment whether the increase in dendritic branching is a compensatory mechanism triggered to counteract early alterations in motoneuronal excitability and electrical properties (see next section) or leads to these electrical alterations. In either case, the increase in dendritic morphology increases the energy demands of mSOD1 expressing motoneurons and may contribute to motoneuron degeneration in familial ALS.
In addition to intrinsically high energy demands, calcium homeostasis is especially precarious in motoneurons. First, motoneurons have a large number of Ca2+ permeable AMPA receptors, which aid in fast synaptic transmission and allow large influxes of Ca2+ to the cell (Carriedo et al., 1996; Van Den Bosch et al., 2000; Vandenberghe et al., 2000a,b; Kawahara et al., 2003; Corona and Tapia, 2007). Second, most motoneurons lack the calcium binding proteins calbindin and parvalbumin which, in other neurons, neutralize large Ca2+ influxes produced by glutamate stimulation (Ren and Ruda, 1994). Instead, motoneurons rely heavily on mitochondria for Ca2+ buffering (Carriedo et al., 2000; Grosskreutz et al., 2007). Stressors that induce mitochondrial damage and dysfunction are therefore especially detrimental to motoneurons causing energy deficiencies which result in improper Ca2+ buffering and increased excitability (Bergmann and Keller, 2004). Interestingly, mitochondria Ca2+ loading capacity is decreased presymptomatically in the brain and spinal cord of the G93A model (high expressor line) (Damiano et al., 2006).
The coupling of high energy demands and low Ca2+ buffering capabilities make motoneurons particularly susceptible to excitotoxic damage (Robberecht, 2000; Van Damme et al., 2005). Excitotoxicity occurs when excessive Ca2+ overwhelms the cell’s buffering systems and activates cell death signals. Although generally associated with increased glutamate receptor activation, excitotoxicity can occur during normal glutamate receptor activation if conditions of energy depletion are present (Novelli et al., 1988; Henneberry et al., 1989). Due to the PIC’s role in setting motoneuron excitability, an increase in PIC amplitude could contribute to excitotoxic damage through a number of mechanisms. First, an increase in the Ca2+ PIC will directly increase Ca2+ entry. Second, an increase in either the Na+ or Ca2+ component of the PIC may lead to an increased F–I gain and higher firing rates (Heckman et al., 2005) which increase Ca2+ entry through activation of high-threshold L-type Ca2+ channels during the action potential. In both cases, the Ca2+ buffering capabilities of mitochondria will be further stressed. Third, an increase in PIC amplitude will raise energy demands by increasing motoneuron excitability and/or possibly by increasing dendritic branching (see section above). Therefore, the PIC amplitude may contribute to motoneuron death through raising both cytosolic Ca2+ levels and the energy demands of the cell.
4.4. Alteration of motoneuron excitability and role of PICs in ALS
In ALS, the excitability of motoneurons is altered, and evidence for both hypoexcitability (Bories et al., 2007; Pambo-Pambo et al., 2009) and hyperexcitability (Pieri et al., 2003, 2009; Kuo et al., 2004, 2005; Zona et al., 2006; Van Zundert et al., 2008; Pambo-Pambo et al., 2009) has been reported in mouse models. Hypoexcitability of mSOD1 motoneurons in the G85R model was expressed as reduction in the input resistance and gain of the F–I relationship relative to WT in response to long current pulses in neonatal motoneurons recorded in vitro in the whole spinal cord preparation (Bories et al., 2007). Similarly, a reduction in the F–I gain of neonatal mSOD1 motoneurons was seen in the G85R and G93A (low expressor line) models in response to slow current ramps recorded in vitro in slices (Pambo-Pambo et al., 2009). On the other hand, hyperexcitability was seen in the G93A (high expressor line) model as an increase in the F–I gain in response to current pulses and ramps in cultured motoneurons (Kuo et al., 2004, 2005) and in the G93A (low expressor line) in response to current pulses recorded in vitro in slices (Pambo-Pambo et al., 2009). Also, neonatal hypoglossal motoneurons and cultured cortical neurons in the G93A model (high expressor line) exhibited an increased Na+ PIC and higher firing frequency to injected current (Van Zundert et al., 2008; Pieri et al., 2009). It is unclear whether the discrepancies between the alterations in excitability (hypo-versus hyperexcitability) observed in mSOD1 motoneurons is due to the type of the genetic model of ALS (G85R versus G93A low or high expressor lines), the type of preparation (whole cord versus slices versus cultured cells), or the protocol used to activate the motoneuron (long current pulse versus slow current ramp). Slice preparations usually lack the full contribution of dendritic voltage-sensitive ion channels because the dendrites are substantially truncated by the slice, and cultured motoneurons lack the physiological environment and full development of motoneuron dendrites. Also, long current pulses and slow current ramps could have differential effects on the ionic conductances involved in triggering spikes in mSOD1 motoneurons (Pambo-Pambo et al., 2009). Clinically, mounting evidence suggests that motoneuronal hyperexcitability is a feature of ALS in patients and is likely present before symptom onset (Kostera-Pruszczyk et al., 2002; Zanette et al., 2002; Piotrkiewicz et al., 2008; Vucic and Kiernan, 2008; Vucic et al., 2008, 2009). Furthermore, riluzole, the only FDA approved drug to treat ALS, is known to decrease the excitability of motoneurons through both intrinsic (i.e., reduction of PIC, see next section) and synaptic mechanisms (i.e., reduction of glutamate release) (Bensimon et al., 1994; Urbani and Belluzzi, 2000; Andreadou et al., 2008; Fumagalli et al., 2008).
Neuronal excitability also has profound effects on dendritic structure and morphology. It has been recently shown that altering the excitability of motoneurons by genetic manipulations of their ion channels resulted in dendritic overgrowth via more than one mechanism (Duch et al., 2008). For instance, when the motoneuronal excitability was increased by knocking-down the potassium channel subunits, the dendrites exhibited increased dendritic branch and node formation. In contrast, when the motoneuronal excitability was reduced by increasing the expression of persistent potassium channels, the dendrites exhibited increased dendritic branch elongation. The changes in dendritic morphology observed in neonatal mSOD1 motoneurons in the G85R model are consistent with those resulting from increased motoneuronal excitability (Amendola and Durand, 2008). Furthermore, it is likely that the motoneuronal excitability and spiking activity are reflected as global calcium signals, which can also effect dendritic growth through transcriptional regulation (Redmond and Ghosh, 2005).
As stated above, data from transgenic mice expressing mSOD1 suggest that the PIC amplitude is altered in very young animals and may play an early role in motoneuron dysfunction. For instance, in studies from cell cultures and in neonatal slice preparations from the G93A (high expressor line) model, the Na+ PIC amplitude was increased in spinal motoneurons (Pieri et al., 2003; Kuo et al., 2004, 2005). Comparable results were also obtained in mSOD1 upper motoneurons and other cortical cells using similar preparations in the G93A (high expressor line) (Van Zundert et al., 2008; Pieri et al., 2009) suggesting that an increase in Na+ PIC amplitude is an early feature of mSOD1 associated ALS. In addition, Na+ channels in mSOD1 mice display a rapid recovery from fast inactivation, which would allow mSOD1 motoneurons to fire at higher firing rates (Zona et al., 2006) and in ALS patients the nodal NaP (persistent Na+ currents at the nodes of ranvier) are increased (Tamura et al., 2006). The ALS drug, riluzole, which is known for its effect in reducing the Na+ PIC, gives further support for the involvement of Na+ currents in ALS (Bensimon et al., 1994; Urbani and Belluzzi, 2000; Andreadou et al., 2008; Fumagalli et al., 2008).
Taken collectively, it is evident that motoneuronal excitability and PICs are altered in ALS, but more work is needed to elucidate the nature of these alterations in the various genetic models of ALS using various protocols of current injection and in preparations having intact motoneuron dendrites. Computer simulations of reconstructed morphologies could be an important tool to assist in resolving these issues (ElBasiouny et al., 2010b). Also, studies looking specifically at the development of both the Na+ and Ca2+ PIC and motoneuron morphology are needed to distinguish the primary versus secondary changes, and to determine if the neonatal increase in PIC amplitude is sustained throughout the disease and contributes to motoneuron degeneration in ALS.
5. Conclusion
The intrinsic excitability and electrical properties of spinal motoneurons are continuously regulated during various daily motor tasks. This regulation is attained through supraspinal control over the intrinsic membrane mechanisms of spinal motoneurons (e.g., PICs) and various spinal mechanisms (e.g., Ia-reciprocal inhibition and presynaptic inhibition). After injury or a neurological disease, pathological changes in these mechanisms occur that contribute to the resulting motor deficit. Specifically, the excitability of motoneurons and the magnitude of their PICs appear to be altered in both SCI and ALS, and are suggested to play major role in the pathophysiology of these conditions. After SCI, rehabilitation interventions targeting PICs or other likely mechanisms are expected to be effective in reducing the severity of spasticity. In ALS, the early alterations in dendritic morphology, motoneuronal excitability, and increase in the PIC amplitude may play a key role in subsequent pathology and motoneuron death. More work is needed in both disorders to study the temporal changes in PIC properties during the progression of these conditions.
Acknowledgements
The authors were supported by grants from the National Institutes of Health (F31 NS060532 to J.E.S., R01 NS051462 to CJH) and the Canadian Institutes of Health Research and the ALS Society of Canada (Tim E. Noel postdoctoral fellowship to S.M.E).
References
- Alaburda A, Perrier JF, Hounsgaard J. Mechanisms causing plateau potentials in spinal motoneurones. Adv Exp Med Biol. 2002;508:219–226. doi: 10.1007/978-1-4615-0713-0_27. [DOI] [PubMed] [Google Scholar]
- Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- Amendola J, Durand J. Morphological differences between wild-type and transgenic superoxide dismutase 1 lumbar motoneurons in postnatal mice. J Comp Neurol. 2008;511:329–341. doi: 10.1002/cne.21818. [DOI] [PubMed] [Google Scholar]
- Amendola J, Verrier B, Roubertoux P, Durand J. Altered sensorimotor development in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2004;20:2822–2826. doi: 10.1111/j.1460-9568.2004.03745.x. [DOI] [PubMed] [Google Scholar]
- Andreadou E, Kapaki E, Kokotis P, Paraskevas GP, Katsaros N, Libitaki G, et al. Plasma glutamate and glycine levels in patients with amyotrophic lateral sclerosis: the effect of riluzole treatment. Clin Neurol Neurosurg. 2008;110:222–226. doi: 10.1016/j.clineuro.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Ashby P, McCrea D. Neurophysiology of spinal spasticity. In: Davidoff RA, editor. Handbook of the spinal cord. New York: Marcel Dekker Inc.; 1987. pp. 120–143. [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Barnes MP. An overview of the clinical management of spasticity. In: Barnes MP, Johnson GR, editors. Upper motor neurone syndrome and spasticity. Clinical management and neurophysiology. Cambridge University Press; 2001. pp. 1–11. [Google Scholar]
- Bastian H. On the symptomatology of total transverse lesions of the spinal cord, with special reference to the condition of the various reflexes. Med-Chir Trans Lond. 1890;73:151–217. doi: 10.1177/095952879007300113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beghi E, Logroscino G, Chio A, Hardiman O, Mitchell D, Swingler R, Traynor BJ. The epidemiology of ALS and the role of population-based registries. Biochim Biophys Acta. 2006;1762:1150–1157. doi: 10.1016/j.bbadis.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Bendotti C, Calvaresi N, Chiveri L, Prelle A, Moggio M, Braga M, et al. Early vacuolization and mitochondrial damage in motor neurons of FALS mice are not associated with apoptosis or with changes in cytochrome oxidase histochemical reactivity. J Neurol Sci. 2001;191:25–33. doi: 10.1016/s0022-510x(01)00627-x. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini MA. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma. 1999;16:69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA. Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol. 2004;91:2247–2258. doi: 10.1152/jn.00946.2003. [DOI] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Berger A, Bayliss D, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Bergmann F, Keller BU. Impact of mitochondrial inhibition on excitability and cytosolic Ca2+ levels in brainstem motoneurones from mouse. J Physiol. 2004;555:45–59. doi: 10.1113/jphysiol.2003.053900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman G, Hulliger M, Lee RG, Tako K, Tanaka R. Reciprocal Ia inhibition in patients with spinal spasticity. Neurosci Lett. 1991;127:57–60. doi: 10.1016/0304-3940(91)90894-y. [DOI] [PubMed] [Google Scholar]
- Boorman GI, Lee RG, Becker WJ, Windhorst UR. Impaired “natural reciprocal inhibition” in patients with spasticity due to incomplete spinal cord injury. Electroencephalogr Clin Neurophysiol. 1996;101:84–92. doi: 10.1016/0924-980x(95)00262-j. [DOI] [PubMed] [Google Scholar]
- Bories C, Amendola J, Lamotte d'Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:451–459. doi: 10.1111/j.1460-9568.2007.05306.x. [DOI] [PubMed] [Google Scholar]
- Bras H, Jankowska E, Noga B, Skoog B. Comparison of effects of various types of NA and 5-HT agonists on transmission from group II muscle afferents in the cat. Eur J Neurosci. 1990;2:1029–1039. doi: 10.1111/j.1460-9568.1990.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Brett MM, William GJ, Jon WG, John HM. Time course of neuropathology in the spinal cord of G86R superoxide dismutase transgenic mice. J Comp Neurol. 1998;391:64–77. doi: 10.1002/(sici)1096-9861(19980202)391:1<64::aid-cne6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Brooks BR. Risk factors in the early diagnosis of ALS: North American epidemiological studies. ALS CARE Study Group. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1 Suppl. 1:S19–S26. doi: 10.1080/14660820052415871. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Bui TV, Ter-Mikaelian M, Bedrossian D, Rose PK. Computational estimation of the distribution of L-type Ca2+ channels in motoneurons based on variable threshold of activation of persistent inward currents. J Neurophysiol. 2006;95:225–241. doi: 10.1152/jn.00646.2005. [DOI] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK. Multiple modes of amplification of synaptic inhibition to motoneurons by persistent inward currents. J Neurophysiol. 2008;99:571–582. doi: 10.1152/jn.00717.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. Spasticity as an adaptation to pyramidal tract injury. Adv Neurol. 1988;47:401–423. [PubMed] [Google Scholar]
- Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriedo SG, Sensi SL, Yin HZ, Weiss JH. AMPA exposures induce mitochondrial Ca(2+) overload and ROS generation in spinal motor neurons in vitro. J Neurosci. 2000;20:240–250. doi: 10.1523/JNEUROSCI.20-01-00240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol. 2006;80:241–268. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Charcot J, Joffroy A. Deux cas d’atrophie musculaire progressive avec lésions de la substance grise et de faisceaux anté rolatéraux de la moelle épinie‘re. Archives de Physiologie normale et pathologique. 1869:744–757. [Google Scholar]
- Chattopadhyay M, Valentine JS. Aggregation of copper-zinc superoxide dismutase in familial and sporadic ALS. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu AY, Zhai P, Dal Canto MC, Peters TM, Kwon YW, Prattis SM, et al. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995;6:349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- Clarke RW, Eves S, Harris J, Peachey JE, Stuart E. Interactions between cutaneous afferent inputs to a withdrawal reflex in the decerebrated rabbit and their control by descending and segmental systems. Neuroscience. 2002;112:555–571. doi: 10.1016/s0306-4522(02)00093-3. [DOI] [PubMed] [Google Scholar]
- Cleland CL, Rymer WZ. Neural mechanisms underlying the clasp-knife reflex in the cat. I. Characteristics of the reflex. J Neurophysiol. 1990;64:1303–1318. doi: 10.1152/jn.1990.64.4.1303. [DOI] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci. 2001;21:4059–4065. doi: 10.1523/JNEUROSCI.21-11-04059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Gorassini MA, Bennett DJ, Burke D, Gandevia SC. Recent evidence for plateau potentials in human motoneurones. Adv Exp Med Biol. 2002;508:227–235. doi: 10.1007/978-1-4615-0713-0_28. [DOI] [PubMed] [Google Scholar]
- Corona JC, Tapia R. Ca2+-permeable AMPA receptors and intracellular Ca2+ determine motoneuron vulnerability in rat spinal cord in vivo. Neuropharmacology. 2007;52:1219–1228. doi: 10.1016/j.neuropharm.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain. 2003;126:495–507. doi: 10.1093/brain/awg036. [DOI] [PubMed] [Google Scholar]
- Dal Canto MC, Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu, Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS) Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, et al. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem. 2006;96:1349–1361. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- De Vos KJ, Grierson AJ, Ackerley S, Miller CC. Role of axonal transport in neurodegenerative diseases. Annu Rev Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier J-F, Hounsgaard J. Local facilitation of plateau potentials in dendrites of turtle motoneurones by synaptic activation of metabotropic receptors. J Physiol (Lond) 1999;515:203–207. doi: 10.1111/j.1469-7793.1999.203ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide P. Human monosynaptic reflexes and presynaptic inhibition. In: Desmedt J, editor. New developments in electromyography and clinical neurophysiology. Basel: Karger; 1973. pp. 508–522. [Google Scholar]
- Delwaide P, Pennisi G. Tizanidine and electrophysiologic analysis of spinal control mechanisms in humans with spasticity. Neurology. 1994;44:S21–S27. [discussion S27–28] [PubMed] [Google Scholar]
- Dolphin AC. Facilitation of Ca2+ current in excitable cells. Trends Neurosci. 1996;19:35–43. doi: 10.1016/0166-2236(96)81865-0. [DOI] [PubMed] [Google Scholar]
- Duch C, Vonhoff F, Ryglewski S. Dendrite elongation and dendritic branching are affected separately by different forms of intrinsic motoneuron excitability. J Neurophysiol. 2008;100:2525–2536. doi: 10.1152/jn.90758.2008. [DOI] [PubMed] [Google Scholar]
- Dunckley T, Huentelman MJ, Craig DW, Pearson JV, Szelinger S, Joshipura K, et al. Whole-genome analysis of sporadic amyotrophic lateral sclerosis. N Engl J Med. 2007;357:775–788. doi: 10.1056/NEJMoa070174. [DOI] [PubMed] [Google Scholar]
- Durand J, Amendola J, Bories C, Lamotte d’Incamps B. Early abnormalities in transgenic mouse models of amyotrophic lateral sclerosis. J Physiol-Paris. 2006;99:211–220. doi: 10.1016/j.jphysparis.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Edwards S. Neurological physiotherapy: a problem-solving approach. 2nd ed. Churchill Livingstone; 2002. [Google Scholar]
- ElBasiouny SM, Mushahwar VK. Suppressing the excitability of spinal motoneurons by extracellularly applied electrical fields: insights from computer simulations. J Appl Physiol. 2007;103:1824–1836. doi: 10.1152/japplphysiol.00362.2007. [DOI] [PubMed] [Google Scholar]
- ElBasiouny SM, Bennett DJ, Mushahwar VK. Simulation of dendritic Cav1.3 channels in cat lumbar motoneurons: spatial distribution. J Neurophysiol. 2005;94:3961–3974. doi: 10.1152/jn.00391.2005. [DOI] [PubMed] [Google Scholar]
- ElBasiouny SM, Bennett DJ, Mushahwar VK. Simulation of Ca+2 persistent inward currents in spinal motoneurons: mode of activation and integration of synaptic inputs. J Physiol (Lond) 2006;570:355–374. doi: 10.1113/jphysiol.2005.099119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElBasiouny SM, Moroz D, Bakr MM, Mushahwar VK. Management of spasticity after spinal cord injury: current techniques and future directions. Neurorehabil Neural Repair. 2010a;24:23–33. doi: 10.1177/1545968309343213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElBasiouny SM, Amendola J, Durand J, Heckman CJ. Evidence from computer simulations for alterations in the membrane biophysical properties and dendritic processing of synaptic inputs in mutant SOD1 motoneurons. J Neuroscience. 2010 doi: 10.1523/JNEUROSCI.0434-10.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott P, Wallis DI. Serotonin and l-norepinephrine as mediators of altered excitability in neonatal rat motoneurons studied in vitro. Neuroscience. 1992;47:533–544. doi: 10.1016/0306-4522(92)90163-v. [DOI] [PubMed] [Google Scholar]
- Faist M, Mazevet D, Dietz V, Pierrot-Deseilligny E. A quantitative assessment of presynaptic inhibition of la afferents in spastics: differences in hemiplegics and paraplegics. Brain. 1994;117:1449–1455. doi: 10.1093/brain/117.6.1449. [DOI] [PubMed] [Google Scholar]
- Fellows SJ, Ross HF, Thilmann AF. The limitations of the tendon jerk as a marker of pathological stretch reflex activity in human spasticity. J Neurol, Neurosurg Psychiatry. 1993;56:531–537. doi: 10.1136/jnnp.56.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran E, Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1587–1602. doi: 10.1089/ars.2009.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol. 2008;578:171–176. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Garraway SM, Hochman S. Modulatory actions of serotonin, norepinephrine, dopamine, and acetylcholine in spinal cord deep dorsal horn neurons. J Neurophysiol. 2001;86:2183–2194. doi: 10.1152/jn.2001.86.5.2183. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Granit R, Kernell D, Shortess G. Quantitative aspects of repetitive firing of mammalian motoneurones, caused by injected currents. J Physiol. 1963;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros-Louis F, Gaspar C, Rouleau GA. Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:956–972. doi: 10.1016/j.bbadis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Grosskreutz J, Haastert K, Dewil M, Van Damme P, Callewaert G, Robberecht W, et al. Role of mitochondria in kainate-induced fast Ca2+ transients in cultured spinal motor neurons. Cell Calcium. 2007;42:59–69. doi: 10.1016/j.ceca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. Non-volatile general anaesthetics reduce spinal activity by suppressing plateau potentials. Neuroscience. 1999;88:353–358. doi: 10.1016/s0306-4522(98)00371-6. [DOI] [PubMed] [Google Scholar]
- Gurney M, Pu H, Chiu A, Dal Canto M, Polchow C, Alexander D, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hammar I, Jankowska E. Modulatory effects of alpha 1-, alpha 2-, and beta -receptor agonists on feline spinal interneurons with monosynaptic input from group I muscle afferents. J Neurosci. 2003;23:332–338. doi: 10.1523/JNEUROSCI.23-01-00332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2006a;96:1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol. 2006b;96:1171–1186. doi: 10.1152/jn.00341.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath PR, Shaw PJ. Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:438–458. doi: 10.1002/mus.10186. [DOI] [PubMed] [Google Scholar]
- Heckman C, Gorassini M, Bennett D. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Hyngstrom AS, Johnson MD. Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol. 2008a;586:1225–1231. doi: 10.1113/jphysiol.2007.145078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist. 2008b;14:264–275. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Mottram C, Quinlan K, Theiss R, Schuster J. Motoneuron excitability: the importance of neuromodulatory inputs. Clin Neurophysiol. 2009;120:2040–2054. doi: 10.1016/j.clinph.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28:154–164. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Tyreman N, Gordon T. Preferential motor unit loss in the SOD1G93A transgenic mouse model of amyotrophic lateral sclerosis. J Physiol. 2008 doi: 10.1113/jphysiol.2007.149286. doi:jphysiol.2007.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberry RC, Novelli A, Cox JA, Lysko PG. Neurotoxicity at the N-methyl-d-aspartate receptor in energy-compromised neurons. An hypothesis for cell death in aging and disease. Ann N Y Acad Sci. 1989;568:225–233. doi: 10.1111/j.1749-6632.1989.tb12512.x. [DOI] [PubMed] [Google Scholar]
- Hiersemenzel L-P, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology. 2000;54:1574–1582. doi: 10.1212/wnl.54.8.1574. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Ca++ dependent bistability induced by serotonin in spinal motoneurons. Exp Brain Res. 1985;57:422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol. 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res. 1984;55:391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O, Mintz I. Response properties of motoneurones in a slice preparation of the turtle spinal cord. J Physiol. 1988a;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988b;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CF, Trueblood PR, Levine MS, Chandler SH. Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. J Neurophysiol. 1997;77:2910–2924. doi: 10.1152/jn.1997.77.6.2910. [DOI] [PubMed] [Google Scholar]
- Hsiao C-F, Negro CAD, Trueblood PR, Chandler SH. Ionic basis for serotonin-induced bistable membrane properties in guinea pig trigeminal motoneurons. J Neurophysiol. 1998;79:2847–2856. doi: 10.1152/jn.1998.79.6.2847. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog Brain Res. 1999;123:39–48. doi: 10.1016/s0079-6123(08)62842-3. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Kiehn O. Neuromodulation of vertebrate motor neuron membrane properties. Curr Opin Neurobiol. 1992;2:770–775. doi: 10.1016/0959-4388(92)90132-5. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Nielsen J. Modulation of transmitter release from Ia afferents by their preceding activity – a ‘postactivation depression’. In: Rudomin P, Romo R, Mendell LM, editors. Presynaptic inhibition and neural control. New York: Oxford University Press; 1998. pp. 178–191. [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol (Lond) 2003;552:945–952. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input–output gain across the motoneuron pool. Prog Brain Res. 2004;143:77–95. doi: 10.1016/s0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Dalton MJ, Gurney ME, Kopito RR. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2000;97:12571–12576. doi: 10.1073/pnas.220417997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, Kwak S, Sun H, Ito K, Hashida H, Aizawa H, et al. Human spinal motoneurons express low relative abundance of GluR2 mRNA: an implication for excitotoxicity in ALS. J Neurochem. 2003;85:680–689. doi: 10.1046/j.1471-4159.2003.01703.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. High-frequency repetitive firing of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiol Scand. 1965;65:74–86. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. The motoneurone and its muscle fibres. New York: Oxford University Press; 2006. [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kieran D, Hafezparast M, Bohnert S, Dick JR, Martin J, Schiavo G, et al. A mutation in dynein rescues axonal transport defects and extends the life span of ALS mice. J Cell Biol. 2005;169:561–567. doi: 10.1083/jcb.200501085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman P. Changes in vesicular glutamate transporter 2, vesicular GABA transporter and vesicular acetylcholine transporter labeling of sacrocaudal motoneurons in the spastic rat. Exp Neurol. 2006;197:407–419. doi: 10.1016/j.expneurol.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Kitzman P. VGLUT1 and GLYT2 labeling of sacrocaudal motoneurons in the spinal cord injured spastic rat. Exp Neurol. 2007;204:195–204. doi: 10.1016/j.expneurol.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Kostera-Pruszczyk A, Niebroj-Dobosz I, Emeryk-Szajewska B, Karwanska A, Rowinska-Marcinska K. Motor unit hyperexcitability in amyotrophic lateral sclerosis vs amino acids acting as neurotransmitters. Acta Neurol Scand. 2002;106:34–38. doi: 10.1034/j.1600-0404.2002.00149.x. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Macht M. Some manifestations of reflex activity in spinal man with particular reference to the occurrence of extensor spasm. Bull Johns Hopkins Hosp. 1948;84:43–85. [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Johnson MD, Heckman HM, Heckman C. Active dendritic integration of inhibitory synaptic inputs in vivo. J Neurophysiol. 2003;90:3617–3624. doi: 10.1152/jn.00521.2003. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Schonewille M, Siddique T, Schults ANA, Fu R, Bar PR, et al. Hyperexcitability of cultured spinal motoneurons from presymptomatic ALS mice. J Neurophysiol. 2004;91:571–575. doi: 10.1152/jn.00665.2003. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na+ current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol (Lond) 2005;563:843–854. doi: 10.1113/jphysiol.2004.074138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance J. Pathophysiology of spasticity and clinical experience with Baclofen. In: Lance J, Feldman R, Young R, Koella W, editors. Spasticity: disordered motor control. Chicago: Yearbook; 1980. pp. 185–204. [Google Scholar]
- Lance J, Burke D. Mechanisms of spasticity. Arch Phys Med Rehabil. 1974;55:332–337. [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol. 1996;76:2107–2110. doi: 10.1152/jn.1996.76.3.2107. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998a;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998b;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha 1 agonist methoxamine. J Neurophysiol. 1999;81:2164–2174. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. J Neurophysiol. 2001;85:472–475. doi: 10.1152/jn.2001.85.1.472. [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004a;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol. 2004b;91:2236–2246. doi: 10.1152/jn.01010.2003. [DOI] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2007;97:1236–1246. doi: 10.1152/jn.00995.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay A, Feldman J. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol. 1993:461. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J, Micklesen P, Umlauf R, Britell C. Lower extremity manifestations of spasticity in chronic spinal cord injury. Am J Phys Med Rehabil. 1989;68:32–36. doi: 10.1097/00002060-198902000-00009. [DOI] [PubMed] [Google Scholar]
- Magrane J, Manfredi G. Mitochondrial function, morphology, and axonal transport in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque P, Simonetta-Moreau M, Maupas E, Roques CF. Facilitation of transmission in heteronymous group II pathways in spastic hemiplegic patients. J Neurol, Neurosurg, Psychiatry. 2001;70:36–42. doi: 10.1136/jnnp.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell L, Maxwell DJ, Neilson M, Kerr R. A confocal microscopic survey of serotoninergic axons in the lumbar spinal cord of the rat: co-localization with glutamate decarboxylase and neuropeptides. Neuroscience. 1996;75:471–480. doi: 10.1016/0306-4522(96)00366-1. [DOI] [PubMed] [Google Scholar]
- Miller JF, Paul KD, Rymer WZ, Heckman CJ. 5-HT1B/1D agonist CGS-12066B attenuates clasp knife reflex in the cat. J Neurophysiol. 1995;74:453–456. doi: 10.1152/jn.1995.74.1.453. [DOI] [PubMed] [Google Scholar]
- Moritz AT, Newkirk G, Powers RK, Binder MD. Facilitation of somatic calcium channels can evoke prolonged tail currents in rat hypoglossal motoneurons. J Neurophysiol. 2007;98:1042–1047. doi: 10.1152/jn.01294.2006. [DOI] [PubMed] [Google Scholar]
- Mourelatos Z, Gonatas N, Stieber A, Gurney M, Dal Canto M. The Golgi apparatus of spinal cord motor neurons in transgenic mice expressing mutant Cu, Zn. Proc Natl Acad Sci USA. 1996;93:5472–5477. doi: 10.1073/pnas.93.11.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacimiento W, Noth J. What, if anything, is spinal shock? Arch Neurol. 1999;56:1033–1035. doi: 10.1001/archneur.56.8.1033. [DOI] [PubMed] [Google Scholar]
- Nelson L, McGuire D. Epidemiology of amyotrophic lateral sclerosis. 2nd ed. London, UK: Informa Healthcare; 2006. [Google Scholar]
- Newton B, Hamill R. The morphology and distribution of rat serotoninergic intraspinal neurons: an immunohistochemical study. Brain Res Bull. 1988;20:349–360. doi: 10.1016/0361-9230(88)90064-0. [DOI] [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC. Forces consistent with plateau-like behaviour of spinal neurons evoked in patients with spinal cord injuries. Brain. 2004;127:660–670. doi: 10.1093/brain/awh073. [DOI] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC, Gorassini MA. Changes in sensory-evoked synaptic activation of motoneurons after spinal cord injury in man. Brain. 2008;131:1478–1491. doi: 10.1093/brain/awn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli A, Reilly JA, Lysko PG, Henneberry RC. Glutamate becomes neurotoxic via the N-methyl-d-aspartate receptor when intracellular energy levels are reduced. Brain Research. 1988;451:205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- Pambo-Pambo A, Durand J, Gueritaud JP. Early excitability changes in lumbar motoneurons of transgenic SOD1G85R and SOD1G93A-Low mice. J Neurophysiol. 2009;102:3627–3642. doi: 10.1152/jn.00482.2009. [DOI] [PubMed] [Google Scholar]
- Pandyan A, Gregoric M, Barnes M, Wood D, Van Wijck F, Burridge J, et al. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil. 2005;27:2–6. doi: 10.1080/09638280400014576. [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Patel R, Kerr R, Maxwell DJ. Absence of co-localized glutamic acid decarboxylase and neuropeptides in noradrenergic axons of the rat spinal cord. Brain Res. 1997;749:164–169. doi: 10.1016/s0006-8993(96)01399-6. [DOI] [PubMed] [Google Scholar]
- Perrier J-F, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol. 2003;89:954–959. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Mejia-Gervacio S, Hounsgaard J. Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol. 2000;528(Pt 1):107–113. doi: 10.1111/j.1469-7793.2000.t01-1-00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier J-F, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain Res Rev. 2002;40:223–229. doi: 10.1016/s0165-0173(02)00204-7. [DOI] [PubMed] [Google Scholar]
- Pieri M, Albo F, Gaetti C, Spalloni A, Bengtson CP, Longone P, et al. Altered excitability of motor neurons in a transgenic mouse model of familial amyotrophic lateral sclerosis. Neurosci Lett. 2003;351:153–156. doi: 10.1016/j.neulet.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Pieri M, Carunchio I, Curcio L, Mercuri NB, Zona C. Increased persistent sodium current determines cortical hyperexcitability in a genetic model of amyotrophic lateral sclerosis. Exp Neurol. 2009;215:368–379. doi: 10.1016/j.expneurol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The pathophysiology of spasticity and parkinsonian rigidity. In: Pierrot-Deseilligny E, Burke D, editors. The circuitry of the human spinal cord: its role in motor control and movement disorders. New York: Cambridge University Press; 2005. pp. 556–599. [Google Scholar]
- Piotrkiewicz M, Kudina L, Mierzejewska J, Hausmanowa-Petrusewicz I. Analysis of double discharges in amyotrophic lateral sclerosis. Muscle Nerve. 2008;38:845–854. doi: 10.1002/mus.20997. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC. Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol. 2008;100:292–303. doi: 10.1152/jn.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Nixon RA. Defective neurofilament transport in mouse models of amyotrophic lateral sclerosis: a review. Neurochem Res. 2003;28:1041–1047. doi: 10.1023/a:1023259207015. [DOI] [PubMed] [Google Scholar]
- Redmond L, Ghosh A. Regulation of dendritic development by calcium signaling. Cell Calcium. 2005;37:411–416. doi: 10.1016/j.ceca.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Ren K, Ruda MA. A comparative study of the calcium-binding proteins calbindin-D28K, calretinin, calmodulin and parvalbumin in the rat spinal cord. Brain Res. 1994;19:163–179. doi: 10.1016/0165-0173(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Robberecht W. Oxidative stress in amyotrophic lateral sclerosis. J Neurol. 2000;247 Suppl. 1:I1–I6. doi: 10.1007/s004150050551. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Russo R, Hounsgaard J. Short-term plasticity in turtle dorsal horn neurons mediated by L-type Ca2+ channels. Neuroscience. 1994;61:191–197. doi: 10.1016/0306-4522(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Plateau-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. J Physiol. 1996;493(Pt 1):39–54. doi: 10.1113/jphysiol.1996.sp021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia. 2005;51:241–253. doi: 10.1002/glia.20210. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. A persistent negative resistance in cat lumbar motoneurons. Brain Res. 1977;120:173–178. doi: 10.1016/0006-8993(77)90510-8. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980a;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Role of a persistent inward current in motoneuron bursting during spinal seizures. J Neurophysiol. 1980b;43:1296–1318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol. 1982;48:875–890. doi: 10.1152/jn.1982.48.4.875. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Neurotransmission by neurons that use serotonin, noradrenaline, glutamate, glycine, and gamma-aminobutyric acid in the normal and injured spinal cord. Neurosurgery. 1997;40:168–176. doi: 10.1097/00006123-199701000-00037. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Observations on the scratch-reflex in the spinal dog. J Physiol. 1906;34:1–50. doi: 10.1113/jphysiol.1906.sp001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Gal J, Kwinter DM, Liu X, Zhu H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbadis.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swash M, Ingram D. Preclinical and subclinical events in motor neuron disease. J Neurol, Neurosurg, Psychiatry. 1988;51:165–168. doi: 10.1136/jnnp.51.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N, Kuwabara S, Misawa S, Kanai K, Nakata M, Sawai S, et al. Increased nodal persistent Na+ currents in human neuropathy and motor neuron disease estimated by latent addition. Clin Neurophysiol. 2006;117:2451–2458. doi: 10.1016/j.clinph.2006.07.309. [DOI] [PubMed] [Google Scholar]
- Thompson F, Parmer R, Reier P. Alteration in rate modulation of reflexes to lumbar motoneurons after midthoracic spinal cord injury in the rat. I. Contusion injury. J Neurotrauma. 1998;15:495–508. doi: 10.1089/neu.1998.15.495. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Lopes EC, Cheema SS. Neuromuscular accumulation of mutant superoxide dismutase 1 aggregates in a transgenic mouse model of familial amyotrophic lateral sclerosis. Neurosci Lett. 2003;350:132–136. doi: 10.1016/s0304-3940(03)00893-0. [DOI] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Van Damme P, Dewil M, Robberecht W, Van Den Bosch L. Excitotoxicity and amyotrophic lateral sclerosis. Neuro-degener Dis. 2005;2:147–159. doi: 10.1159/000089620. [DOI] [PubMed] [Google Scholar]
- Van Den Bosch L, Vandenberghe W, Klaassen H, Van Houtte E, Robberecht W. Ca(2+)-permeable AMPA receptors and selective vulnerability of motor neurons. J Neurol Sci. 2000;180:29–34. doi: 10.1016/s0022-510x(00)00414-7. [DOI] [PubMed] [Google Scholar]
- Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:1068–1082. doi: 10.1016/j.bbadis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Van Zundert B, Peuscher MH, Hynynen M, Chen A, Neve RL, Brown RH, Jr, et al. Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Neurosci. 2008;28:10864–10874. doi: 10.1523/JNEUROSCI.1340-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe W, Robberecht W, Brorson JR. AMPA receptor calcium permeability, GluR2 expression, and selective motoneuron vulnerability. J Neurosci. 2000a;20:123–132. doi: 10.1523/JNEUROSCI.20-01-00123.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe W, Ihle EC, Patneau DK, Robberecht W, Brorson JR. AMPA receptor current density, not desensitization, predicts selective motoneuron vulnerability. J Neurosci. 2000b;20:7158–7166. doi: 10.1523/JNEUROSCI.20-19-07158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Kiernan MC. Cortical excitability testing distinguishes Kennedy’s disease from amyotrophic lateral sclerosis. Clin Neurophysiol. 2008;119:1088–1096. doi: 10.1016/j.clinph.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008;131:1540–1550. doi: 10.1093/brain/awn071. [DOI] [PubMed] [Google Scholar]
- Vucic S, Cheah BC, Krishnan AV, Burke D, Kiernan MC. The effects of alterations in conditioning stimulus intensity on short interval intracortical inhibition. Brain Res. 2009;1273:39–47. doi: 10.1016/j.brainres.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Wohlfart G. Clinical considerations on innervation of skeletal muscle. Am J Phys Med. 1959;38:223–230. [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Young RR. Spasticity: a review. Neurology. 1994;44:S12–S20. [PubMed] [Google Scholar]
- Zanette G, Tamburin S, Manganotti P, Refatti N, Forgione A, Rizzuto N. Different mechanisms contribute to motor cortex hyperexcitability in amyotrophic lateral sclerosis. Clin Neurophysiol. 2002;113:1688–1697. doi: 10.1016/s1388-2457(02)00288-2. [DOI] [PubMed] [Google Scholar]
- Zoccolella S, Beghi E, Palagano G, Fraddosio A, Samarelli V, Lamberti P, et al. Predictors of delay in the diagnosis and clinical trial entry of amyotrophic lateral sclerosis patients: a population-based study. J Neurol Sci. 2006;250:45–49. doi: 10.1016/j.jns.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Zona C, Pieri M, Carunchio I. Voltage-dependent sodium channels in spinal cord motor neurons display rapid recovery from fast inactivation in a mouse model of amyotrophic lateral sclerosis. J Neurophysiol. 2006;96:3314–3322. doi: 10.1152/jn.00566.2006. [DOI] [PubMed] [Google Scholar]